Abstract
Summary
This study aimed to compare the vitamin D status in healthy Moroccan men and women aged 50 years and older. A total of 186 Moroccan women and 68 men, who had no previous diagnosis of osteoporosis, were recruited prospectively. We found in this study a high prevalence of hypovitaminosis D with no difference between men and women.
Purpose
The main purpose of this study was to describe and compare the vitamin D status, parathormone, calcium, and phosphate of healthy Moroccan men and women aged 50 years and older.
Methods
We conducted two cross-sectional studies, in postmenopausal women from October 2008 to November 2009 and in men over 50 years old, from December 2009 to August 2010. A total of 186 Moroccan women and 68 men, who had no previous diagnosis of osteoporosis, were recruited prospectively. For the definition of hypovitaminosis D, the preferred level for 25-hydroxyvitamin D (25(OH)D) insufficiency, which is now recommended by many experts, is 30 ng/mL (75 nmol/L), and the levels below 10 ng/ml (25 nmol/L) indicate deficiency.
Results
The prevalence of vitamin D deficiency in men and women was 4.4 and 8.6 %, respectively, and the prevalence of vitamin D(25(OH) D) insufficiency in men and women were 85.2 and 77.4 %, respectively. In men and women, no correlations were found between intact parathormone (PTHi) and 25(OH) D (r = 0.056).
Conclusions
Despite a sunny environment, we found in this study a high prevalence of hypovitaminosis D (insufficiency + deficiency) in Moroccan men over 50 years old and postmenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D (25(OH)D) plays an important role in bone and calcium metabolism and may participate in the the prevention of diabetes, cancers, heart diseases, respiratory diseases, and many other diseases [1]. Reports from across the world indicate that hypovitaminosis D is widespread and is reemerging as a major health problem globally. The serum level of 25-hydroxyvitamin D (25(OH)D), which is the main circulating metabolite of vitamin D, is considered the best indicator of vitamin D status in humans [2]. For the definition of the hypovitaminosis D, the preferred level for 25(OH)D insufficiency and that is now recommended by many experts is 30 ng/mL (75 nmol/L), and the levels below 10 ng/ml (25 nmol/L) indicate deficiency [3, 4]. Levels below 10 ng/ml are most common in regions such as South Asia and the Middle East [5–8]. Studies across Europe (n = 55,844) in different age groups revealed widespread vitamin D deficiency (13 %)[9]. A British study reported that approximately 15 % of the population had serum 25(OH)D levels below 10 ng/ml [10]. The vitamin D status is influenced by many factors like older age, female sex, higher latitude, winter season, darker skin pigmentation, less sunlight exposure, dietary habits, and absence of vitamin D fortification [1]. The main purpose of this study was to describe and compare levels of vitamin D, intact parathormone, calcium, and phosphate status of healthy Moroccan men and women aged 50 years and older.
Methods
Subjects
This was a cross-sectional study conducted from October 2008 to November 2009 in postmenopausal women and from December 2009 to August 2010 in men over 50 years old (Fig. 1). A total of 186 postmenopausal women and 68 men, who had no previous diagnosis of osteoporosis, were recruited prospectively from our laboratory department. All subjects who volunteered to participate in the study were enrolled after obtaining informed written consent. They were screened using a detailed questionnaire, history, behaviors, and physical examination. Subjects were excluded from the study if they had conditions affecting bone metabolism, such as diseases of kidney, liver, parathyroid, thyroid, diabetes mellitus, hyperprolactinemia, rheumatoid arthritis, ankylosing spondylitis, malabsorption syndromes, malignant tumors, hematological diseases, or previous pathological fractures. Subjects were also excluded if they smoke cigarette or had been receiving corticosteroids, thyroid hormone, fluoride, bisphosphonate, calcitonin, thiazide diuretics, barbiturates, antiseizure medications, 25(OH)D, or calcium-containing drugs. Body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared.
