Abstract
Purpose
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease that primarily affects the axial skeleton and typically has an early onset. Although earlier onset is associated with worse prognosis, there have been few studies of bone mineral density (BMD) in adolescent patients with axSpA.
Methods
We analysed the clinical characteristics of 43 adolescent patients with axSpA at a baseline assessment and at a follow-up 2 years later. The baseline assessment included age, disease duration, treatment agents, and clinical, radiologic, and laboratory data. BMD of the lumbar spine, femoral neck, and total hip were measured by dual-energy X-ray absorptiometry during both the baseline assessment and the 2-year follow-up. We performed multivariate linear regression analyses to identify factors independently associated with BMD. We analysed the associations between changes in BMD and reductions in inflammatory markers.
Results
The average age of participants was 17.9 years and the mean disease duration was 2.2 years. Of the 43 patients, 10 (23%) had low BMD at any site (lumbar spine, femoral neck, and/or total hip). At baseline, multivariate analysis showed that body mass index (BMI), erythrocyte sedimentation rate (ESR), and spinal structural damage were associated with lumbar spine Z-scores. Increases in BMD in the lumbar spine were correlated with reductions in ESR (r = 0.40, P = 0.02) and C-reactive protein (CRP) (r = 0.40, P = 0.02). Increases in BMD in the total hip were correlated with reductions in CRP (r = 0.38, P = 0.03).
Conclusion
In adolescent axSpA patients, bone health was associated with systemic inflammation and the severity of structural damage. Reduced systemic inflammation was associated with improvements in bone health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spondyloarthritis (SpA) is an umbrella term for a group of heterogeneous conditions, occurring in both adults and children, that differ from other types of inflammatory arthritis in genetic predisposition, pathogenesis, and outcome [1]. Juvenile SpA is a group of human leukocyte antigen (HLA) B27-associated disorders that begin at age 16 years or younger [2]. Axial SpA (axSpA) is a chronic inflammatory disease that primarily affects the axial skeleton. Patients present with chronic inflammatory pain, predominantly of the pelvis and lower back. Patients with axSpA onset at a young age may have worse functional outcomes and more comorbidities than those with later onset [3].
Bone loss and structural damage can occur in the axial skeleton as a consequence of inflammation. The increased risk of osteoporosis in axSpA is related to systemic inflammation, decreased mobility, and mineralization defects due to subclinical gut involvement [4, 5]. In patients with axSpA, bone mineral density (BMD) is lower than in the age- and sex-matched general population, and the fracture risk is greater [6, 7]. Monitoring the bone status of axSpA patients is therefore important for the prevention of fractures and osteoporosis [8]. A recent study showed that the prevalence of low BMD in children and adolescents with juvenile onset AS was 16.1% (18/112) [3].
Measurement of BMD at the hip and spine using dual-energy X-ray absorptiometry (DXA) is commonly used to establish or confirm a diagnosis of osteoporosis and to monitor patients [5]. Most data related to bone loss in patients with axSpA are based on measurements of BMD [9].
There are few studies of the changes in BMD in adolescents with axSpA. The aims of the present study were to identify the factors related to low BMD in adolescent patients with axSpA and to examine the association between systemic inflammation and bone health over 2 years.
Patients and methods
Participants
The study included 43 patients with axSpA who fulfilled the imaging arm of the Assessment of SpondyloArthritis International Society (ASAS) axSpA criteria [10] and who were followed up consecutively between September 2013 and June 2019. After retrospective review of the clinical records, we included patients who had more than two BMD evaluations. No patients had thyroid, parathyroid, renal, or liver disease.
The patients included in the study were not treated with glucocorticoids during the study period. The study was reviewed and approved by the local Institutional Review Board, which has jurisdiction over the local study populations (IRB number: OC20RISI0069).
Clinical data
We collected disease-related data, disease activity scores, and treatment agents for patients with axSpA. The clinical data included age, gender, body mass index (BMI), disease duration, age of disease onset, and HLA-B27 status. Disease activity was measured with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [11]. All scores were recorded on a visual analogue scale from 0 to 10. The Bath Ankylosing Spondylitis Functional Index (BASFI) [12] and patient global assessment (PGA) scores were also recorded. The Ankylosing Spondylitis Disease Activity Score (ASDAS) was calculated as described previously [13]. The erythrocyte sedimentation rate (ESR) and level of C-reactive protein (CRP) were measured during the baseline and follow-up evaluations.
