Abstract
This study evaluated the Cretaceous (Campanian–Maastrichtian) kaolinitic sediments of the Ajali/Mamu and Enugu/Nkporo Formations from the Lower Benue Trough of Nigeria. A combined method of inductively coupled plasma–mass spectrometry and isotope ratio mass spectrometry was used to investigate trace and rare-earth element geochemistry and hydrogen and oxygen isotopic compositions. These data were then used to infer the sediments’ provenance and paleoclimatic conditions during their deposition. The sediments contained low concentrations of most trace elements, with the exceptions of Zr (651–1352 ppm), Ba (56–157 ppm), V (38–90 ppm), and Sr (15.1–59.6 ppm). Average values of Co and Ni were 1.5 and 0.7 ppm, respectively. Trace and rare earth element values were lower than corresponding values for upper continental crust and Post-Archean Australian Shale, with the exception of Zr. The samples showed only slight light rare-earth enrichment and nearly flat heavy rare-earth depletion patterns, with negative Eu and Tm anomalies, typical of felsic sources. Geochemical parameters such as La/Sc, Th/Sc, and Th/Co ratios support that the kaolinitic sediments were derived from a felsic rock source, likely deposited in an oxic environment. 18O values ranged from + 15.4 to + 21.2‰ for the investigated samples, consistent with a residual material derived from chemical weathering of felsic rock and redeposited in a sedimentary basin (typical values of + 19 to + 21.2‰). While in the basin, the sediments experienced extended interactions with meteoric water enriched in δD and δ16O. However, the variation in δD and δ16O values for the investigated samples is attributed to the high temperature of formation (54–91 °C). The δD and δ18O values suggest that the sediments, although obtained from different localities within the Lower Benue Trough, formed under similar hot, tropical climatic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The chemical composition of weathered rocks depends largely on the parent materials, the rate of weathering, and well-established concepts on element mobility during weathering (Singh et al. 2005). The relative distribution of immobile elements—such as La and Th (felsic rocks); and Sc, Cr, and Co (basic rocks)—has been used to infer relative contributions, from sources within or outside the depocenter, of kaolinitic sediments from different environments (Wronkiewicz and Condie 1990). Larger light rare earth element (LREE)/heavy rare earth element (HREE) ratios and negative Eu anomalies are generally found in deposits originating from felsic rocks, whereas mafic rocks produce deposits that have lower LREE/HREE ratios and small Eu anomalies (Cullers 1995).
The hydrogen and oxygen isotopic compositions of kaolinite are determined primarily by temperature and by the isotopic composition of the water present during crystallization (Savin and Epstein 1970; Lawrence and Taylor 1971, 1972). Because δD and O values of meteoric water vary systematically with latitude and altitude (Craig 1961; Dansgaard 1964; Yurtsever and Gat 1981), the stable isotope composition of kaolinite formed during weathering is normally considered to reflect the location, and hence the mean temperature, of the landscape surface (Lawrence and Taylor 1971, 1972). For example, lower kaolinite δD and O values are characteristic of cooler regions, typically located at higher latitudes and/or altitudes. For oxygen, post-depositional isotopic exchange between kaolinite and water is virtually non-existent (O’Neil and Kharaka 1976). Thus, this climatic signature is typically preserved. Measured hydrogen (H2/H1) and oxygen (18O/16O) isotope ratios for kaolinites in weathering profiles can be used to estimate the isotope fractionation factor (Savin and Epstein 1970a). Indeed, a “supergene/hypogene” line has been established to distinguish between clay minerals formed under normal Earth surface conditions and those formed under higher burial temperatures (Sheppard et al. 1969). Thus, hydrogen (2H/1H) and oxygen (18O/16O) isotope studies can be a powerful tool in understanding the genesis, temperature of formation, and paleoclimatic conditions under which a clay mineral formed (Sheppard and Gilg 1996; Savin and Hsieh 1998).
While several researchers (e.g., Reyment 1965; Akande et al. 1992; Zaborski 1998; Idakwo, et al. 2013; and Odoma et al. 2015) have studied Lower Benue Trough stratigraphy, mineralization, and petroleum potential, there is a paucity of published work on trace elements, REEs, and O and H isotopic characteristics of the Cretaceous kaolinitic sediments from the area. The aim of this study was to determine the source of the sediments and the paleoclimatic conditions under which the sediments were deposited using REEs and trace elements, as well as O and H isotopic compositions. This study used a combination of inductively coupled plasma–mass spectrometry and isotope ratio mass spectrometry method to evaluate kaolinitic sediments from the Lower Benue Trough to provide insight into the temperature of formation and the preserved climatic signature.
2 Geologic setting
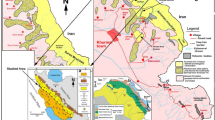
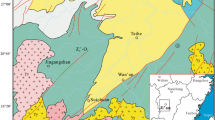
The study area is near the Gulf of Guinea, south of the Chad Basin (6°00′00″N to 8°20′00″N and 6°50′00″E to 8°00′00″E) (Figs. 1 and 2). We evaluated portions of the Ajali/Mamu and Enugu/Nkporo Formations, which are exposed at Aloji, Udene-Biomi, Agbenema, Ofejiji, Otukpa, Okpokwu, and Enugu, in the Lower Benue Trough (Fig. 2).