BMD measurement
Bone mineral density (BMD) was determined by a Lunar Prodigy Vision DXA system (Lunar Corp., Madison, WI). The DXA scans were obtained by standard procedures supplied by the manufacturer for scanning and analysis. All BMD measurements were carried out by two experienced technicians. Daily quality control was carried out by measurement of a Lunar phantom. At the time of the study, phantom measurements showed stable results. The phantom precision expressed as the coefficient of variation percentage was 0.08. Moreover, reproducibility has been assessed by the same two technicians in clinical practice and showed a smallest detectable difference of 0.04 g/cm2 (spine) and 0.02 (hips). Patient BMD was measured at the lumbar spine and at the femurs (i.e., femoral neck and total hip). Patient BMD was measured at the lumbar spine (anteroposterior projection at L1–L4) and at the femurs (i.e., femoral neck, trochanter, and total hip). The World Health Organization (WHO) classification system was applied, defining osteoporosis as T-score ≤−2.5 and osteopenia as −2.5 < T-score < −1.
Biological measurements
All subjects had fasting blood taken in the morning. The samples were frozen and stored at −80 °C and subsequently thawed and analyzed in one batch. Serum vitamin D and intact parathyroid hormone (PTHi) were measured using electrochemiluminescence immunoassay (ECLIA) (Cobas e601, Roche Diagnostics 125GmBH, Mannheim, Germany). This 25(OH)D assay measures only the 25(OH)D3 and not the 25(OH)D2. The intra- and interassay variation coefficients for vitamin D in our laboratory were 10.5 and 17.8 %, respectively, and for the PTH, they were 2.5 and 4.8 % respectively. Calcium and phosphate was measured by the Dimension® RxL Max® Integrated Chemistry System of Siemens. All the laboratory tests were subject to validation using National External Quality Assurance Schemes.
Statistical analysis
Continuous variables are presented as the mean and the standard deviation (SD). To compare anthropometric and biological parameters in men and women, the analysis of variance (ANOVA) was used for quantitative variables and the chi-square test for qualitative variables. For the comparison of patients with high and low levels of 25(OH)D, statistical comparison of patients according to quartiles of 25(OH)D was used. Associations between continuous variables were examined by Pearson correlation coefficient. Significance is usually accepted at <0.05. Excel 2007 and SPSS 15.0 were used for statistical analysis.
Results
The mean (SD) age was 59.0 (8.2) in women and 64.7 (8.6) in men. The prevalence of vitamin D deficiency in men and women were respectively 4.4 and 8.6 %, and vitamin D insufficiency in men and women were respectively 85.2 and 77.4 %. The prevalence of osteoporosis in men and women were 8.8 and 35.4 %, respectively (Table 1).
Mean (SD) BMI was significantly higher in women 30.9 (4.9) than in men 26.75 (4.26). We found low mean vitamin D levels in nanograms per milliliter in both men (20.1 ng/ml (9.2)) and women (20.7 ng/ml (6.5)). Calcium and phosphate levels were significantly higher in women than in men; conversely, the mean PTHi level was significantly higher in men (Table 1).
In the Table 2, we compared the mean (SD) of age, BMI, calcium, phosphate, and PTHi in women and men within three intervals of serum vitamin D level (vitamin D < 10 ng/ml, 10 ≤ vitamin D < 30 ng/ml, vitamin D ≥ 30 ng/ml). Indeed, no significant difference was found between patients with deficiency and insufficiency and those with normal vitamin D status.
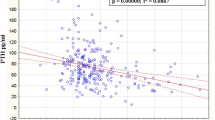
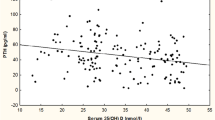
No correlation was found between PTHi and 25(OH)D in women or in men (Fig. 2). Only two positive correlations were found among men. Indeed, the PTHi correlates positively with phosphate and the BMI correlates positively to the calcium level. Comparison of patients according to quartiles of vitamin D levels showed that only men in the lowest quartile were shorter (p = 0.027) (Tables 3 and 4).