BMD measurement
Areal BMD was measured using DXA (Lunar Prodigy densitometer, GE Healthcare, Madison, WI, USA). All measurements were taken by experienced operators using the same machine and standardized procedures for participant positioning. BMD was measured at the lumbar spine (L1–L4) and the left hip (femoral neck and total proximal femur) and expressed as the number of grams of bone mineral per square centimetre (g/cm2). A position statement by the International Society for Clinical Densitometry (ISCD) recommends the use of Z-scores in females prior to menopause and in males younger than age 50 years. A Z-score less than or equal to − 2.0 is defined as “below the expected range for age” and a Z-score greater than − 2.0 is “within the expected range for age” [14]. As recommended by the ISCD, we also use Z-scores in our analysis of BMD measurements. We used the manufacturer’s reference values. Low BMD was defined as a Z-score less than or equal to − 2.0.
Radiographic scoring
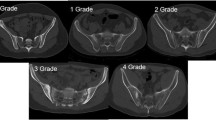
For all patients, radiographs of the cervical spine, lumbar spine, and pelvis were obtained. Lateral views of the cervical and lumbar spines were scored according to the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) [15]. Sacroiliitis was scored from right- and left-sided pelvic radiographs using the modified New York criteria [16]. We used the average score from both sides in our analysis. Sacroiliitis and the mSASSS were scored by a single trained expert who was blinded to the patient characteristics.
Statistical analysis
Statistical analysis was performed in SPSS version 21. Continuous data are expressed as the mean (SD), and categorical data are expressed as percentages. Clinical variables were compared with an independent t-test and categorical variables were compared with a chi-squared test. Pearson’s correlation coefficient was used to analyse the correlations between variables. Multiple linear regression models were used to assess the associations between lumbar spine Z-scores and the clinical variables. Linear regression analysis was used to evaluate the factors affecting BMD at baseline. Pearson’s correlation analysis was performed to assess the influence of disease activity and severity on BMD in the follow-up. The Wilcoxon signed-rank test was used to compare measurements of BMD and inflammatory markers at baseline and at the 2-year follow-up. P < 0.05 was considered statistically significant.
Results
Patient characteristics
Table 1 shows the characteristics of the axSpA patients at baseline, for all patients combined and for the groups with normal BMD and low BMD. Forty-three patients (36 male, seven female) were included in this study. The mean age at disease onset was 17.9 ± 1.6 years. The mean duration of disease symptoms was 2.2 ± 2.2 years. Thirty-seven (86.4 %) patients were positive for HLD-B27. The mean mSASSS and sacroiliitis scores were 0.7 ± 1.7 and 2.1 ± 0.8. Of the 43 patients, eight had low BMD in the lumbar spine, three had low BMD in the femoral neck, and two had low BMD in the total hip. Ten patients (23.3%) had low BMD at one or more sites (lumbar spine, femoral neck, and/or total hip). ESR and mSASSS were higher in the set of patients with low BMD (P = 0.03 and P = 0.013, respectively).
Correlations between clinical data, inflammatory markers, and BMD at baseline
BMD in the lumbar spine was negatively correlated with ESR. BMD in the femoral neck and total hip was positively correlated with age and BMI, and negatively correlated with ESR (Table 2).
Linear regression analyses of lumbar spine Z-scores
Table 3 shows the results of univariate and multivariate analyses of BMD at baseline. Univariate analysis revealed that lumbar spine Z-score was associated with BMI, peripheral arthritis, ESR, and mSASSS. In the multivariate analysis, BMI (β, − 0.10; 95% CI, − 0.21 to − 0.01), ESR (β, − 0.03; 95% CI, − 0.05 to − 0.01), and mSASSS (β, − 0.25; 95% CI, − 0.49 to − 0.01) were significantly associated with lumbar spine Z-scores (P = 0.032, P = 0.018, and P = 0.042, respectively) (Table 3).
