Abstract
Summary
Osteosarcopenic obesity is a new syndrome that has been recently discussed in the scientific community. It is a condition that affects the elderly and involves the loss of bone, muscle, and fat tissue. The few studies that have been done on this disease showed that it has a high prevalence among the elderly and can cause various complications. This study was the first one to investigate this syndrome in Iran, and found that its prevalence was 19.83%.
Background
Osteosarcopenic obesity syndrome (OSO) is a condition that involves osteopenia/osteoporosis, sarcopenia, and obesity. It leads to a reduction in the quality of life of the elderly and an increase in hospitalization which has attracted the attention of physicians. This study aimed to determine the prevalence and risk factors of osteosarcopenic obesity in people over 60 years of age in Bushehr.
Materials and methods
We used data from the Bushehr study, which included 2426 participants aged ≥ 60 years. We assessed osteoporosis/osteopenia based on T-score; sarcopenia based on hand grip strength, skeletal muscle mass index (SMI), and walking speed; and obesity based on fat mass and BMI for diagnosing OSO. We first examined the factors related to OSO in the univariable analysis and then fitted the multiple logistic regression model, separately for women and men. The result was summarized as adjusted odds ratios with a 95% confidence interval.
Results
In total, 2339 elderly were examined in our study, of which 464 elderly were suffering from osteosarcopenic obesity. The standardized prevalence of OSO was 23.66% (95% CI: 21.15–26.16) in women and 18.53% (95% CI 16.35–20.87) in men. Age was positively linked to osteosarcopenic obesity in both genders and so was diabetes in men. However, education, physical activity, and protein intake were negatively linked to osteosarcopenic obesity in both genders, as well as hypertriglyceridemia and hypertension in women.
Conclusion
The prevalence of OSO among men and women of Bushehr city is high and is related to increasing age, low levels of education, physical activity, and protein intake among women and men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As life expectancy rises in different societies, the middle age population also shows a marked increase. By 2050, the elderly are expected to account for 21.4% of the world’s population [1]. However, aging also increases the risk of various diseases [2]. Osteoporosis is a condition that causes bone density and strength to decline [3]. In addition, aging is associated with a reduction in muscle mass, strength, and function, leading to sarcopenia [4]. On the other hand, aging is associated with the gradual accumulation of body fat and obesity [5, 6]. Although fat was thought to protect bones from fractures, new evidence suggests that fat mass has a negative effect on bone mass [7]. The coexistence of all three conditions results in osteosarcopenic obesity [8].
This syndrome can increase the risk of falls, fractures, and hospitalization in the elderly population [9]. Therefore, it can impair their quality of life and also lead to significant healthcare costs. In light of these major effects, OSO has recently received attention [10]. Several studies have estimated the prevalence of OSO to be between 10 and 19% of older people in Turkey and Mexico, respectively [11, 12].
There are some studies to determine the associated factors of OSO in different populations. For example, an association between OSO and insulin resistance [13], hypertension [14], dyslipidemia [15], protein intake [16], and reduced physical activity [17] was reported. Understanding the factors associated with OSO is essential for developing effective prevention and treatment strategies.
As the number of elderly people will increase in the future, this geriatric syndrome might be a public health challenge that requires urgent interventions to prevent or manage its progression and complications in old age. Unfortunately, the prevalence of this disease and its associated factors have not been yet studied in Iran. Thus, in the present study, we investigated the prevalence and also actual associated factors of OSO in a large elderly population of Bushehr, Iran.
Method
This cross-sectional study was conducted in the framework of the Bushehr Elderly Health (BEH) program [18], which is a population-based study to investigate the risk factors of non-communicable diseases in a representative urban population of Bushehr, Iran. Our study was conducted in the second stage of the protocol study in which 2426 participants aged ≥ 60 years were included to estimate the prevalence of musculoskeletal disorders and their risk factors.
