Abstract
Melt spun ribbons of Fe95−x Zr x B4Cu1 with x = 7 (Z7B4) and 9 (Z9B4) alloys have been prepared, and their structure and magnetic properties have been evaluated using XRD, DSC, TEM, VSM, and Mössbauer spectroscopy. The glass forming ability (GFA) of both alloys has been calculated theoretically using thermodynamical parameters, and Z9B4 alloy is found to possess higher GFA than that of Z7B4 alloy which is validated by XRD results. On annealing, the amorphous Z7B4 ribbon crystallizes into nanocrystalline α-Fe, whereas amorphous Z9B4 ribbon shows two-stage crystallization process, first partially to bcc solid solution which is then transformed to nanocrystalline α-Fe and Fe2Zr phases exhibiting bimodal distribution. A detailed phase analysis using Mössbauer spectroscopy through hyperfine field distribution of phases has been carried out to understand the crystallization behavior of Z7B4 and Z9B4 alloy ribbons. In order to understand the phase transformation behavior of Z7B4 and Z9B4 ribbons, molar Gibbs free energies of amorphous, α-Fe, and Fe2Zr phases have been evaluated. It is found that in case of Z7B4, α-Fe is always a stable phase, whereas Fe2Zr is stable at higher temperature for Z9B4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fe88Z7B4Cu1 (NANOPERM) alloys with improved soft magnetic properties developed by Suzuki et al.[1] exhibit unique combination of soft magnetic properties such as high saturation, high Curie temperature, high permeability, low coercivity, and high resistivity which are superior to other conventional soft magnetic materials. The ultra-soft magnetic properties arises in these materials due to the coexistence of amorphous and nanograins. According to random anisotropy theory, the exchange coupling between nanocrystalline grains and amorphous matrix results in averaging out of magnetocrystalline anisotropy which forces the magnetization vector to be aligned parallel to the field direction.[2] The saturation magnetostriction becomes almost zero due to the positive and negative contributions from the amorphous and nanocrystalline phases, respectively.[3] These alloys are generally processed by rapid solidification (melt spinning) method to produce an amorphous phase followed by controlled heat treatment to obtain nanocrystalline precipitates in amorphous matrix.
Our earlier work has shown that, on decreasing the wheel speed (lower cooling rate), the structure of Fe88Z7B4Cu1 alloy changes from completely amorphous phase to the cellular structure of bcc solid solution coexisting with the amorphous phase at the intercellular region, thus affecting the soft magnetic properties in the as-spun condition.[4] Shih et al.[5] have estimated the volume fraction of nanocrystalline bcc α-Fe phase, residual amorphous phase, and Fe23Zr6 phase in melt spun ribbon of Fe89Z7B3Cu1 alloy annealed at 898 K (625 °C) using Mössbauer technique and also reported that these estimates are consistent with atom probe study reported by Hono.[6] In a similar way, Mössbauer technique was used by us to find out the composition and volume fraction of nanocrystalline bcc α-Fe(Co) phase in (Fe1−x Co x )88Z7B4Cu1 melt spun ribbons in an earlier report.[7]
Recently, we have reported the structure and soft magnetic properties of two series of alloys, namely Fe99−x−y Zr x B y Cu1 with x + y = 11 and x + y = 13.[8] In this study, the glass forming ability (GFA) of the alloys has been calculated based on thermodynamic properties and verified with experimental results. Moreover, a soft magnetic property vs Zr concentration map has been established and alloys having better soft magnetic properties have been identified. Interestingly, it was found that Fe86Zr9B4Cu1 (Z9B4) alloy has very low coercivity and low saturation magnetization in the as-spun condition. Unlike other alloys, upon annealing, the coercivity increases drastically and saturation magnetization increases gradually. In this paper, we also report a detailed study on phase transformation behavior of amorphous Fe86Zr9B4Cu1 (Z9B4) ribbon and compare the results with Fe88Zr7B4Cu1 (Z7B4) ribbon which has been studied extensively. Thermodynamic calculations have been carried out for understanding the stability of the phases.
2 Experimental Details
Two alloys of nominal compositions Fe88Z7B4Cu1 (termed as Z7B4) and Fe86Z9B4Cu1 (termed as Z9B4) have been prepared by melting the eutectic Fe-Zr master alloy together with high-purity Fe, B, and Cu in a vacuum induction furnace (2 × 10−4 mbar). Rapidly solidified ribbons (2 to 5 mm wide and 30 to 100 µm thick) of all alloys were produced in an argon atmosphere using a vacuum melt spinner (initially evacuated up to a vacuum level of 1 × 10−4 mbar) at different wheel speed conditions. All ribbons were then vacuum annealed (2 × 10−5 mbar) at different temperatures ranging from 773 K to 858 K (500 °C to 585 °C) for 1 hour.
