Abstract
Summary
Bisphosphonates are common treatment for osteoporosis. Among patients admitted with hip fracture, atypical femoral fractures (AFF) were more prevalent in those who were treated with Bisphosphonates for five or more years. Five years of Bisphosphonates treatment may signify an increased risk for AFF, though the absolute risk remains very low.
Purpose
Atypical femoral fractures (AFF) are a rare complication of bisphosphonate (BP) treatment. We evaluated the correlation between BP exposure and AFF risk among hip fracture patients.
Methods
This retrospective nested case–control study included patients over age 50 years, operated for osteoporotic hip fracture between July 2014 and November 2018, who attended our Fracture Liaison Service. We classified fracture radiographs and compared demographic, clinical, biochemical, and drug purchase data between patients with AFF and those with typical osteoporotic hip fracture (controls). To correct for the younger age of patients with AFF, we matched each case (AFF) with three controls according to age (\(\pm\) 1 year) and sex and performed a conditional logistic regression model.
Results
Of 989 patients, 31 (3%) had AFF. Patients with AFF were younger than those with inter-trochanteric fractures (mean ± SD: 72.3 ± 10.3 vs. 80.2 ± 9.6 years, p < 0.001). Following matching, the mean Charlson’s Comorbidity Index (CCI) was lower in the AFF than in the control group (2.9 ± 3.7 vs. 4.7 ± 4.2; p = 0.030) and a higher proportion of them were treated with BP for 5 years or more (58.1 vs. 16.0%; p < 0.001). Among patients admitted with hip fracture who were treated with BP for 5 years or more, the odds ratio of this fracture being atypical was significantly higher compared with no BP treatment (21.7; 95% CI-4.1–113.9).
Conclusions
Patients with AFF compared to typical hip fractures showed better baseline medical conditions irrespective of their younger age. Five years of BP treatment may be associated with an increased risk for AFF, though the absolute risk remains very low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic skeletal disorder, manifested by increased fracture risk [1]. About one of every two Caucasian women and one of five Caucasian men experience an osteoporosis-related fracture during their lifetimes [2] . Bisphosphonates (BP), bone antiresorptive drugs, are the pillar stones of the medical treatment of osteoporosis. These drugs reduce osteoclasts number and activity and thus decrease bone turnover. This results in increased bone mineral density and reduction in fracture risk by 30–70% [3,4,5,6,7].
In the past decade, BP treatment has been shown to be associated with atypical femoral fractures (AFFs), which are rare fractures with a transverse morphology [8]. AFF pathogenesis is considered as the development of an unhealed stress fracture [9]. In the physiological state, a stress fracture is usually followed by normal bone remodeling and healing. Long-term use of BP impairs the capacity of this natural repair process and rarely results in atypical fragility fractures [8, 10,11,12,13]. The estimated incidence of AFF is 1.8/100,000 patient years after 2 years of BP treatment, 16/100,000 patient years after 5 years of treatment, and 113/100,000 after 10 years of treatment [14,15,16]. It should be emphasized however that the absolute risk of AFF remained very low as compared with reductions in the risk of hip and other fractures with BP treatment [17]. Due to this established correlation between the duration of BP treatment and AFF, current guidelines suggest a “drug holiday,” after 3–5 years of BP treatment [7, 15]. It was shown that even few months of “drug holiday” may reduce significantly the risk of AFF among patients who were treated with BP [17]. Additional risk factors for AFF may include diabetes mellitus, rheumatoid arthritis, and medical treatment with glucocorticoid and proton pump inhibitors (PPI) [18,19,20]. Asian ancestry, higher BMI, and specific femoral geometry (e.g., proximal femoral varus) have also been associated with increased risk of AFF [15, 17, 18, 21].