Discussion
If the location of Morocco is theoretically between latitudes of 32° 0′ N and longitudes of 5° 00′ W, then it gets plenty of sunlight throughout the year and should not experience poor vitamin D status. However, the results of the present paper showed no difference in the prevalence of vitamin D deficiency in men and women (4.41 vs 8.61 %) and also no difference in the prevalence of vitamin D insufficiency in men and women (85.29 vs 77.41 %). Although, women have a higher prevalence of osteoporosis and a higher BMI and men have a higher mean PTHi level. Many factors can contribute to poor vitamin D status in Moroccan men and women as measured by low circulating levels of 25(OH)D. Indeed, Moroccan people avoid the sun and have a more pigmented skin, also their clothing style prevents exposure of the body to direct sunlight which results in less vitamin D production in the skin. Also, Moroccans have low consumption of vitamin D-rich foods and the absence of fortification. Another Moroccan study in 178 Moroccan menopausal women aged 50 years old and over have found higher deficiency rate. Thus, 152 patients (85.3 %) had levels <30 ng/ml (insufficiency) and 92 (51.6 %) had levels <10 ng/ml (deficiency) [11]. In another study among 415 Moroccan women aged between 24 and 77 years, the prevalence of vitamin D insufficiency was 91 % [12]. Moreover, the results of this study are in line with previous reports from other Arabian studies. Thus, in healthy Saudi Arabian men, vitamin D deficiency and insufficiency were common. About 87.8 % exhibited a serum 25(OH)D level less than that commonly considered to represent deficiency (<50 nmol/L), 9.7 % showing levels considered insufficient (≥50– < 75 nmol/L), and with only 2.5 % showing levels of sufficiency (≥75.0 nmol/L) [13]. Worldwide vitamin D deficiency seems to be highly prevalent. Mean serum 25(OH)D of 30 nmol/l or even lower has been reported from surveys in India, China, the Middle East, and some African countries. In addition, poor vitamin D status is common in industrialized countries like North America, Oceania, and Europe and especially in risk groups such as children, pregnant women, elderly, and immigrants [14–16]. In Irish adults, the year-round prevalence estimates of serum 25(OH)D is <30 and <50 nmol/l (6.7 and 40.1 %, respectively) [17]. Let us remember that blacks consistently have lower levels of total 25-hydroxyvitamin D than whites do; they are frequently given a diagnosis of vitamin D deficiency [18]. Thus, in black Americans, low levels of total 25-hydroxyvitamin D probably do not indicate true vitamin D deficiency when levels of vitamin D-binding protein are also low [18]. So, to indicate the supplementation in persons with low levels of vitamin D status, we must take into account the levels of vitamin D-binding proteins.
In this study, there was no correlation between vitamin D and age. The mean age of the study population was 59.0 (8.28) in women and 64.7 (8.6) in men, and this remains low compared to other cohorts of the literature. Classically, increasing age is associated with the decrease of the concentration of vitamin D. This is due to the decrease in the synthesis of vitamin D by the skin, the decrease in the dietary intake, the decrease in renal function, and the reduced time spent outdoors.
Saliba et al. found that serum parathyroid hormone levels were inversely and weakly correlated with 25(OH)D levels (r = −0.176), and the parathyroid hormone plateau was attained at a significantly lower 25(OH)D cut point of 46.2 nmol/L [19]. The serum levels of 25(OH)D sufficient to keep the parathyroid hormone level at a range that will prevent bone loss are still unclear. In the present study, there was no correlation between serum 25(OH)D and intact PTH levels, perhaps because our study population only included relatively young postmenopausal women who were under 70 years old since older men and women are more prone to develop secondary hyperparathyroidism resulting in greater bone turnover, bone loss, and increased risk of fractures. Moreover, it could be related to the fact that PTHi is regulated by several modulators including calcium intake and the magnesemia. In agreement with our results, two Moroccan studies failed to show any significant correlation with serum vitamin D and PTHi in postmenopausal women [11], and women aged between 24 and 77 years [12].