Structure of the as-spun and annealed ribbons was determined by X-ray diffractometer (PHILIPS PW1830 XRD) using Cu-Kα (λ = 1.54056 Å) radiation. A differential scanning calorimeter (NETSCH STA 409 PC DSC) was used to evaluate the thermal stability of the as-spun ribbons. Microstructural characterization was carried out using transmission electron microscope (FEI Tecnai-20UT TEM). Mössbauer experiments were carried out using a constant acceleration spectrometer (FAST ComTech, Germany) in transmission mode with 25 mCi, 57Co(Rh) source. Thermomagnetic curves for the as-spun ribbons have been measured using a vibrating sample magnetometer (ADE EV7 VSM) with an interval of 5 K (5 °C) equilibrating for 3 seconds up to 823 K (550 °C), keeping a constant field of 500 Oe.
3 Results
3.1 Structural Evolution
Figures 1(a) and (b) show the XRD patterns of melt spun Z7B4 and Z9B4 ribbons, respectively, with increasing order of thickness obtained by reducing the wheel speed. XRD pattern of 30-μm-thick Z7B4 ribbon (Figure 1(a)) exhibits fully amorphous phase, whereas for 40-μm-thick ribbon, the coexistence of bcc α-Fe phase along with amorphous phase can be observed. In case of Z9B4 ribbon (Figure 1(b)), the XRD patterns exhibit fully amorphous phase even up to a ribbon thickness of 100 μm. This experimentally proves that the Z9B4 ribbon has better GFA than that of Z7B4. The GFA of these alloys were calculated theoretically using a recent approach, proposed by Rao et al.[9] Accordingly, thermodynamic and topological models are combined to evolve a parameter, P HSS, which is defined as
where ΔH chem is the enthalpy of chemical mixing, ΔS σ is the mismatch entropy, ΔS c is the configurational entropy, R is the gas constant, and k B is Boltzmann constant. It was reported that the parameter, P HSS, correlates well with the GFA of bulk glass forming alloy systems.[10,11] The P HSS values of Z7B4 and Z9B4 alloys are −0.78 and −0.88 kJ/mol, respectively, revealing that Z9B4 alloy (more negative) has higher GFA than that of Z7B4.
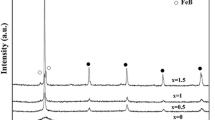
For further investigation on structure and soft magnetic properties of the as-spun and annealed ribbons, only 30-μm-thick ribbons of both alloys have been used as both are completely amorphous in the as-spun condition. Figure 2 shows DSC thermograms of the as-spun ribbons of Z7B4 and Z9B4 ribbons obtained at a heating rate of 20 K/min (20 °C/min). The Z7B4 as-spun ribbon exhibits two exothermic peaks with onset temperatures of 781 K and 1002 K (508 °C and 729 °C), whereas the Z9B4 ribbon has three exothermic peaks with onset temperatures, namely 699 K, 843 K, and 1001 K (426 °C, 570 °C, and 728 °C), indicating a three-stage crystallization process. Figure 3(a) shows the XRD pattern of Z7B4 ribbon annealed at 813 K (540 °C) for 1 hour. The XRD pattern can be indexed with bcc α-Fe phase. This indicates that the DSC peak for Z7B4 ribbon with onset at 781 K (508 °C) corresponds to the crystallization of α-Fe phase. Figure 3(b) shows the XRD patterns of Z9B4 ribbon, annealed at different temperatures [773 K to 858 K (500 °C to 585 °C)]. The indexing of the ribbon annealed at 773 K (500 °C) (which is between 1st and 2nd exothermic peaks in DSC thermogram) revealed the precipitation of bcc from amorphous phase. This implies that the 1st broad DSC peak of Z9B4 ribbon with onset at 699 K (426 °C) corresponds to the crystallization of bcc α-Fe phase. Comparing the result with Z7B4, it can be observed that the onset temperature of bcc phase decreases with Zr content. It is interesting to note that, although the GFA of Z9B4 is higher than that of Z7B4, the former crystallizes earlier. Indexing the XRD pattern of Z9B4 ribbon annealed at 813 K (540 °C) revealed the existence of Fe2Zr phase (C15 Laves phase with fcc structure) along with strong peak of bcc α-Fe phase. Further heat treating the ribbon at 858 K (585 °C), the XRD peak intensity of Fe2Zr phase is found to be high as compared to that of bcc phase. Since no other phase was found by indexing the XRD pattern of ribbon annealed at 858 K (585 °C), the 2nd DSC peak of Z9B4 ribbon with onset at 843 K (570 °C) can be assigned to be the crystallization of Fe2Zr phase. Detailed structural evolution of these two ribbons at different conditions and their crystallization temperatures are shown in Table I. Further, it can be observed that onset temperature of 2nd exothermic peak of Z7B4 ribbon [1002 K (729 °C)] and 3rd exothermic peak of Z9B4 ribbon [1001 K (728 °C)] are almost same. Comparing our results with the reported literature, it can be concluded that this higher temperature exothermic peak for both Z7B4 and Z9B4 ribbons corresponds to the formation Fe3Zr phase (C14 Laves phase) from the residual amorphous matrix.[12]
The precision lattice parameters (a 0) have been determined using Nelson–Riley (N–R) extrapolation method[13] in which lattice parameter, calculated from each peak, is plotted against the N–R function ((cos2 θ/sinθ) + (cos2 θ/θ)). A straight line is drawn by regression analysis and extrapolated up to zero value of N–R function. The intercept value gives the measure of the precision lattice parameter. The a 0 values of different specimens are given in Table II. The a 0 value of bcc phase of Z7B4 ribbon, annealed at 813 K (540 °C), is 2.8586 Å which is close to the a 0 of pure Fe (2.86 Å). In case of Z9B4 ribbon, the a 0 value of specimen annealed at 773 K (500 °C) is 2.8710 Å, whereas it becomes 2.8569 and 2.8587 Å for the specimens annealed at 813 K and 858 K (540 °C and 585 °C), respectively.