Data collection
The data collection method for the elderly population has already been described in the study protocol Bushehr Elderly Health (BEH) [18]. Trained interviewers used a questionnaire to collect information on participants’ demographic status, such as age, education, and smoking status. Also, height and weight were measured with a fixed stadiometer and a digital scale, respectively, according to the standard protocol. In addition, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice on the right arm. Lipid indices such as total cholesterol (TC) and triglycerides (TG), as well as high-density lipoprotein (HDL-C), low-density lipoprotein cholesterol (LDL-C), and fasting plasma glucose (FPG), were measured using fasting blood samples. The level of physical activity was evaluated using a valid self-report questionnaire including sports, work, and leisure time, on the average on weekdays [18, 19]. The 24-h dietary recall is one of the most common dietary assessment questionnaires that was used in the Bushehr study to examine the participants’ diets. The nutritionist IV packages, modified for Iranian foods, were used to obtain daily energy, nutrient intakes, and servings of foods consumed. Protein intakes were converted to grams using standard reference tables.
Body composition, fat mass, bone mineral density, and muscle mass were measured using dual X-ray absorptiometry (DXA, Discovery WI, Hologic, Inc., USA) [20]. Fat mass was computed for accounting the obesity of the participants. The bone mineral density for the lumbar spine, femoral neck, and total hip was measured in the correct position. Muscle strength was measured by handgrip strength, using a digital Seahan dynamometer. The measurement was carried out three times for each hand, and maximum grip strength was calculated by taking the average of the highest measurement from both hands. Usual walking speed (m/s) on a 4.57-m course was used as an objective measure of physical performance.
Definitions
Osteopenia/osteoporosis component was defined based on the WHO standard criteria of a T-score ≤ − 1.0in each site [21], considering the Caucasian women aged 20–29 years as the reference population. Sarcopenia refers to reduced skeletal muscle mass plus low muscle strength and/or low physical performance. Appendicular skeletal muscle mass (ASM) for each participant was derived as the sum of upper and lower limb muscle mass. The skeletal muscle mass index (SMI) was defined as ASM/height2 (kg/m2). The cutoff values for low SMI were 7.0 kg/m2 and 5.4 kg/m2 among men and women, respectively [22]. The muscle strength was handgrip strength < 26 kg for men and < 18 kg for women; while the cutoff value for low physical performance was a usual walking speed < 0.8 m/s for both genders [23].
Body fat percentage and BMI were used to determine obesity in this study. The American Council on Body Fat Classification has set a body fat percentage cutoff point of ≥ 25% for men and ≥ 32% for women, and we used the American Council cutoff point in this study [10]. The body mass index (BMI) was calculated using a person’s weight in kilograms divided by the square of height in meters. We considered individuals with a body mass index ≥ 30 kg/m2 to be obese [24]. Accordingly, individuals were considered obese if they had either an abnormal body fat percentage or a BMI ≥ 30.
Type 2 diabetes mellitus was identified in participants who had an FPG ≥ 126 mg/dl or were taking antidiabetic drugs or had an HbA1C > 6.5 mmol/mol. Hypertension was defined as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or taking antihypertensive medication [22]. Hypercholesterolemia was defined as TC ≥ 200 mg/dl, high LDL-C as > 110 mg/dl, low HDL-C as < 40 mg/dl in men and < 50 mg/dl in women, and hypertriglyceridemia as TG ≥ 150 mg/dl [25]. Current smoking was defined as participants who smoked cigarettes daily or occasionally and participants who used hookah or pipes. The physical activity level was calculated using the total energy expenditure divided to the basal energy expenditure. Accordingly, four lifestyle categories were explained as sedentary (1–1.39), low active (1.4–1.59), active (1.6–1.89), and very active (1.9–2.5) [26, 27]. In this study, we considered the participants in the sedentary and low active categories as the low physical activity, and individuals in the active and very active categories as the high physical activity group. TG/HDL were defined as the ratio of triglyceride to high-density lipoprotein cholesterol (TG/HDL-c).
Statistical analysis
Descriptive statistics are reported as means (SD) and median (IQR) for normal and non-normal distributions, respectively. The categorical variables were reported as frequency (percentage). The crude prevalence of osteosarcopenic obesity in the whole population was estimated by sex. All comparisons were performed separately for men and women. To measure age-standardized prevalence in men and women, direct standardization was performed using the population distribution of the 2015 Iranian census data. The Pearson chi-squared test was used for categorical variables, and the Mann–Whitney U test and independent t-test were used for quantitative variables.