In this retrospective study, we aimed to characterize AFF among patients who attended a tertiary care hospital fracture liaison service (FLS). We compared the rates and durations of BP exposure, as well as demographic, clinical, biochemical, and radiological characteristics, and drug purchase data between patients with AFF (cases) and patients with typical osteoporotic hip fractures (controls). We examined two hypotheses. Our first hypothesis was that patients with AFF would have distinct demographic and clinical characteristics compared with patients with typical osteoporotic hip fractures, regardless of the specific site of these typical fractures. Second, we hypothesized that the risk of AFF among patients admitted to our institution with osteoporotic hip fracture would be greater among those treated with BP and would increase from a certain point of time during the course of BP exposure.
Methods
Study population
This is a retrospective, nested case–control study of patients treated at Soroka University Medical Center, a 1100-bed teaching, tertiary care referral hospital in Beer-Sheva, Israel. Patients over age 50 years, admitted to our institution with low trauma hip fracture from July 2014 to November 2018, were offered evaluation, follow-up, and treatment by our FLS. Patients were not offered to attend the service if they had a severe comorbidity (e.g., active malignancy, end-stage kidney disease treated by hemodialysis), had not undergone an operation for the fracture, had difficulty communication, or were not living in the district. In addition, patients did not attend our FLS if they were insured by a health maintenance organization other than Clalit Health Services. This is because we did not have pre-admission data for those patients, and follow-up and treatment in our institution were not covered by their health maintenance organizations. We excluded from the current analysis patients with device-associated fractures and patients with incomplete data.
Data captured
Demographic, clinical, biochemical, radiological, and drug purchase data of all the individuals in the cohort were collected from a unified electronical medical file and reviewed. The unified medical files included all the hospital and community records of each patient. Past medical diagnoses were used to calculate Charlson’s Comorbidity Index (CCI) [22]. Laboratory results of hemoglobin and creatinine were retrieved from the computerized data of admissions with hip fracture. Estimated glomerular filtration rate (eGFR) was calculated using the MDRD equation [23]. Parathyroid hormone (PTH) and 25(OH)-vitamin D serum levels were retrieved from 6 months prior and up to 1 month after the index admission. Drug purchase data were retrieved and analyzed up to 5 years prior to the index admission with an osteoporotic hip fracture. One year of BP treatment was defined as a period of at least 6 months of purchase of oral preparations or one purchase of zoledronic acid per year, as was defined by others [24,25,26]. For medications other than BP, we classified any purchase during 5 years before the fracture as a positive result and no purchase as a negative result.
Radiographic assessment
From review of lower extremity radiographs and surgical records of patients admitted with low trauma hip fracture, we classified AFF based on the revised definition of the American Society for Bone and Mineral Research [9]. Accordingly, the fracture is located along the femoral diaphysis, from just distal to the lesser trochanter to just proximal to the supracondylar flare. In addition, at least three of four major radiographic features must be present: (1) the fracture line originates at the lateral cortex and is substantially transverse in its orientation; (2) complete fractures extend through both cortices and may be associated with a medial spike, while incomplete fractures involve only the lateral cortex; (3) the fracture is non-comminuted or minimally comminuted; and (4) localized periosteal or endosteal thickening of the lateral cortex is present at the fracture site.
All fractures that were not classified as AFF were considered typical osteoporotic hip fractures, based on the guidelines of the American Association of Clinical Endocrinologists [27]. To address our first hypothesis that patients admitted with AFF had distinct demographic, clinical, and biochemical characteristics compared with patients admitted with typical osteoporotic hip fractures, regardless of the specific site of these typical fractures, we subdivided the latter according to the site of fracture: (1) femur neck (sub-capital and trans-cervical, not involving the trochanteric area); (2) inter-trochanteric; and (3) sub-trochanteric, not classified as AFF, and not involving the greater or lesser trochanter. Bilateral and recurrent fractures were documented separately.
Hip fracture images of all the patients were reviewed by our research team, which included a radiologist and an endocrinologist. The reviewers were blinded to BP treatment status. Images were viewed on our hospital picture archival and communication system (PACS). Radiographic images were magnified in order to detect and accurately classify the fractures. Following review by the study team, fracture classifications were compared with the ICD-9 code of the discharge diagnosis from the orthopedic department. In cases of discordance between the ICD-9 discharge code and our diagnosis, we used a consensus decision method among our research team members.