The activation energy of crystallization (E) was calculated from DSC thermograms obtained at different heating rates using Kissinger method.[14] The slope of plot of ln(β/T 2P ) vs 1/T P, for different β (heating rate) values, multiplied by universal gas constant yields the activation energy, where T P is peak temperature of the transformation. The activation energy of all phases is also shown in Table II. The activation energy of phase transformation corresponding to bcc phase for Z7B4 and Z9B4 is 299 and 64 kJ/mol, respectively, whereas the activation energy of phase transformation corresponding to Fe2Zr phase in Z9B4 alloy is 351 kJ/mol.
The enthalpy of crystallization per mol (∆H) for all phases has been evaluated from the area under the crystallization peak of DSC thermogram divided by the molecular weight of the particular phase. Again the weight fraction of individual phase was calculated from the volume fraction multiplied by the density of the respective phase. The volume fractions of α-Fe ((110) peak), Fe2Zr ((440) peak), and residual amorphous phase have been obtained from the area fraction of different phases which had been evaluated by deconvoluting the intense overlapping peak in the XRD patterns of annealed Z7B4 and Z9B4 ribbons. Figure 3(c) shows a typical deconvolution of intense XRD peak of Z9B4 ribbon annealed at 813 K (540 °C). The densities of α-Fe and Fe2Zr, obtained from the literature, are 7.87[15] and 7.82 g/cc, respectively.[16] Table II gives the calculated volume fraction of α-Fe, and residual amorphous phases for Z7B4 ribbon annealed at 813 K (540 °C) and α-Fe, Fe2Zr and residual amorphous phases for Z9B4 ribbon annealed at 858 K (585 °C). The ΔH per mol calculated for bcc α-Fe and Fe2Zr phases of Z9B4 ribbon are also shown in Table I. It can be observed that the enthalpy of bcc α-Fe phase reduces drastically with Zr content.
3.2 Transmission Electron Microscopy
Figures 4(a) and (b) show TEM bright field (BF) micrographs of the as-spun and 813 K (540 °C) annealed ribbons of Z7B4 alloy, respectively. The insets in both the figures show the selected area diffraction (SAD) pattern of the respective regions. The featureless contrast in Figure 4(a) and corresponding halo ring in SAD pattern indicate that the as-spun ribbon is fully amorphous. The BF image of Z7B4 ribbon annealed at 813 K (540 °C) shows the nanocrystalline precipitates in a featureless matrix (Figure 4(b)). The indexing of the corresponding ring pattern in SAD has revealed the formation of bcc α-Fe phase from the amorphous matrix. The size of these grains was measured within a BF image area of 500 nm × 600 nm for about 100 to 200 grains, and the histogram of grain size distribution has been plotted and is shown in Figure 4(c). The histogram was fitted with Lorentzian distribution curve and the fitted parameters are given in Table II. The average grain size (d av) obtained from the histogram is found to be 7.8 nm with a scatter in the grain size (FWHM) of 3 nm.
Figure 5(a) shows the BF image of Z9B4 as-spun ribbon and the inset is the corresponding SAD pattern. In this case also, the featureless contrast in BF image and corresponding halo ring in the SAD pattern reveal the presence of fully amorphous phase. Figure 5(b) shows the BF image of Z9B4 ribbon annealed at 773 K (500 °C) with the corresponding SAD pattern as inset. One can observe the formation of uniformly distributed nanocrystalline grains which have been indexed as bcc α-phase in the remaining amorphous matrix. Figure 5(c) shows the histogram of grain size distribution for Z9B4 ribbon annealed at 773 K (500 °C) which has been evaluated in the similar way to that for annealed Z7B4 ribbon. The parameters using Lorentzian fitting are given in Table II. The average grain size is 9.1 nm with the scatter in the grain size of 3.2. One can observe that the average grain size of bcc α-Fe phase in Z9B4 ribbon annealed at 773 K (500 °C) is larger than that the one obtained in Z7B4 ribbon annealed at 813 K (540 °C).