All variables with p-value < 0.2 in the univariate analysis were included in the multiple logistic regression model [28,29,30,31,32] fitted separately in women and men. Backward selection with alpha = 0.157 (consistent with AIC criteria) was used to identify the best model among all possible subsets. The linearity assumption between the quantitative variables and the outcome variable was checked using multiple fractional polynomials. Finally, the results were summarized as adjusted odds ratios with 95% confidence interval. The Stata version 17 was used to perform the statistical analysis.
Results
After data collection, participants who had missing values for each component of osteosarcopenic obesity (n = 62) and elderly people who had cancer (n = 25) were excluded from the study. Finally, 2339 elderly people were included in the study. Of these, 464 participants had osteosarcopenic obesity. The flowchart of this study is shown in Fig. 1. The clinical characteristics of women and men participating in Bushehr Elderly Health (BEH) program are shown in Tables 1 and 2. Age, calories, protein, physical activity, education, hypertriglyceridemia, and TG/HDL were compatibly different in participants with and without osteosarcopenic obesity in women. Also, variables such as age, calories, protein, physical activity, education, and hypertension were compatibly different in participants with and without OSO in men.
The prevalence of OSO was 19.83% (n = 464) in the overall population: of which 22.13% (n = 266) were women and 17.41% (n = 198) were men. Age-standardized OSO was also nearly identical to crude prevalence in both sexes. The age-standardized prevalence was 23.66% (95% CI 21.15–26.16%) in women and 18.53% (95% CI 16.35–20.87) in men (Table 3).
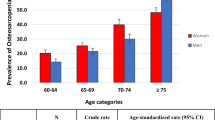
Figure 2 shows the prevalence of OSO in four different age groups in men and women. Among women in the age group of 60–64 years, 65–69 years, and 70–74 years, the prevalence of OSO was higher than that of men. But in the age group of 75 years and above, the prevalence increases in men so that it becomes more than in women.
As shown in Table 4, age is directly related to OSO. With each unit of protein intake, the odds of developing OSO decreases by 0.98 (95% CI: 0.97–0.99) and 0.98 (95% CI: 0.98–0.99) in women and men, respectively. Diabetes was also directly related to OSO as men with diabetes were 1.74)95% CI: 1.20–2.52) times more likely to develop OSO than nondiabetic men. However, education, protein, and physical activity were inversely related to OSO in both sexes, while hypertension and hypertriglyceridemia were also inversely related to OSO in women. With increased education, the odds of OSO decreased by 6% in women (OR 0.94, 95% CI: 0.93–1.75) and by 4% in men (OR 0.96, 95% CI: 0.92–0.99). In addition, with each unit of protein intake, the odds of developing OSO decreases by 0.98 (95% CI: 0.97–0.99) and 0.98 (95% CI: 0.98–0.99) in women and men, respectively.
Among older people who were less physically active, the odds of developing OSO increased for both women and men. Women were at 34% lower odds to have OSO (OR 0.66, 95% CI: 0.45–0.97) and men were at 42% lower odds to have OSO (OR 0.58, 95% CI: 0.35–0.98). The odds of OSO were lower for hypertriglyceridemia and hypertension in women (0.65, 95% CI: 0.47–0.90 and OR 0.62, 95% CI: 0.45–0.84, respectively) and the healthy population was considered the reference category.
Discussion
This cross-sectional study aimed to estimate the prevalence and factors associated with osteosarcopenic obesity (OSO) in a large sample of elderly people in Bushehr. We found that the overall prevalence of OSO was 19.83%, with 22.13% in women and 17.41% in men. We also found that OSO was positively associated with age and negatively associated with education, physical activity, and protein intake in both genders. In addition, diabetes was positively associated with OSO in men, while hypertension and hypertriglyceridemia were negatively associated with OSO in women.
The studies that determined the prevalence of osteosarcopenic obesity yielded different values, ranging from 4 to 19% of prevalence. A cross-sectional study in Mexico in 2018 determined the prevalence of OSO in 434 women over 50 years old (mean age = 71.3 years). The study defined osteoporosis as a T-score of less than − 2.5, and obesity was defined as body fat mass ≥ 35%. Sarcopenia was diagnosed by ALMBMI of less than 0.512 and grip strength of less than 16 kg in women. Finally, the prevalence of OSO in women was 19% [12]. The prevalence of OSO among women in the Mexican study was almost similar to our study (22.13%), but the slight difference might be due to the younger participants included in the Mexican study since we showed that the prevalence of OSO increases with age.