According to the World Health Organization (WHO) classification of BMI, in this study, men were overweight and women were obese. For the BMI and the vitamin D level, no correlation was found between these two parameters, unlike the data of the literature that reports that obese individuals have lower bioavailability of vitamin D [20]. In fact, obese individuals have decreased sun exposure compared with their lean counterparts, negative feedback from increased 1, 25(OH)2D concentration in obese individuals decreases 25(OH)D concentrations, and as a lipid-soluble molecule, vitamin D3 can be taken up by adipocytes and stored in fat for later use. Vitamin D is sequestered within the adipose tissue [21]. Many observational studies have demonstrated a negative association between indicators of obesity and serum levels of vitamin D [22, 23]. The expression of both the vitamin D receptor and 25-hydroxyvitamin D 1α-hydroxylase (CYP27B1) genes has been shown in human adipocytes [22]. In fact, vitamin D inhibits in adipocytes the active form of adipogenic transcription factors and fat accumulation during the differentiation phase. Some recent studies demonstrate that vitamin D metabolites also influence adipokine production and the inflammatory response in adipose tissues [22].
The interpretation of a serum level of 25(OH)D is challenging. Indeed, the studies are not always comparable, since different assays have been used and the interlaboratory variation is considerable. The vitamin D status was determined in this study by using the test vitamin D3 (25-OH-vitamin D3) and not the total vitamin D (25-OH-vitamin D2 + 25-OH-vitamin D3). However, none of our patients was under D2 therapy.
Our study has strengths and limitations. The strengths of the present study include very strict detailed inclusion criteria, and all of the DXA and biochemical measurements were conducted with a single bone densitometer and a single biochemistry laboratory, with very careful quality controls in place. The main limitations lie in the cross-sectional nature of the study. The 25(OH)D assay used in our study was the Roche electrochemiluminescence assay that only measures 25(OH)D3. Indeed, the total 25-hydroxyvitamin D level is the appropriate indicator of vitamin D body stores.
Conclusion
Hypovitaminosis D is widespread in Moroccan men over 50 years and postmenopausal women. The prevalence of vitamin D deficiency in men and women were respectively 4.41 and 8.61 %, and the prevalence of vitamin D insufficiency in men and women were respectively 85.29 and 77.41 %. Indeed, no significant correlation was found between 25(OH)D and PTHi levels, and no significant difference was found between quartiles of vitamin D levels in men and women.
References
1. Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J; IOF Committee of Scientific Advisors (CSA) Nutrition Working Group (2009) Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 20(11):1807-20. doi:10.1007/s00198-009-0954-6. Epub 2009 Jun 19. Review. Erratum in: Osteoporos Int. 20(11):1821
Seamans KM, Cashman KD (2009) Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr 89(6):1997S–2008S. doi:10.3945/ajcn.2009.27230D
Holick MF (2006) Resurrection of vitamin D deficiency and rickets. J Clin Invest 116(8):2062–2072. doi:10.1172/JCI29449
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B (2006) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84(1):18–28
Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, Pierroz DD, Weber P, Hoffmann KA (2014) Systematic review of vitamin D status in populations worldwide. Br J Nutr 111(1):23–45. doi:10.1017/S0007114513001840, Epub 2013 Aug 9. Review
Yu S, Fang H, Han J, Cheng X, Xia L, Li S, Liu M, Tao Z, Wang L, Hou L, Qin X, Li P, Zhang R, Su W, Qiu L (2015) The high prevalence of hypovitaminosis D in China: a multicenter vitamin D status survey. Medicine (Baltimore). 2015 Feb;94(8):e585. doi: 10.1097/MD.0000000000000585. Erratum in: Medicine (Baltimore). 2015 Mar;94(11):1. PubMed PMID: 25715263; PubMed Central PMCID: PMC4554140.
Al-Faris NA (2016) High prevalence of vitamin D deficiency among pregnant Saudi women. Nutrients. 4;8(2):77. doi:10.3390/nu8020077. PubMed PMID: 26861386; PubMed Central PMCID: PMC4772041.
Jeong H, Hong S, Heo Y, Chun H, Kim D, Park J, Kang MY (2014) Vitamin D status and associated occupational factors in Korean wage workers: data from the 5th Korea national health and nutrition examination survey (KNHANES 2010-2012). Ann Occup Environ Med. 16;26:28. doi: 10.1186/s40557-014-0028-x. eCollection 2014. PubMed PMID: 25852939; PubMed Central PMCID: PMC4387784.