TEM bright field images of the as-spun ribbon (a) and ribbons annealed at 773 K (500 °C) (b), 813 K (540 °C) (d), and 858 K (585 °C) (f) of Z9B4 alloy. The corresponding histograms of grain size distribution of ribbons annealed at 773 K (500 °C) (c), 813 K (540 °C) (e) and 858 K (585 °C) (g). The insets show corresponding SAD patterns
Figure 5(d) shows the BF image and corresponding SAD pattern (inset) of Z9B4 ribbon annealed at 813 K (540 °C) revealing that the number density and size of nanocrystalline grains have increased considerably as compared to that of ribbon annealed at 773 K (500 °C). The indexing of SAD pattern has revealed the existence of Fe2Zr phase along with bcc α-phase. The fitted histogram of grain size (Figure 5(e)) shows a bimodal distribution with smaller grains having the average grain size of 5.3 nm and bigger grains having the average grain size of 14 nm (Table II). Figures 5(f) and (g) show BF image of ribbon annealed at 858 K (585 °C) with SAD pattern as inset and corresponding histogram of grain size respectively. Figure 5(g) also shows bimodal distribution of grain size with smaller grains having the average grain size of 11.6 nm and the bigger ones having the average grain size of 33 nm. The electron dispersive X-ray analysis (EDAX) results obtained from the bigger grain (33 nm) revealed the composition to be, Fe: 99.06, Zr: 0.66 and Cu: 0.28 at. pct. The composition of smaller grains could not be measured accurately because of the interference from the contribution of surrounding amorphous phase.
3.3 Magnetic Properties
Figures 6(a) and (b) show the Mössbauer spectra of the as-spun and 813 K (540 °C) annealed Z7B4 ribbons, respectively. All recorded spectra were analyzed using Lecaer program to get probability distribution of magnetic hyperfine fields P(H). Figures 6(c) and (d) show the probability distribution of hyperfine field of the as-spun and annealed Z7B4 ribbons corresponding to Figures 6(a) and (b), respectively. The as-spun Z7B4 ribbon shows a broad peak in hyperfine field distribution at 90 kOe which is the characteristic of amorphous phase and similar to the results published elsewhere.[17,18] The probability distribution of hyperfine field of ribbon annealed at 813 K (540 °C) exhibits three peaks, namely a sharp and intense peak at 325 kOe and broad and low intensity peaks at 266 and 131 kOe ,respectively. The peak at 325 kOe is due to the presence of α-Fe phase, whereas the peak at 266 kOe can be attributed to contribution from the interface of bcc α-Fe and amorphous phase and the peak at 131 kOe can be assigned to the contribution from residual amorphous phase.
Figures 7(a) through (d) show the Mössbauer spectra of the as-spun, 773 K, 813 K, and 858 K (500 °C, 540 °C, and 585 °C), annealed ribbons of Z9B4 alloy, respectively. The corresponding probability distributions of hyperfine field are shown in Figures 7(e) through (h), respectively. Similar to Z7B4 ribbon, the hyperfine field distribution (Figure 7(e)) of the as-spun Z9B4 ribbon exhibits a broad peak at 88 kOe, which is the typical characteristics of amorphous phase. In case of hyperfine field distribution of Z9B4 ribbon annealed at 773 K (500 °C) (Figure 7(f)), two small peaks can be observed at 287 and 249 kOe, in addition to the amorphous peak at 86 kOe. These small peaks at 287 and 249 kOe may be attributed to the contribution from the Fe-based bcc solid solution and interface between the amorphous and bcc phase, respectively. Due to the presence of alloying elements such as Zr, B, and Cu, inside the bcc α-Fe lattice, the hyperfine field value of Fe-based bcc solid solution (287 kOe) is far less than that of pure Fe (330 kOe). The hyperfine field distribution of Z9B4 ribbon annealed at 813 K (540 °C) is shown in Figure 7(g). In addition to the amorphous peak at 91 kOe and almost pure Fe phase peak at 324 kOe, two kinks at 184 and 221 kOe can be observed. These are attributed to two distinguishable iron sites in Fe2Zr phase.[19] It can be pointed out that the contribution from interface could not be deconvoluted. Figure 7(h) shows the hyperfine field distribution of ribbon annealed at 858 K (585 °C) which is almost similar to that of ribbon annealed at 813 K (540 °C) except for the fact that the intensities of hyperfine field correspond to Fe2Zr phase have increased.