In addition, in South Korea, a cross-sectional study was conducted on 3504 women and 3385 men over 50 years old. The study used the WHO criteria to classify osteopenia as a − 1.0 ≥ T-score > − 2.5 SD, and osteoporosis as a T-score ≤ − 2.5 SD. Sarcopenia was diagnosed by an ASM/Ht2 with the cutoff value as 7.26 kg/m2 in male and 5.45 kg/m2 in female. Obesity was defined by a fat mass > 30% in male and > 40% in female. A total of 293 participants in the study had OSO, and the overall prevalence of OSO was 4.2% (3.1% in men and 5.4% in women) [33]. Although the abovementioned study included both genders with a large sample size, the prevalence they reported was much lower than ours. Besides the younger ages of the study population, the other reason to explain the difference might be the different definitions and cutoffs for obesity and sarcopenia in these two studies. We defined obesity by a BMI ≥ 30, and a fat mass of ≥ 25% for men and ≥ 32% for women. We diagnosed sarcopenia by an SMI index (ASM/height2) with cutoff 7.0 kg/m2 and 5.4 kg/m2 among men and women, respectively, a handgrip strength < 26 kg for men and < 18 kg for women, and usual walking speed < 0.8 m/s for both sexes.
Another study in 2020 in Turkey reported the prevalence of osteosarcopenic obesity (OSO) in 460 elderly individuals (337 women and 123 men) aged 65 years or older. The overall prevalence of osteosarcopenic obesity in that study was 10.7% (11% in women and 9.8% in men) [11]. The study reported a lower prevalence of OSO than ours. A possible reason for this difference is the small sample size of the study. A larger sample size can increase the precision of the results.
The higher prevalence reported in this study may be due to some factors, such as (1) the high prevalence of sarcopenia, osteopenia, and obesity found in previous Bushehr studies; (2) the larger sample size of this study compared to other studies, which increases the precision of the estimates; (3) the older age of the participants in this study compared to some other studies; and (4) the different criteria used to define OSO in different studies.
This study found that age was positively associated with osteosarcopenic obesity (OSO), which is consistent with the study by Kim et al. [34]. Aging leads to a decrease in bone mineral density and muscle mass, as shown by several studies [35, 36]. Our study also found that education level was negatively associated with OSO. This is similar to the findings of a study among people over 50 in South Korea. They have also showed that in men and women, those having a OSO tended to be less educated [37]. This negative association may be due to better nutrition and lifestyle in educated people.
Our study showed that protein intake was negatively associated with the osteosarcopenic obesity (OSO) in both genders. This is in line with a study in South Korea by Choi et al. [16] which examined 1351 people aged over 50 years using a 24-h recall questionnaire. They found that protein intake was associated with a lower risk of OSO in older Korean men. A 2017 review [38] also showed that high carbohydrate consumption and excess glycemia, lower protein intake, and low intake of long-chain polyunsaturated fats may play a role in osteosarcopenic obesity. It has been suggested that the prevention and treatment of OSO require a balanced and adequate daily intake of total nutrients [39].
We found that physical activity (PA) was negatively associated with osteosarcopenic obesity (OSO) in both women and men. This is consistent with the study by Dos Santos et al. [17], which was conducted in Brazil on 152 women over 60 years of age. PA tends to decline with aging, and aging also leads to neuromuscular and cardiovascular changes that impair physical performance and increase the stiffness of cartilage, tendons, and ligaments. These changes, along with low PA levels, may reduce the functional capacity of the elderly. Therefore, our study suggests that PA may have a protective effect against OSO [12, 40].
This study examined the association between type 2 diabetes and osteosarcopenic obesity (OSO). We found that diabetes was positively associated with OSO, but only in men. We could not find any study to show the association between diabetes and OSO, but some studies have reported that diabetes increases the risk of sarcopenia, one of the components of OSO [41]. Moreover, several studies have shown that diabetes can also affect bone tissue and fracture risk in both men and women through different mechanisms [42]. Furthermore, obesity is a major risk factor for diabetes, and it has been estimated that obesity increases the risk of diabetes by seven times [43].