Comparison of baseline and follow-up data
Table 4 shows the BMD, ESR, and CRP data from the baseline assessment and from the follow-up assessment 2 years later. Lumbar spine and femoral neck Z-scores were significantly greater in the follow-up assessment than at baseline. Both ESR and CRP levels were lower in the follow-up assessment, but the differences were not statistically significant. The changes in lumbar spine and femoral neck Z-scores were not related to age, therapy, or baseline clinical data (BASFI, BASDAI; data not shown). Table 5 shows the relationships between changes in BMD and the changes in inflammatory markers. The increase in lumbar spine BMD was correlated with the reductions in ESR (r = 0.40, P = 0.02) and CRP (r = 0.40, P = 0.02). The increases in lumbar spine and total hip Z-scores were significantly correlated with the reduction in ESR (lumbar spine Z-score: r = 0.37, P = 0.04; total hip Z-score: r = 0.37, P = 0.046). The increase in the total hip Z-score was positively correlated with the reduction in CRP (r = 0.40, P = 0.03).
Discussion
Our study found a significant improvement in BMD in adolescent axSpA patients after treatment. The data further revealed a relationship between the gains in bone mass and reductions in inflammatory activity in these patients.
Currently, classification of SpA is approached differently in adults and children. In juveniles, the International League of Associations for Rheumatology (ILAR) system for juvenile idiopathic arthritis (JIA) is used for classification, and most childhood cases of SpA are classified as enthesitis-related arthritis (ERA) [17]. Unlike the adult form, in which inflammatory lower back pain is the predominant clinical symptom, the juvenile form has more severe peripheral enthesitis and arthritis (predominantly of the lower extremities) as its main clinical features [18]. While HLA-B27 positivity is reported in more than 95% of adults with ankylosing spondylitis, juvenile SpA studies demonstrate a lower prevalence of approximately 50% [19].
Systemic bone loss with osteoporosis and increased fracture rates are observed in axSpA patients. In the study by Robinson et al. conducted in Sweden, the rate of spinal fractures in AS patients increased from 0.82% in 1987 to 11.3% in 2008 [20]. Kang et al. reported an incidence of vertebral fractures of 4.7% and 13.6% at 2 and 4 years, respectively [21]. Previous studies have reported bone health in JIA patients, including those with ERA, but there have been no prior reports on bone health in adolescent patients who fulfil the criteria for axSpA. The current study observed both bone density and bone quality in adolescent patients with axSpA.
In our study, low BMD had a prevalence of 23.3% in adolescents with axSpA. A different study showed a low BMD prevalence of 16.1% in children and adolescents with juvenile onset ankylosing spondylitis [3]. Forty-three axSpA patients from rheumatology clinic at Incheon St. Mary’s Hospital (tertiary centre) were recruited to this study; therefore, it may include relative severe patients. In adults, a high prevalence (47%) of low BMD has been reported for both femur and lumbar spine in SpA patients with early onset disease [22]. Furthermore, in a recent study, the prevalence of low BMD was about five times higher in axSpA patients than in the general population. Together, these results suggest that evaluation and treatment of BMD is important in both young and older axSpA patients [23].
Although osteoporosis is a well-recognized feature of axSpA, its pathogenesis remains unclear. Various factors such as treatment type, the presence of hormone disorders, alteration of the patient’s vitamin D status, and immobility or decreased physical activity may all contribute to the loss of bone activity [24,25,26]. Other risk factors for low bone mass in JIA include chronic inflammation, delayed pubertal maturation, malnutrition, and muscle weakness.
A number of longitudinal studies demonstrated that significant loss of bone mass in axSpA was strongly associated with inflammatory activity [4, 27, 28]. In our present study of adolescent patients with axSpA, markers of systemic inflammation, such as ESR and CRP, were negatively correlated with BMD. Increases in BMD in the lumbar spine and total hip were correlated with reductions in ESR and CRP, respectively. In a previous cross-sectional study, there was no correlation between bone density and serum CRP levels. However, several longitudinal studies found that the loss of bone mass in axSpA was related to inflammatory activity [28, 29], and Maillefer et al. showed that the BMD gains in the femoral neck were related to persistent systemic inflammation. Our results are thus in accordance with previous data showing that a reduction in systemic inflammation is correlated with gains in BMD.