Mössbauer spectra of Z9B4 ribbons: (a) as-spun ribbon and ribbons annealed at (b) 773 K (500 °C), (c) 813 K (540 °C), and (d) 858 K (585 °C). The corresponding probable distribution of hyperfine field for (e) as-spun ribbon and ribbons annealed at (f) 773 K (500 °C), (g) 813 K (540 °C) and (h) 858 K (585 °C)
Figure 8(a) shows the thermomagnetic curves (obtained at 500 Oe applied field) of the as-spun and 813 K (540 °C) annealed ribbon of Z7B4 alloy up to 823 K (550 °C). In case of the as-spun ribbon, one can observe that the thermomagnetic curve suddenly drops down at 329 K (56 °C), remains constant at near zero value up to around 773 K (500 °C), and then increases sharply. The initial decrease of thermomagnetic curve is associated with the ferro to paramagnetic transition (T c) of amorphous phase, whereas the sharp increase in magnetization at high temperature is associated with the crystallization of α-Fe phase whose Curie temperature (T c) is higher than 823 K (550 °C). The magnetization of annealed ribbon shows a monotonous decrease till 823 K (550 °C).
Figure 8(b) shows the thermomagnetic curves of the as-spun and different temperature-annealed Z9B4 ribbons. The magnetization behavior of the as-spun Z9B4 ribbon is similar to that of the as-spun Z7B4 ribbon. The Curie temperature of amorphous phase is 346 K (73 °C), whereas the crystallization of α-Fe phase from amorphous matrix is 808 K (535 °C). The thermomagnetic curve of ribbon annealed at 813 K (540 °C) shows a 1st Curie temperature around 374 K (101 °C), subsequently forms a step followed by dropping down the magnetization value up to 0.1 T around 349 K (176 °C) (second T c), and then remains almost the same up to 823 K (550 °C). The thermomagnetic curve of ribbon annealed at 858 K (585 °C) also shows two T c: the first kink around 393 K (120 °C) and other around 475 K (202 °C). The magnetization curve then reaches at 0.2 T and remains almost the same up to 823 K (550 °C).
4 Discussion
4.1 Phase Transformation
Our results indicate that upon heating, the amorphous phase in both Z7B4 and Z9Z4 has partially been transformed into bcc phase. In case of Z7B4 ribbon, the detailed Mössbauer analysis has revealed the hyperfine field of bcc phase in ribbon annealed at 813 K (540 °C) is as high as 325 kOe. Comparing the hyperfine field value of pure α-Fe to be 330 kOe, it can be inferred that the composition of bcc phase is close to pure Fe which has less amount of other alloying elements such as Zr, B, and Cu. Therefore, it can be concluded that the crystallization of amorphous Z7B4 ribbon occurred through the following sequence:
The crystallization behavior of Z9B4 ribbon is different from that of Z7B4 ribbon. In this case, annealing at 773 K (500 °C) which is above the 1st crystallization peak obtained from DSC thermogram resulted in forming bcc phase. Large differences in crystallization temperatures and the enthalpies of crystallization between bcc phases obtained in both annealed Z7B4 and Z9B4 ribbons reveal that the compositions are different. This observation is complemented with the Mössbauer spectroscopy result where the hyperfine field of bcc phase in Z9B7 ribbon annealed at 773 K (500 °C) is found to be less (287 kOe) than that for annealed Z7B4 ribbon. From this result, it can be inferred that the bcc phase in Z9B4 ribbon annealed at 813 K (540 °C) is Fe-based solid solution (SS) which contains alloying elements such as Zr, B, and Cu. This is also supported by the low value of activation energy (64 kJ/mol) of bcc SS revealing the ease of crystallization. Since the kinetics of crystallization process for bcc phases in both ribbons is different, the average grain size of bcc SS obtained in Z9B4 ribbon annealed at 773 K (500 °C) (9.1 nm) is found to be larger than that of bcc phase of Z7B4 ribbon annealed at 813 K (540 °C) (7.8 nm).