Hypertension and hypertriglyceridemia were the other factors that led to different results in women. We found that hypertension and hypertriglyceridemia were less common in women with OSO. In a Chinese study of 1939 participants over 50 years of age, hypertension in women with OSO was 3.15 (95% CI: 1.13 to 8.78) [14]. However, in some studies, there are conflicting results about hypertension and sarcopenia. A Japanese study found an inverse relationship between hypertension and sarcopenia (OR: 0.60, 95% CI: 0.40–0.90) [44], while a Chinese study found that the prevalence of sarcopenia increased with hypertension [45]. In addition, hypertension may have led to decreased bone turnover, which may be one of the mechanisms of hypertension-induced osteoporosis. Some studies found that the prevalence of osteoporosis was higher in patients with hypertension [46]. Therefore, more studies are needed to clarify the association between hypertension and OSO.
We found no study that determined the relationship between hypertriglyceridemia and OSO. But, in a study by Dan et al. in China [15], dyslipidemia was investigated in women with OSO. Dyslipidemia was defined by the presence of one or more abnormal serum lipid concentrations (hypercholesterolemia, hypertriglyceridemia, decreased HDL-C, and increased LDL-C). The results of this study showed that the likelihood of dyslipidemia was much higher in women older than 60 years with OSO than in women younger than 60 years with OSO.
Strengths and limitations
To our knowledge, this is the first study conducted in Iran to determine the prevalence and factors associated with osteosarcopenic obesity using a large population-based study in the older adults. Another strength of our study is that we considered both genders and investigated the possible association between different factors in people with and without OSO. However, this study also had some limitations that need to be acknowledged. One of the limitations of our study is that this is a cross-sectional study that is limited in determining causal relationships and therefore may not reflect the true cause-and-effect relationship of osteosarcopenic obesity. In addition, as the study was conducted in one of the cities of Iran, some caution should be exercised when generalizing the results to other populations.
Conclusion
Osteosarcopenic obesity (OSO) is a common condition among elderly population in Iran. The results showed that age was positively correlated with OSO in both men and women, and diabetes was positively correlated with OSO in men. On the other hand, physical activity, education, and protein intake were negatively correlated with OSO in both genders. These findings suggest that OSO is associated with the various biological and lifestyle factors in the elderly population of Iranian. Considering the high prevalence of OSO in the elderly population, it seems prospective research with follow-up is necessary to access the risk factor related to this syndrome more comprehensively. Comprehensive programs are needed to address the consequences and burdens that are imposed by this syndrome.
Data Availability
The dataset analyzed in this paper is provided by BEH study that is not publicly available, however it could be accessible upon reasonable request.
References
Olshansky SJ, Goldman DP, Zheng Y, Rowe JW (2009) Aging in America in the twenty-first century: demographic forecasts from the MacArthur foundation research network on an aging society. Milbank Q 87(4):842–862
Jaul E, Barron J (2017) Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health 5:335
Coughlan T, Dockery F (2014) Osteoporosis and fracture risk in older people. Clin Med (Lond) 14(2):187–191
Dodds RM, Roberts HC, Cooper C, Sayer AA (2015) The epidemiology of sarcopenia. J Clin Densitom: Off J Int Soc Clin Densitom 18(4):461–466
Jura M, Kozak LP (2016) Obesity and related consequences to ageing. Age (Dordr) 38(1):23
Reyes-Farias M, Fos-Domenech J, Serra D, Herrero L, Sánchez-Infantes D (2021) White adipose tissue dysfunction in obesity and aging. Biochem Pharmacol 192:114723
Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J et al (2008) Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res: Off J Am Soc Bone Miner Res 23(1):17–29
Ilich JZ, Kelly OJ, Inglis JE (2016) Osteosarcopenic obesity syndrome: what is it and how can it be identified and diagnosed? Curr Gerontol Geriatr Res 2016:7325973
Keramidaki K, Tsagari A, Hiona M, Risvas G (2019) Osteosarcopenic obesity, the coexistence of osteoporosis, sarcopenia and obesity and consequences in the quality of life in older adults ≥65 years-old in Greece. J Frailty, Sarcopenia Falls 4(4):91–101
Ma Y, Zhang W, Han P, Kohzuki M, Guo Q (2020) Osteosarcopenic obesity associated with poor physical performance in the elderly Chinese community. Clin Interv Aging 15:1343–1352
Kolbaşı EN, Demirdağ F (2020) Prevalence of osteosarcopenic obesity in community-dwelling older adults: a cross-sectional retrospective study. Arch Osteoporos 15(1):166
Szlejf C, Parra-Rodríguez L, Rosas-Carrasco O (2017) Osteosarcopenic obesity: prevalence and relation with frailty and physical performance in middle-aged and older women. J Am Med Dir Assoc 18(8):733.e1-e5
Lee K (2020) Association of osteosarcopenic obesity and its components: osteoporosis, sarcopenia and obesity with insulin resistance. J Bone Miner Metab 38(5):695–701
Chen X, Kong C, Yu H, Gong J, Lan L, Zhou L et al (2019) Association between osteosarcopenic obesity and hypertension among four minority populations in China: a cross-sectional study. BMJ Open 9(7):e026818
Mo D, Hsieh P, Yu H, Zhou L, Gong J, Xu L et al (2018) Osteosarcopenic obesity and its relationship with dyslipidemia in women from different ethnic groups of China. Arch Osteoporos 13(1):65
Choi MK, Bae YJ (2020) Protein intake and osteosarcopenic adiposity in Korean adults aged 50 years and older. Osteoporos Int: J Established Result Cooperation Between Eur Found Osteoporos Natl Osteoporos Found USA 31(12):2363–2372
Dos Santos VR, Gobbo LA (2020) Physical activity is associated with functional capacity of older women with osteosarcopenic obesity: 24-month prospective study. Eur J Clin Nutr 74(6):912–919
Shafiee G, Ostovar A, Heshmat R, Darabi H, Sharifi F, Raeisi A et al (2017) Bushehr Elderly Health (BEH) programme: study protocol and design of musculoskeletal system and cognitive function (stage II). BMJ Open 7(8):e013606
Aadahl M, Jørgensen T (2003) Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc 35(7):1196–1202
Rothney MP, Brychta RJ, Schaefer EV, Chen KY, Skarulis MC (2009) Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity (Silver Spring, Md) 17(6):1281–1286
Sheu A, Diamond T (2016) Bone mineral density: testing for osteoporosis. Aust Prescr 39(2):35–39
Jang HN, Moon MK, Koo BK (2022) Prevalence of diabetic retinopathy in undiagnosed diabetic patients: a nationwide population-based study. Diabetes Metab J 46(4):620–629
Shafiee G, Ostovar A, Heshmat R, Keshtkar AA, Sharifi F, Shadman Z et al (2018) Appendicular skeletal muscle mass reference values and the peak muscle mass to identify sarcopenia among Iranian healthy population. Int J Prev Med 9:25
Li Y, Wang H, Wang K, Wang W, Dong F, Qian Y et al (2017) Optimal body fat percentage cut-off values for identifying cardiovascular risk factors in Mongolian and Han adults: a population-based cross-sectional study in Inner Mongolia, China. BMJ Open 7(4):e014675
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA et al (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart. Lung, and Blood Institute scientific statement. 112(17):2735–52
Brooks GA, Butte NF, Rand WM, Flatt JP, Caballero B (2004) Chronicle of the Institute of Medicine physical activity recommendation: how a physical activity recommendation came to be among dietary recommendations. Am J Clin Nutr 79(5):921s-s930
Mahan LK, Raymond JL (2016) Krause and Mahan’s food and the nutrition are process-e-book, 15th edn. Elsevier Health Sciences
Mansournia MA, Collins GS, Nielsen RO, Nazemipour M, Jewell NP, Altman DG et al (2021) A CHecklist for statistical Assessment of Medical Papers (the CHAMP statement): explanation and elaboration. Br J Sports Med 55(18):1009–1017