Statistical analyses
Baseline demographic, clinical, and medical characteristics and blood test results were summarized using descriptive statistics. Patients with AFF were compared with the 3 subgroups of patients with typical osteoporotic hip fractures using the ANOVA test or the Kruskal–Wallis test, as appropriate. Categorical data were compared using the χ2 test or Fisher’s exact test. A p value < 0.05 was considered statistically significant. We aimed to detect differences in BP exposure between patients with a fracture classified as an AFF and patients with typical osteoporotic hip fractures (the control group). The probability for AFF in patients admitted with hip fractures according to their BP exposure time was assessed in two models. The first model compared the AFF group (n = 31) with the entire typical osteoporotic hip fracture group (n = 958). To correct for the younger age of patients with AFF, a second model was used. We randomly matched each case (AFF) with three controls according to age (\(\pm\) 1 year) and sex. We used conditional logistic regression models for the multivariable analysis. Each time period of BP treatment prior to fracture has been analyzed separately. We examined four time-periods: any BP exposure, 2 years or less out of the 5 years prior to fracture, between 2 and 5 years prior to fracture and lastly, and 5 years or longer prior to fracture. We presented the models once with a BP exposure window without adjustment and second, following adjustment to the variables that were found to differ significantly between cases (AFF) and controls. Statistical analysis of pooled data was performed using SPSS version 25.0 (SPSS, Chicago, IL).
Ethics statement
The study was approved by the Helsinki committee of Soroka University Medical Center (SCRC-170–19). The requirement for informed consent was waived due to the retrospective nature of the study.
Results
Baseline characteristics
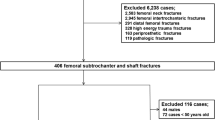
During the study period, 1616 patients over age 50 years were admitted to our institution with low trauma hip fracture. Of these, 1123 patients were offered to attend our FLS. Following exclusion of 23 patients with device-associated fractures and 111 patients with missing data (mainly due to low-quality radiographs), the final study cohort consisted of the 989 patients whose full data were available to us (Fig. 1). Radiographic assessment classified 31 (3%) as having an AFF, of whom 1 was incomplete fracture, 330 (33%) with femur neck fractures, 600 (61%) with inter-trochanteric fractures, and 28 (3%) with sub-trochanteric fractures not classified as AFF and not involving the greater or lesser trochanter. Baseline characteristics according to the site of fracture are presented in Table 1. Overall, 71% of the patients were women. Patients who presented with AFF (mean age ± SD: 72.3 ± 10.3 years) were younger than those who presented with femur neck fractures (78.0 ± 10.3) and younger than those with inter-trochanteric fractures (80.2 ± 9.6); p = 0.004 and p < 0.001 for the respective comparisons. Patients with AFF showed a better medical condition at baseline as was demonstrated by a statistically significant lower mean CCI (2.9 ± 3.7) than patients with femur neck fractures (4.9 ± 5.1), inter-trochanteric fractures (5.5 ± 4.3), and non-AFF sub-trochanteric fractures (5.2 ± 4.3), p = 0.028, p = 0.001, and p = 0.029 for the respective comparisons. Rates of any duration of PPI treatment within the 5 years prior to fracture were lower in patients with AFF (18.8%) than in patients with femur neck fractures (46.7%) and lower than in patients with inter-trochanteric fractures (50.9%), p = 0.004 and p < 0.001 for the respective comparisons. Other suggested risk factors for AFF, such as diabetes mellitus and treatment with glucocorticoids, were not found to be statistically different between the AFF group and each of the subgroups of typical osteoporotic hip fractures. The mean hemoglobin level at admission was higher among patients with AFF (13.4 ± 1.2 gr/dL) than among those with inter-trochanteric fractures (12.04 ± 1.9 gr/dL) and higher than among those with non-AFF sub-trochanteric fractures (11.9 ± 1.2), p = 0.018 and p = 0.024 for the respective comparisons. Other lab test results, including creatinine, eGFR, PTH, and 25(OH)-vitamin D levels, did not differ between the groups.