Although the second crystallization temperature of Z9B4 ribbons obtained from DSC measurements at a heating rate of 20 °C/min is ~843 K (570 °C), the thermomagnetic measurements revealed that the magnetization increases sharply at 808 K (535 °C) due to the crystallization of α-Fe phase. Both XRD and TEM studies indicate that increasing the annealing temperature to 813 K (540 °C) and then to 858 K (585 °C) resulted in the precipitation of both α-Fe and Fe2Zr phases. The value of activation energy, as high as 351 kJ/mol, reveals the higher stability of the phases. The hyperfine field peak around 287 kOe that could be observed due to the formation of iron solid solution during decomposition of amorphous phase when Z9B4 ribbon is annealed at 773 K (500 °C) could not be observed when annealed at these higher temperatures. Instead a new peak around 324 kOe appears. This corresponds to hyperfine field of pure Fe. This suggests that with the increase in annealing temperature, alloying elements such as Zr, B, and Cu get partitioned from the bcc Fe phase. The results also corroborate with the precision lattice parameter (a 0) calculations. The a 0 value for Z9B4 ribbon annealed at 773 K (500 °C) is higher (2.8710 Å) than that of ribbons annealed at high temperatures (2.8569 to 2.8587 Å) and Z7B4 ribbon annealed at 813 K (540 °C) (2.8586 Å). This implies the presence of other alloying elements in bcc phase of Z9B4 ribbon annealed at 773 K (500 °C) which get partitioned at higher annealing temperatures. The bimodal distribution of grain size observed in bright field TEM micrographs was further explored. The detailed EDAX analysis reveals that the bigger grains are almost pure Fe. The value of enthalpy estimated from the second exothermic peak is 28.8 kJ/mol. Therefore, a suggested pathway for the three-stage phase transformation process is as follows:
4.2 Phase Stability
The results presented show significant difference in the decomposition behavior of amorphous phase in the two ribbons where the chemical composition differs by 2 at. pct Zr. To understand the phase stability in Z7B4 and Z9B4 alloys, the molar Gibbs free energies of amorphous (G amorm ), bcc (G bccm ), and Fe2Zr (\( G_{\text{m}}^{{{\text{Fe}}_{2} {\text{Zr}}}} \)) phases have been calculated based on regular solution model. Since only 1 at. pct Cu has been added in both alloys, the calculation of molar free energy of all phases has been simplified by omitting Cu in the solution. Therefore, in the present study, only Fe-Zr-B ternary alloy system has been considered. Accordingly, G of any phase (ϕ) can be defined as[20]
where x i (where i = Fe/Zr/B) is the mole fraction of the ith element, G ϕ i is the Gibbs free energy of the ith element in ϕ phase, R is the gas constant, T is the temperature, L ϕ ij is interaction energy between i and j elements in ϕ phase, and L ϕB,Fe,Zr is the interaction energy between B, Fe, and Zr elements. It is to be noted that the ternary interaction between B, Fe, and Zr is not considered in this study. The Gibbs free energy values (G ϕ i ) of B, Zr, and Fe were taken from thermodynamical data of various elements compiled by Hultgreen et al.[21] The interaction energy between i and j elements in the ϕ phase was calculated using Redlich–Kister polynomial of nth degree using the formula as[22]
where
Since amorphous phase is considered to be as a supercooled liquid whose structure is similar to that of liquid, the G amorm has been calculated from the liquid free energy data of Fe, Zr, and B and extrapolated to the supercooled region even up to room temperature.
For calculating the \( G_{\text{m}}^{{{\text{Fe}}_{2} {\text{Zr}}}} \), a sublattice model has been used. Since the Fe2Zr is a Laves phase consisting of C15 structure with varying composition, sublattice with the formula of (Fe,Zr)2 (Fe,Zr)1 has been considered for the calculation. Therefore, the molar Gibbs free energy of Fe2Zr (\( G_{\text{m}}^{{{\text{Fe}}_{2} {\text{Zr}}}} \)) can be obtained using the following formula[20] as
where y (1) i and y (2) i are the site fractions of ith element in first (Fe,Zr)2 and second (Fe,Zr)1 sublattices, respectively. \( G_{i:j}^{{{\text{Fe}}_{2} {\text{Zr}}}} \) is the molar Gibbs free energy of Fe2Zr compound where sublattice 1 and 2 are occupied by i and j elements, respectively. The ex \( G^{{{\text{Fe}}_{2} {\text{Zr}}}} \) is the interaction energy between unlike atoms and is given by the following formula:
where \( L_{ij:k}^{{{\text{Fe}}_{2} {\text{Zr}}}} \) is the interaction parameter between unlike atoms on the same sublattice and is expressed by an equation similar to Eq. [3].
Using the above equations, room temperature molar free energy (G) for amorphous, bcc, and Fe2Zr phases as a function of Fe content in (Fe x Zr1−x )96B4 alloy system has been evaluated and is shown in Figure 9. The composition of both Z7B4 and Z9B4 alloys, considered in the present investigation has also been marked in the diagram. As expected, the G amor is always higher than that of other two phases. The \( G_{\text{m}}^{{{\text{Fe}}_{2} {\text{Zr}}}} \) is lower than that of bcc phase within the composition between Fe = 0.53 to 0.85 at. pct. It is interesting to note that composition of equal molar free energies of bcc and Fe2Zr phases is 0.85 at. pct Fe which is very close to the composition of Z9B4 alloy. G bcc phase is lower beyond this composition from which it can be concluded that the bcc phase in Z7B4 alloy is more stable whereas both bcc and Fe2Zr can coexist in Z9B4 alloy.