Our longitudinal assessment suggests that axSpA patients show significant improvements in lumbar spine and femoral neck Z-scores after anti-inflammatory treatment. Most patients were treated with NSAIDs and about half of the patients were treated with sulfasalazine, a tumour necrosis factor (TNF) inhibitor. The BMD gains were significantly correlated with the reductions in systemic inflammatory markers. This suggests that the inflammatory activity of the disease itself plays a major role in the pathophysiology of bone loss in adolescent patients with axSpA. However, a previous study reported that JIA patients experienced no significant improvements in bone mass at a follow-up assessment [29].
The peak bone mass attained at the end of adolescent growth is critical in determining the risk of osteoporosis in adult life [30]. Decreased bone mass is common in children and adolescents with JIA and has been observed at the onset of the disease, throughout childhood, and into adulthood, thus increasing the risk of osteoporosis and fracture later in life [31]. TNF inhibitors appear to be associated with improvements in BMD, as well as a decrease of disease activity in adult AS [32]. This suggests that regulation of inflammation through treatment with biologic agents improves bone health in axSpA. In the present study, there was a trend toward greater Z-score increases at the femoral neck and hip in the TNF inhibitor and NSAID treatment group than in the only NSAID treatment group (data not shown). New potential drugs that target the IL-12/23 and IL-17 axis include usterkinmab, an anti-IL-12/23 human monoclonal antibody [33, 34]. In addition, there are multiple promising biologic agents on the horizon for the treatment of SpA patients, including the PDE4 inhibitor apremilast [35], but additional clinical trials are warranted prior to their use in the paediatric population.
In conclusion, bone health in adolescent axSpA patients was associated with systemic inflammation and the severity of structural damage. Reductions in systemic inflammation were associated with improvements in bone health. To reduce the risk of osteoporosis and fracture in adolescent axSpA patients, we advocate for close monitoring of bone density and bone quality and better control of disease activity.
References
Weiss PF, Colbert RA (2018) Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin N Am 65:675–690
Gmuca S, Weiss PF (2015) Juvenile spondyloarthritis. Curr Opin Rheumatol 27:364–372
Bao J, Chen Y, Bao Y-X (2014) Prevalence and risk factors of low bone mineral density in juvenile onset ankylosing spondylitis. Calcif Tissue Int 95:108–111
Maillefert JF, Aho LS, El Maghraoui A, Dougados M, Roux C (2001) Changes in bone density in patients with ankylosing spondylitis: a two-year follow-up study. Osteoporos Int 12:605–609
Mitra D, Elvins DM, Collins AJ (1999) Biochemical markers of bone metabolism in mild ankylosing spondylitis and their relationship with bone mineral density and vertebral fractures. J Rheumatol 26:2201–2204
Magrey M, Khan MA (2010) Osteoporosis in ankylosing spondylitis. Curr Rheumatol Rep 12:332–336
Kang KY, Kim IJ, Park S-H, Hong YS (2018) Associations between trabecular bone score and vertebral fractures in patients with axial spondyloarthritis. Rheumatology (Oxford) 57:1033–1040
Thornton J, Pye SR, O’Neill TW, Rawlings D, Francis RM, Symmons DPM et al (2011) Bone health in adult men and women with a history of juvenile idiopathic arthritis. J Rheumatol 38:1689–1693
Kang KY, Chung MK, Kim HN, Hong YS, Ju JH, Park S-H (2018) Severity of sacroiliitis and erythrocyte sedimentation rate are associated with a low trabecular bone score in young male patients with ankylosing spondylitis. J Rheumatol 45:349–356
Rudwaleit M, Landewé R, van der Heijde D, Listing J, Brandt J, Braun J et al (2009) The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 68:770–776
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 21:2286–2291
Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P et al (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 21:2281–2285
van der Heijde D, Lie E, Kvien TK, Sieper J, Van den Bosch F, Listing J et al (2009) ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 68:1811–1818