According to the matched groups (Table 2), the mean CCI of patients with AFF was significantly lower than that of patients with typical osteoporotic hip fractures (2.9 ± 3.7 vs. 4.7 ± 4.2; p = 0.030). In addition, rates of PPI treatment were lower in the AFF group (18.8% vs. 51.6% p = 0.002). Differences between the groups were not significant in the other parameters examined, including hemoglobin level, eGFR, PTH and 25(OH)-vitamin D levels, diabetes mellitus status, and glucocorticoid treatment.
BP exposure
Computerized data of BP purchases were available for 5 years prior to the fracture date for patients with all fracture types. We compared the duration of BP exposure between patients diagnosed with AFF (n = 31) and those with typical osteoporotic hip fractures (n = 958) (Table 3). The proportion of patients treated with BP for 5 years or more was higher among those with AFF than among those with typical osteoporotic hip fractures (58.1 vs. 16.0%; p < 0.001). The only incomplete AFF patient was under prolonged BP treatment of 5 years or more.
Odds ratios (ORs) for the risk for AFF at admission with hip fracture for the entire cohort and for the matched group, according to the length of BP exposure time, are presented in Table 4. In both models, the adjusted OR of being admitted with AFF increased as the number of BP treatment years increased, becoming statistically significant for 5 years of treatment or more (adjusted OR using the model that included the entire cohort- 19.34; 95% CI-7.84–47.93 and 21.7; 95% CI-4.1–113.9 for the matched cohort model). For patients treated with BP for more than 2 years and less than 5 years, the trend for increased risk did not reach statistical significance. The risk of being admitted with AFF was higher in the adjusted models than in the unadjusted models (Table 4).
Discussion
This case control study showed that patients with AFF (cases) were younger and in better medical condition at baseline than patients with all other sites of typical osteoporotic hip fracture (controls). This was evident from a statistically significant lower value of CCI. CCI remained significantly lower (p = 0.03) following 1:3 matching for age (\(\pm\) 1 year) and sex, of cases with controls. These results, taken together, concur with our first hypothesis that patients admitted with AFF had distinct demographic and clinical characteristics compared with patients admitted with typical osteoporotic hip fractures. We also found that a higher proportion of cases than controls had been treated with BP for 5 years or more (58.1 vs. 16.0%; p < 0.001). A multivariate analysis of patients matched for age and sex found that the OR for being admitted with an AFF, adjusted for CCI and any duration of PPI treatment, increased as the number of BP treatment years increased and reached statistical significance after 5 years or more of treatment (OR-21.7; 95% CI-4.1–113.9). A second multivariant analysis for the entire cohort, adjusted for sex, age, CCI, and any duration of PPI treatment showed comparable results (adjusted OR-19.34; 95% CI-7.84–47.93) and, thus, reassure the concern regarding selection bias of using the matched group model. These findings address our second hypothesis, as to the risk of AFF in relation to the length of BP exposure. Our results demonstrated that 5 years of BP treatment may represent a point of time at which the risk of AFF should become a significant consideration in decisions about treatment management. Moreover, though not statistically significant, our results suggest that the critical point for decision making may occur even earlier, between 2 and 5 years of treatment. These results are congruent with previous studies that suggested an exponential correlation between the duration of BP exposure and the risk of AFF [14, 15, 17].
Our study is consistent with a number of reports that showed a younger age of patients with AFF than with typical osteoporotic hip fractures [9, 11, 18]. Associations of other risk factors with AFF are controversial. We found a lower rate of any duration of PPI treatment among patients admitted with AFF. This contrasts with others who demonstrated higher rates of PPI treatment among patients admitted with AFF, compared with typical osteoporotic hip fracture [11, 18]. However, since according to our methods any duration of PPI treatment within the 5 years prior to hip fracture was considered as “positive” for PPI treatment, it is questionable whether this observation has clinical significance.