Mansournia MA, Collins GS, Nielsen RO, Nazemipour M, Jewell NP, Altman DG et al (2021) CHecklist for statistical Assessment of Medical Papers: the CHAMP statement. Br J Sports Med 55(18):1002–1003
Greenland S, Mansournia MA, Joffe M (2022) To curb research misreporting, replace significance and confidence by compatibility: a preventive medicine golden jubilee article. Prev Med 164:107127
Mansournia MA, Nazemipour M, Etminan M (2022) P-value, compatibility, and S-value. Glob Epidemiol 4:100085
Etminan M, Brophy JM, Collins G, Nazemipour M, Mansournia MA (2021) To adjust or not to adjust: the role of different covariates in cardiovascular observational studies. Am Heart J 237:62–67. https://doi.org/10.1016/j.ahj.2021.03.008
Chung JH, Hwang HJ, Shin HY, Han CH (2016) Association between sarcopenic obesity and bone mineral density in middle-aged and elderly Korean. Ann Nutr Metab 68(2):77–84
Kim J, Lee Y, Kye S, Chung YS, Kim JH, Chon D et al (2017) Diet quality and osteosarcopenic obesity in community-dwelling adults 50 years and older. Maturitas 104:73–79
Cunha PM, Ribeiro AS, Tomeleri CM, Schoenfeld BJ, Silva AM, Souza MF et al (2018) The effects of resistance training volume on osteosarcopenic obesity in older women. J Sports Sci 36(14):1564–1571
Lee SH, Lee JY, Lim KH, Lee YS, Koh JM (2022) Associations between plasma growth and differentiation factor-15 with aging phenotypes in muscle, adipose tissue, and bone. Calcif Tissue Int 110(2):236–243
Kim J, Lee Y, Kye S, Chung YS, Lee O (2017) Association of serum vitamin D with osteosarcopenic obesity: Korea National Health and Nutrition Examination Survey 2008–2010. J Cachex Sarcopenia Muscle 8(2):259–266
Kelly OJ, Gilman JC, Kim Y, Ilich JZ (2017) Macronutrient intake and distribution in the etiology, prevention and treatment of osteosarcopenic obesity. Curr Aging Sci 10(2):83–105
Lin CL, Lee MC, Hsu YJ, Huang WC, Huang CC, Huang SW (2018) Isolated soy protein supplementation and exercise improve fatigue-related biomarker levels and bone strength in ovariectomized mice. Nutrients 10(11):1792
Hita-Contreras F, Martínez-Amat A, Cruz-Díaz D, Pérez-López FR (2015) Osteosarcopenic obesity and fall prevention strategies. Maturitas 80(2):126–132
Wang T, Feng X, Zhou J, Gong H, Xia S, Wei Q et al (2016) Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci Rep 6:38937
Janghorbani M, Van Dam RM, Willett WC, Hu FB (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166(5):495–505
Grant B, Sandelson M, Agyemang-Prempeh B, Zalin A (2021) Managing obesity in people with type 2 diabetes. Clin Med (Lond) 21(4):e327–e231
Endo T, Akai K, Kijima T, Kitahara S, Abe T, Takeda M et al (2021) An association analysis between hypertension, dementia, and depression and the phases of pre-sarcopenia to sarcopenia: a cross-sectional analysis. PLoS One 16(7):e0252784
Wu HL, Yang J, Wei YC, Wang JY, Jia YY, Li L et al (2022) Analysis of the prevalence, risk factors, and clinical characteristics of osteoporosis in patients with essential hypertension. BMC Endocr Disord 22(1):165
Hu Z, Yang K, Hu Z, Li M, Wei H, Tang Z et al (2021) Determining the association between hypertension and bone metabolism markers in osteoporotic patients. Medicine (Baltimore) 100(24):e26276
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The BEH program has received the ethical approval of both Bushehr University of Medical Sciences and Endocrinology and Metabolism Research Institute. Informed consent was obtained from all participants included in the BEH program. The current study was approved by the ethical approval number of IR.TUMS.SPH.REC.1401.049.
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmadinezhad, M., Mansournia, M.A., Fahimfar, N. et al. Prevalence of osteosarcopenic obesity and related factors among Iranian older people: Bushehr Elderly Health (BEH) program. Arch Osteoporos 18, 137 (2023). https://doi.org/10.1007/s11657-023-01340-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-023-01340-9