Cashman KDA-Ohoo, Dowling KG, Skrabakova Z, Gonzalez-Gross M, Valtuena J, De Henauw S et al (2016) Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 103(4):1033-44. doi:10.3945/ajcn.115.120873. Epub 2016 Feb 10
Hirani V, Mosdol A, Mishra G (2009) Predictors of 25-hydroxyvitamin D status among adults in two British national surveys. Br J Nutr 101(5):760–764. doi:10.1017/S0007114508023416
El Maghraoui A, Ouzzif Z, Mounach A, Rezqi A, Achemlal L, Bezza A, Tellal S, Dehhaoui M, Ghozlani I (2012) Hypovitaminosis D and prevalent asymptomatic vertebral fractures in Moroccan postmenopausal women. BMC Womens Health 12:11. doi:10.1186/1472-6874-12-11
Allali F, El Aichaoui S, Khazani H, Benyahia B, Saoud B, El Kabbaj S, Bahiri R, Abouqal R, Hajjaj-Hassouni N (2009) High prevalence of hypovitaminosis D in Morocco: relationship to lifestyle, physical performance, bone markers, and bone mineral density. Semin Arthritis Rheum 38(6):444–451. doi:10.1016/j.semarthrit.2008.01.009
Ardawi MS, Sibiany AM, Bakhsh TM, Qari MH, Maimani AA (2012) High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 23 (2):675-686. doi:10.1007/s00198-011-1606-1
Ovesen L, Andersen R, Jakobsen J (2003) Geographical differences in vitamin D status, with particular reference to European countries. Proc Nutr Soc 62(4):813–821. doi:10.1079/PNS2003297
Holick MF (2008) Vitamin D: a D-Lightful health perspective. Nutr Rev 66(10 Suppl 2):S182–S194. doi:10.1111/j.1753-4887.2008.00104.x
Lips P (2010) Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol 121(1-2):297–300. doi:10.1016/j.jsbmb.2010.02.021
ashman KD, Muldowney S, McNulty B, Nugent A, FitzGerald AP, Kiely M, Walton J, Gibney MJ, Flynn A. (2013) Vitamin D status of Irish adults: findings from the National Adult Nutrition Survey. Br J Nutr. 14;109(7):1248-56. doi:10.1017/S0007114512003212. Epub 2012 Aug 10.
Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D,Bhan I, Karumanchi SA, Powe NR, Thadhani R. (2013) Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 21;369(21):1991-2000. doi:10.1056/NEJMoa1306357
Saliba W, Barnett O Fau - Rennert HS, Rennert Hs Fau - Lavi I, Lavi I Fau - Rennert G, Rennert G (2011) The relationship between serum 25(OH)D and parathyroid hormone levels. Am J Med. 124(12):1165-70. doi:10.1016/j.amjmed.2011.07.009
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72(3):690–693
Pourshahidi LK (2015) Vitamin D and obesity: current perspectives and future directions. Proc Nutr Soc 74(2):115–124. doi:10.1017/S0029665114001578, Epub 2014 Oct 31. Review
Ding C, Gao D, Wilding J, Trayhurn P, Bing C.(2012) Vitamin D signalling in adipose tissue. Br J Nutr. 108(11):1915-23. doi:10.1017/S0007114512003285.Epub 2012 Oct 9. Review
Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P (2005) Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab 90(7):4119–4123, Epub 2005 Apr 26
Authors’ contributions
Aissam El maataoui conceived the study, carried out the immunoassays and participated in its design and coordination, performed the statistical analysis, and drafted the manuscript.
Abdellah El maghraoui participated in the design of the study and performed the bone mineral density measures.
Asmae Biaz, Samira El Machtani, Sanae Bouhsain and Abdellah Dami participated in the design of the study and the data collection.
Zhor Ouzzif participated in the design and coordination of the study and supervised the biochemistry analysis and helped draft the manuscript All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The procedures of the study were in accordance with the Declaration of Helsinki, and formal ethics committee approval was obtained for the study (Military Hospital Ethics Committee).
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
El Maataoui, A., Biaz, A., El Machtani, S. et al. Vitamin D status in healthy Moroccan men and women aged 50 years and older: a cross-sectional study. Arch Osteoporos 11, 24 (2016). https://doi.org/10.1007/s11657-016-0279-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-016-0279-y