Treatment with glucocorticoids (GC) is another controversial, yet commonly reported risk factor for AFF [9, 11, 17, 18, 21]. We did not find an association of any GC treatment with increased risk of AFF compared with typical osteoporotic hip fractures. Our findings concur with those of a retrospective Korean study [11] but contrast with those of a retrospective Canadian study that showed a higher rate of GC treatment among patients admitted with AFF than with other fractures [18]. A recent, large, well-designed retrospective study, by Black et al., has convincingly shown that the risk of AFF, among women treated with BP, increased significantly after one or more years of GC treatment [17]. A possible explanation for the discrepancy between the studies is the lack of information regarding the duration of GC treatment in our study and others [11, 18]. Accordingly, studies aimed to evaluate the added risk of GC to the development of AFF should include data regarding treatment duration with both drugs [28].
This study has several limitations. First, data collection was retrospective. A prospective evaluation may have achieved better quality data. Second, our cohort does not represent either the general population or the population of patients with osteoporosis. More specifically, our cohort represents high-risk osteoporotic patients [7] who were admitted to our institution with hip fracture. Accordingly, we do not claim that the increased risk found in our study represents the risk of having an AFF following certain BP exposure time in the general osteoporotic population but rather the risk of AFF among patients over age 50 years, admitted with low trauma hip fracture. Third, pre-treatment bone mineral density (BMD) measurements were available for only few patients in our cohort (less than 20%). Therefore, we could not completely exclude the possibility of reverse causality, in which the patients on longer treatment with BP are those with more severe osteoporosis. However, the lower CCI in patients with AFF shown in our study suggests that the overall morbidity load was lower in the AFF group compared with typical osteoporotic hip fractures. We assumed that this better medical condition is also applicable to the AFF patient’s bone health status. The consistency of the CCI trend after adjustment to age and sex strengthens this assumption. Fourth, the number of patients with AFF in our study was small, due to the rarity of this condition. This resulted in numerically unequal “cases” and “controls” groups. We overcame this disparity by creating a control group that was randomly matched 3:1 for sex and age (\(\pm\) 1 year). Fifth, we could not determine the exact adherence of each individual patient to BP treatment, as well as to treatment with other medications of interest, such as PPIs and glucocorticoids. Finally, a possible selection bias arises in that 310 patients were excluded from the analysis due to their being insured by a health medical organization for which we did not have drug purchase data. There is no reason to believe that their characteristics differed from the patients included in the cohort.
Despite the above limitations, this study has important strengths. The unified electronical medical files, used in our hospital and in the community medical services, enabled access to all recorded information of all the patients in our fracture liaison service. Specifically, we could easily calculate the CCI [22], a well validated index for comorbidities. Additionally, the femoral fracture images of all our patients were reviewed by the same research team members, including a radiologist and an endocrinologist, blinded to BP treatment status. The classification of the typical femoral hip fractures into subgroups and the assessment of each group separately strengthen the credibility of our hypothesis, namely that patients admitted with AFF had distinct characteristics, compared with patients admitted with typical osteoporotic hip fractures, regardless of the specific site of these typical fractures.
In conclusion, our study showed that patients with AFF compared to typical hip fractures showed better baseline medical conditions irrespective of their younger age. A higher proportion of patients with AFF than patients with typical osteoporotic hip fractures was treated by BPs for 5 or more years prior to their fractures. Further, the risk of AFF, though absolutely low, increased as the number of BP treatment years increased and reached statistical significance after 5 years of treatment. This finding concurs with the rationale of current guidelines, which suggests a “drug holiday” after 3–5 years of BP treatment, for patients with a mild to moderate fracture risk [7, 15, 29]. Nevertheless, the beneficial effect of BP treatment on the risk of vertebral and non-vertebral typical osteoporotic fractures should be considered when decisions on “drug holiday,” and its duration is taken [17]. To overcome the limitations of our study, a large, prospective study that follows patients treated with BP, including adherence data, is needed. This will enable drawing more precise conclusions regarding the time point during BP treatment at which the risk of AFF becomes significantly elevated.
Availability of data and material
Data is available upon the request and according to the national policy of data sharing requiring authorization by ethics committee.
Code availability
Not applicable.