The molar Gibbs free energy (G) of amorphous, bcc, and Fe2Zr as a function of temperature for Z7B4 and Z9B4 alloys has been evaluated and is shown in Figures 10(a) and (b), respectively. It is evident that for all phases, G decreases with temperature and G amor for both alloys is higher than that of bcc and Fe2Zr. Since amorphous is a non-equilibrium phase at room temperature, it will tend to transform to either bcc or Fe2Zr phase during heating. In case of Z7B4 alloy, it can be observed from the Figure 10(a) that G bcc is always lower than that of Fe2Zr at all temperatures implying that the bcc phase is more stable. Therefore, one can expect the formation of only stable bcc phase during annealing. In case of Z9B4 alloy (Figure 10(b)), G bcc is low till 488 K (215 °C), and beyond this temperature, \( G^{{{\text{Fe}}_{2} {\text{Zr}}}} \) curve crossovers G bcc and remains low throughout the higher temperature range. This implies that lower temperature favors the formation of stable bcc phase, whereas the Fe2Zr phase is more stable at an elevated temperature. This supports our experimental observation.
4.3 Magnetic Properties
The spectral contribution of crystalline phase, amorphous phase, and Fe2Zr phase has been calculated based on area under the curve of hyperfine field distribution peaks responsible for respective phases and is shown in Table II. Initially, the hyperfine field distribution curve is deconvoluted around each peak using Gaussian distribution curve fitting (as shown in Figures 7(g) and (h)), and the area under each peak has been calculated which gives the spectral contribution of relative Fe concentration in each phase. In case of Z7B4 ribbon annealed at 813 K (540 °C), approximately ¾ of the total Fe atoms are contributing toward the formation of α-Fe phase and the remaining ¼ Fe atoms is retained in the amorphous phase. In case of Z9B4 ribbons, the spectral contribution of Fe atoms for bcc phase increases slowly with annealing temperature and reaches 10 pct for ribbon annealed at 858 K (585 °C). The spectral contribution of Fe atoms for Fe2Zr phase is very high as compared to bcc α-Fe phase. In amorphous phase, the spectral contribution of Fe continuously decreases at higher annealing temperature which shows that the amorphous phase is continuously getting depleted with Fe atoms. This result is well corroborated with XRD, DSC, and TEM results.
It is evident from the thermomagnetic curves of Z9B4 ribbons annealed at different temperatures that only amorphous phase T c can be observed for the as-spun ribbon, whereas ribbons annealed at 813 K and 858 K (540 °C and 585 °C) exhibits two transition temperatures. The XRD results reveal that the phases present in both the annealed samples are α-Fe, Fe2Zr, and residual amorphous phases. Since T c of α-Fe is very high [1543 K (770 °C)], the first and second transitions can be associated with the T c of amorphous and Fe2Zr phases, respectively. It can be observed that T c of amorphous phase increases with annealing temperature. Although the amorphous phase is continuously depleted of Fe atoms at high annealing temperatures as shown by Mössbauer results, the exchange field penetration effect of α-Fe and Fe2Zr phase in the amorphous matrix causes an increase of amorphous phase T c.[23,24]
The constant values of magnetization of 0.1 T in case of ribbon annealed at 813 K (540 °C) and 0.2 T in case of ribbon annealed at 858 K (585 °C) beyond the T c of Fe2Zr can be correlated with the existence of ferromagnetic bcc Fe phase. From the knowledge of spectral contribution, the relative Fe concentration in ribbons annealed at 813 K and 858 K (540 °C and 585 °C) are found to be 5.4 and 10 pct, respectively (Table II). Assuming that only α-Fe (whose magnetization value is 2.1 T) contributes toward the magnetization at higher temperature beyond the T c of Fe2Zr, the magnetization values of 813 K and 858 K (540 °C and 585 °C) annealed ribbons at high temperature can be calculated to be 0.11 and 0.21 T, respectively. These are similar to the values obtained experimentally.
5 Conclusions
The GFA (calculated using P HSS = ∆H chem(ΔS σ /k B)(ΔS C /R)) of Z9B4 alloy is found to be higher than that of Z7B4 alloy which is validated by experimental results. On annealing, Z7B4 amorphous ribbon crystallizes to partially bcc α-Fe from amorphous phase while Z9B4 amorphous ribbon crystallizes in two stages as follows: initially, partial bcc solid solution from amorphous phase followed by α-Fe and Fe2Zr phases from bcc solid solution. While annealed Z7B4 ribbon exhibits nanocrystalline bcc α-Fe phase, the annealed Z9B4 ribbon exhibits the bimodal distribution of nanograins of α-Fe and Fe2Zr phases. Molar Gibbs free energy calculations using regular solution model (for amorphous and α-Fe phases) and sublattice model (for Fe2Zr intermetallic phase) reveal that the α-Fe phase is a stable phase for Z7B4 alloy. In case of Z9B4 alloy, low temperature favors the formation of stable bcc phase whereas the Fe2Zr phase is more stable at an elevated temperature.