Baim S, Leonard MB, Bianchi M-L, Hans DB, Kalkwarf HJ, Langman CB, Rauch F (2008) Official positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Pediatric Position Development Conference. J Clin Densitom 11:6–21
Averns HL, Oxtoby J, Taylor HG, Jones PW, Dziedzic K, Dawes PT (1996) Radiological outcome in ankylosing spondylitis: use of the Stoke Ankylosing Spondylitis Spine Score (SASSS). Br J Rheumatol 35:373–376
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368
Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur AM, Suarez-Almazor ME, Woo P, International League of Associations for Rheumatology (2004) International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31:390–392
Gensler L, Davis JC (2006) Recognition and treatment of juvenile-onset spondyloarthritis. Curr Opin Rheumatol 18:507–511
Full Text PDF [Internet]. [cited 2020 Aug 31]. Available from: http://onlinelibrary.wiley.com.ssl.proxy.cuk.ac.kr:8080/doi/pdfdirect/10.1002/acr.22411
Robinson Y, Sandén B, Olerud C (2013) Increased occurrence of spinal fractures related to ankylosing spondylitis: a prospective 22-year cohort study in 17,764 patients from a national registry in Sweden. Patient Saf Surg 7:2
Kang KY, Kim IJ, Jung SM, Kwok S-K, Ju JH, Park K-S et al (2014) Incidence and predictors of morphometric vertebral fractures in patients with ankylosing spondylitis. Arthritis Res Ther 16:R124
van der Weijden MAC, van Denderen JC, Lems WF, Heymans MW, Dijkmans BAC, van der Horst-Bruinsma IE (2011) Low bone mineral density is related to male gender and decreased functional capacity in early spondylarthropathies. Clin Rheumatol 30:497–503
Kang KY, Kwok S-K, Ju JH, Hong YS, Park S-H (2016) Assessment of fracture risk in patients with axial spondyloarthritis: a case–control study using the fifth Korean National Health and Nutrition Examination Survey (KNHANES V). Scand J Rheumatol. Taylor & Francis 45:23–31
Aydin T, Karacan I, Demir SE, Sahin Z (2005) Bone loss in males with ankylosing spondylitis: its relation to sex hormone levels. Clin Endocrinol 63:467–469
Raisz LG (1988) Local and systemic factors in the pathogenesis of osteoporosis. N Engl J Med 318:818–828
Lange U, Teichmann J, Strunk J, Müller-Ladner U, Schmidt KL (2005) Association of 1.25 vitamin D3 deficiency, disease activity and low bone mass in ankylosing spondylitis. Osteoporos Int 16:1999–2004
Gratacós J, Collado A, Pons F, Osaba M, Sanmartí R, Roqué M et al (1999) Significant loss of bone mass in patients with early, active ankylosing spondylitis: a followup study. Arthritis Rheum 42:2319–2324
Kang KY, Lee KY, Kwok S-K, Ju JH, Park K-S, Hong YS, Kim HY, Park SH (2011) The change of bone mineral density according to treatment agents in patients with ankylosing spondylitis. Joint Bone Spine 78:188–193
Tang T, Tang X, Xu L, Huang Y, Zeng J, Li Q (2015) Evaluation of bone mass in children and young adults with juvenile idiopathic arthritis. Clin Exp Rheumatol 33:758–764
Cimaz R (2002) Osteoporosis in childhood rheumatic diseases: prevention and therapy. Best Pract Res Clin Rheumatol 16:397–409
Cetin A, Celiker R, Dinçer F, Ariyürek M (1998) Bone mineral density in children with juvenile chronic arthritis. Clin Rheumatol 17:551–553
Kang KY, Ju JH, Park S-H, Kim H-Y (2013) The paradoxical effects of TNF inhibitors on bone mineral density and radiographic progression in patients with ankylosing spondylitis. Rheumatology. 52:718–726
Poddubnyy D, Hermann K-GA, Callhoff J, Listing J, Sieper J (2014) Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis 73:817–823
Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, McInnes I, van Laar JM, Landewé R, Wordsworth P, Wollenhaupt J, Kellner H, Paramarta J, Wei J, Brachat A, Bek S, Laurent D, Li Y, Wang YA, Bertolino AP, Gsteiger S, Wright AM, Hueber W (2013) Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 382:1705–1713
Pathan E, Abraham S, Van Rossen E, Withrington R, Keat A, Charles PJ et al (2013) Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in ankylosing spondylitis. Ann Rheum Dis 72:1475–1480
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, SH., Kim, K., Kim, MY. et al. A 2-year longitudinal study of bone health in adolescent patients with axial spondyloarthritis. Arch Osteoporos 16, 12 (2021). https://doi.org/10.1007/s11657-020-00860-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-020-00860-y