References
Ensrud KE, Crandall CJ (2017) Osteoporosis. Ann Intern Med 167:17–32. https://doi.org/10.7326/AITC201708010
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R, National osteoporosis foundation (2014) clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381. https://doi.org/10.1007/s00198-014-2794-2
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. The Lancet 348:1535–1541. https://doi.org/10.1016/S0140-6736(96)07088-2
Boonen S, Reginster J, Kaufman J, Lippuner K, Zanchetta J, Langdahl B, Rizzoli R, Lipschitz S, Dimai HP, Witvrouw R, Eriksen E, Brixen K, Russo L, Claessens F, Papanastasiou P, Antunez O, Su G, Bucci-Rechtweg C, Hruska J, Incera E, Vanderschueren D, Orwoll E (2012) Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 367:1714–1723. https://doi.org/10.1056/NEJMoa1204061
Harris ST, Watts NB, Genant HK, Mckeever CD, Hangartner T, Keller M, Chesnut CH III, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD, for the Vertebral efficacy with risedronate therapy, study group (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282:1344–1352. https://doi.org/10.1001/jama.282.14.1344
Black DM, Rosen CJ (2016) Postmenopausal Osteoporosis. N Engl J Med 374:254–262. https://doi.org/10.1056/NEJMcp1513724
Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D (2019) pharmacological management of osteoporosis in postmenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 104:1595–1622. https://doi.org/10.1210/jc.2019-00221
Lloyd AA, Gludovatz B, Riedel C, Luengo EA, Saiyed R, Marty E, Lorich DG, Lane JM, Ritchie RO, Busse B, Donnelly E (2017) Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci U S A 114:8722–8727. https://doi.org/10.1073/pnas.1704460114
Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, Ebeling PR, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Howe TS, van der Meulen MC, Weinstein RS, Whyte MP (2014) Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 29:1–23. https://doi.org/10.1002/jbmr.1998
Larsen MS, Schmal H (2018) The enigma of atypical femoral fractures: A summary of current knowledge. EFORT Open Rev 3:494–500. https://doi.org/10.1302/2058-5241.3.170070
Kim D, Sung YK, Cho SK, Han M, Kim YS (2016) Factors associated with atypical femoral fracture. Rheumatol Int 36:65–71. https://doi.org/10.1007/s00296-015-3323-0
McKenna MJ, Heffernan E, Hurson C, McKiernan FE (2014) Clinician approach to diagnosis of stress fractures including bisphosphonate-associated fractures. QJM 107:99–105. https://doi.org/10.1093/qjmed/hct192
Geissler JR, Bajaj D, Fritton JC (2015) American Society of Biomechanics Journal of Biomechanics Award 2013: cortical bone tissue mechanical quality and biological mechanisms possibly underlying atypical fractures. J Biomech 48:883–894. https://doi.org/10.1016/j.jbiomech.2015.01.032
Dell RM, Adams AL, Greene DF, Funahashi TT, Silverman SL, Eisemon EO, Zhou H, Burchette RJ, Ott SM (2012) Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 27:2544–2550. https://doi.org/10.1002/jbmr.1719
Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, Compston JE, Drake MT, Edwards BJ, Favus MJ, Greenspan SL, McKinney R Jr, Pignolo RJ, Sellmeyer DE (2016) Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the american society for bone and mineral research. J Bone Miner Res 31:16–35. https://doi.org/10.1002/jbmr.2708
van de Laarschot DM, McKenna MJ, Abrahamsen B, Langdahl B, Cohen-Solal M, Guañabens N, Eastell R, Ralston SH, Zillikens MC (2019) Medical management of patients after atypical femur fractures: a systematic review and recommendations from the European calcified tissue society. J Clin Endocrinol Metab 105:1682–1699. https://doi.org/10.1210/clinem/dgz295
Black DM, Geiger EJ, Eastell R, Vittinghoff E, Li BH, Ryan DS, Dell RM, Adams AL (2020) Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med 383(8):743–753. https://doi.org/10.1056/NEJMoa1916525
Mahjoub Z, Jean S, Leclerc J, Brown JP, Boulet D, Pelet S, Grondin C, Dumont J, Belzile ÉL, Michou L (2016) Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 31:767–776. https://doi.org/10.1002/jbmr.2748