References
K. Suzuki, A. Makino, N. Kataoka, A. Inoue and T. Masumoto: Mater. Trans. JIM, 1991, Vol 32, pp. 93-102.
G. Herzer: IEEE Trans. Magn., 1989, Vol 25, pp. 3327-3329.
G. Herzer and H.R. Hilzinger: J. Mag. Mag. Mater., 1986, Vol 62, pp. 143-145.
D. ArvindhaBabu, B. Majumdar, R. Sarkar, D. Akhtar and V. Chandrasekaran: J. Phys. D: Appl. Phys., 2008, Vol 41, pp. 195002-195008.
J.C. Shih, L. Burgeos, K. Suzuki and J.S. Garitaonanda: J. Magn. Magn. Mater. 304 (2006) pp e693-e696.
K. Hono: Prog. Mater. Sci., 2002, Vol 47, pp. 621-729.
D. ArvindhaBabu, B. Majumdar, R. Sarkar, M. ManivelRaja and D Akhtar: J. Mater. Res. 2011, Vol 26, pp. 2065-2071.
D. ArvindhaBabu, B.Majumdar, A.P.Srivastava, B.R. Rao, D. Srivastava, B.S. Murty and D. Akhtar: Metal. Mater. Trans. A, 2011, Vol 42A, pp. 508-516.
B.R. Rao, M. Srinivas, A.K. Shah, A.S. Gandhi and B.S. Murty: Intermetallics, 2012, Vol 35, pp. 73-81.
B. R. Rao, A.S. Gandhi, S. Vincent, J. Bhatt, and B.S. Murty: Trans Ind. Inst. Metals, 2012, Vol 65, pp. 559-563.
S. Vincent, B.S. Murty and Jatin Bhatt: Trans Ind. Inst. Metals, 2012, Vol 65, pp. 827-831.
D.M. Zhu, K. Raviprasad, K. Suzuki and S.P. Ringer: J. Phys. D: Appl. Phys., 2004, Vol 37, pp. 645-652.
J.B. Nelson and D.P. Riley: http://iopscience.iop.org/0959-5309/57/3/302?fromSearchPage=true Proc. Phys. Soc., 1945, vol. 57, p. 160.
H.E. Kissinger: Anal. Chem., 1957, Vol 29, pp. 1702-1706.
E.A. Owen and G.I.Williams: J. Sci. Instruments, 1954, Vol 31, pp. 49-54.
Rhines and Gould: Advan. X-Ray Anal., 1962, Vol 6, pp. 62-73.
M. Kopcewicz, A. Grabias and D.L.Williamson: J. Appl. Phys., 1997, Vol 82, pp. 1747-1758.
G.S. Garitaonandia, D.S. Schmool and J.M. Barandiran: Phys. Rev. B., 1998, Vol 58, pp. 12147-12158.
L.F. Kiss, G. Huhn, T. Kemeny, J. Balogh and D. Kaptas: J. Magn. Magn. Mater., 1996, Vol 160, pp. 229-232.
T. Tokunaga, K. Terashima, H. Ohtani and M. Hasebe: Mater. Trans., 2008, Vol 49, pp. 2534 – 2540.
R. Hultgreen, P.D. Desai, D.T. Hawkins, M. Gleiser, K.K. Kelley and D.D. Wagman: Selected Value of Thermodynamic Properties of the Elements, American Society of Metals, Materials Park 1973, pp. 54 – 581.
O. Redlich and A.T. Kister: Ind. Eng. Chem., 1948, Vol 40, pp. 345-348.
A. Hernando and I. Navarro: Phys. Rev. B. 1995, Vol 51, pp. 3281-3284.
B. Majumdar and D. Akhtar: Bull. Mater. Sci. 2005, Vol 28, pp. 395-399.
Acknowledgments
This work was supported by Defence Research and Development Organization (DRDO), Government of India. The authors thank Dr. A. Bhattacharjee, Dr. A. K. Singh, and Dr. M. Manivel Raja of DMRL for melting alloys, XRD measurements, and Mössbouer spectroscopy, respectively. The authors gratefully acknowledge Dr. A. Gokhale, Director DMRL, and Dr. G. Malakondaiah, DS, CC (R&D) for continued support and permission to publish this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted May 27, 2015.
Rights and permissions
About this article
Cite this article
Arvindha Babu, D., Majumdar, B., Sarkar, R. et al. On the Structural Stability of Melt Spun Ribbons of Fe95−x Zr x B4Cu1 (x = 7 and 9) Alloys and Correlation with Their Magnetic Properties. Metall Mater Trans A 47, 560–571 (2016). https://doi.org/10.1007/s11661-015-3204-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-015-3204-x