Giusti A, Hamdy NAT, Papapoulos SE (2010) Atypical fractures of the femur and bisphosphonate therapy. Bone 47:169–180. https://doi.org/10.1016/j.bone.2010.05.019
Rasmussen NH, Dal J, de Vries F, van den Bergh JP, Jensen MH, Vestergaard P (2020) Diabetes and fractures: new evidence of atypical femoral fractures? Osteoporos Int 31:447–455. https://doi.org/10.1007/s00198-019-05224-y
Koh JH, Myong JP, Yoo J, Lim YW, Lee J, Kwok SK, Park SH, Ju JH (2017) Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years’ experience in a single center. Osteoporos Int 28:3251–3259. https://doi.org/10.1007/s00198-017-4169-y
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47:1245–1251. https://doi.org/10.1016/0895-4356(94)90129-5
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, for the Modification of diet in renal disease study group, (1999) a more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130:461–470. https://doi.org/10.7326/0003-4819-130-6-199903160-00002
Siris ES, Pasquale MK, Wang Y, Watts NB (2011) Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001–2008. J Bone Miner Res 26:3–11. https://doi.org/10.1002/jbmr.189
Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, Cummings SR, Hue TF, Lippuner K, Lakatos P, Leung PC, Man Z, Martinez RLM, Tan M, Ruzycky ME, Su G, Eastell R (2012) The effect of 3 versus 6 years of Zoledronic acid treatment of osteoporosis: A randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27:243–254. https://doi.org/10.1002/jbmr.1494
McClung M, Harris ST, Miller PD, Bauer DC, Davison KS, Dian L, Hanley DA, Kendler DL, Yuen CK, Lewiecki EM (2013) Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 126:13–20. https://doi.org/10.1016/j.amjmed.2012.06.023
Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, Kleerekoper M, Lewiecki EM, Miller PD, Narula HS, Pessah-Pollack R, Tangpricha V, Wimalawansa SJ, Watts NB (2016) American Association Of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis — 2016. Endocr Pract 22:1–42. https://doi.org/10.4158/EP161435.GL
Black DM, Abrahamsen B, Bouxsein ML, Einhorn T, Napoli N (2018) Atypical femur fractures: review of epidemiology, relationship to bisphosphonates, prevention, and clinical management. Endocr Rev 40(2):333–368. https://doi.org/10.1210/er.2018-00001
Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, Kleerekoper M, Luckey MM, McClung MR, Pollack RP, Petak SM (2010) American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis: executive summary of recommendations. Endocr Pract 16:1016–1019. https://doi.org/10.4158/ep.16.6.1016
Funding
None.
Author information
Authors and Affiliations
Contributions
Noa Bareli—study design, radiograph images assessment, analysis and interpretation of data, manuscript writing.
Roni Gat—study design, acquisition of data, analysis and interpretation of data, approval of the manuscript final version.
Victoria Makarov—radiograph images assessment, analysis and interpretation of data, approval of the manuscript final version.
Ethel Siris—analysis and interpretation of data, revision of the manuscript, approval of the manuscript final version.
Merav Fraenkel—study design, analysis and interpretation of data, manuscript writing and approval.
Uri Yoel—study design, radiograph image assessment, analysis and interpretation of data, manuscript writing and approval.
Corresponding author
Ethics declarations
Conflict of interest
Noa Bareli, Roni Gat, Victoria Makarov, Ethel Siris, Merav Fraenkel, and Uri Yoel declare that they have no conflict of interest.
Ethical approval
The study was approved by the Helsinki committee of Soroka University Medical Center (SCRC-170–19). The requirement for informed consent was waived due to the retrospective nature of the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bareli, N., Gat, R., Makarov, V. et al. Bisphosphonate treatment and the risk of atypical femoral fracture among patients participating in a Fracture Liaison Service of a tertiary medical center. Arch Osteoporos 16, 86 (2021). https://doi.org/10.1007/s11657-021-00944-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-021-00944-3