Map of Nigeria showing the position of the Lower Benue Trough (Modified after Obaje 2009)
Sedimentation in the Lower Benue Trough began with the Albian Asu River Group of marine origin, it constitutes the shales, limestones, and sandstone lenses within the Abakaliki Formation of Abakaliki area and the Limestones from Mfamosing in the Calabar Flank (Petters 1982; Figs. 2 and 3). This group preceded the deposition of the Ezeaku Formation (Ezeaku Shales of Turonian age) and Makurdi Formations. These shales of Ezeaku are thick, flaggy, calcareous and non-calcareous with Limestones (sandy or shelly) and calcareous sandstones. Exposures of these shales are observed in the southern part of Otukpa with varying thickness from ~ 305 m in the northern part to ~ 610 m southward. The Ezeaku Formation is overlain by the marine Cenomanian–Turonian Nkalagu Formation, composed of black shales, limestones, and siltstones. Deformation during the Mid-Santonian within the Benue Trough resulted in the displacement of major depositional axis towards the west, thus, leading to the formation of the Anambra Basin. Anambra Basin represents the post-deformational sedimentation in the Lower Benue Trough. The process of sedimentation in the Anambra Basin started with the marine and paralic shales of the Enugu and Nkporo Formations during the Campanian–Maastrichtian, this marks a depositional cycle represented by the brackish marsh and fossiliferous pro-delta facies of the Late Campanian–Early Maastrichtian (Fig. 2). The Ajali and Owelli Formations’ fluvio-deltaic sandstones lie conformably on the Mamu Formation and its lateral equivalents are observed in most places. The Nkporo cycle represents an overall regression for an epoch during which the coal-bearing Mamu Formation and the Ajali Sandstone accumulated while the onset of another transgression was marked during Paleocene with the Nsukka Formation and the Imo. Finally, within the Lower Benue Trough, there was returned regressive conditions marked by the Eocene Nanka sands, thus, suggesting more predominance flood-tidal currents than weak ebb-reverse currents (Zaborski 1998; Obaje 2009).
Idealized N–S stratigraphic cross-section across the Benue Trough and the relationship to the Niger Delta and the Chad Basin (vertical scale exaggerated; erosion and uplift not considered). (After Obaje 2009)
The kaolinitic clay, a unit in the Mama and Ajali Formations, has an exposed, weathered surface at Aloji, along the Ayingba-Itobe road. The mine pit reveals massive dirty white clay about 9.90 m thick, brown and gray clays about 0.75 m thick, brown clay about 1.55 m thick, and conglomeritic sandstone about 0.50 m thick (Fig. 4a) The exposed kaolinitic clay within the Enugu/Nkporo Formations at Enugu is massive, and has a thickness of about 61 m, including a 20-m thick dirty white layer at the bottom, a grayish layer of 1 m, a mixed brown and dirty white layer of 38 m, and a lateritic capping of 2 m (Fig. 4b).
The kaolinitic Cretaceous (Campania-Maastrichtian) sediments of the Ajali/Mamu and Enugu/Nkporo Formations from the Lower Benue Trough of Nigeria were selected for study because of the abundance of sediment within these formations and the comparative lack of published data on its hydrogen (2H/1H) and oxygen (18O/16O) isotopic compositions and trace element and REE compositions.
3 Methods
Thirty-four sediment samples were collected from different vertical sections of the exposures in Aloji, Udene-Biomi, Agbenema, Ofejiji, Otukpa, Okpokwu, and Enugu in the Lower Benue Trough (Fig. 2). Efforts were made to avoid weathered horizons (Fig. 4). Samples for chemical analyses were selected based on variety and location within the Lower Benue Trough for a representative distribution. The samples were pulverized and packaged for trace element and REE analyses at the ACME Analytical Laboratories Ltd, Canada, using an inductively coupled plasma–mass spectrometer (Perkin-Elmer Elan 6000). Trace element and REE analyses used powdered and pressed pellets prepared from 3 to 5 g samples that were digested by weighing 0.2 g aliquots in a graphite crucible mixed with 1.5 g lithium metaborate/tetraborate (LiBO2/LiB4O7) flux. The crucibles were placed in an oven and heated at 980 °C for 30 min. The cooled bead was dissolved in 5% HNO3 (ACS grade nitric acid diluted in demineralized water). Calibration standards and reagent blanks were added to sample sequences. A selection of 34 elements (Ba, Co, Cs, Ga, Hf, Nb, Rb, Sn, Sr, Ta, Th, U, V, Y, Zr, La, Ce Pr, Nd, Sm, Eu, Gd, Lu, etc.) was determined for the clay samples. Hydrogen and oxygen isotopic analyses were carried out at the Department of Geological Sciences, Indiana University, Bloomington, Indiana, U.S.A. Extraction of H for isotopic analysis followed the principle of Bigeleisen et al. (1952). Samples (from 0.741 to 2.767 mg) were degassed at 50 °C in a vacuum to remove adsorbed moisture; H2O was extracted from the clays by heating the samples in a Mo crucible with an induction furnace to 1404 °C, and H2O was converted to H2 gas by reduction over hot U. Yield ranges of 100 ± 2% of the theoretical amounts were determined monometrically. Hydrogen isotopic composition was measured on a Delta Plus XP isotope ratio mass spectrometer (IRMS) with a TCEA inlet spectrometer calibrated using Vienna Standard Mean Ocean Water (VSMOW).
Oxygen for isotopic analysis was liberated quantitatively from dried and thoroughly out-gassed samples (2.9–4.2 mg) that were dried at 200 °C for 12 h before reaction with bromine pentafluoride at 550 °C, following Clayton and Mayeda (1963). Oxygen was subsequently converted to CO2 by reaction with hot graphite at a temperature of about 550–600 °C. The δ18O value of this gas was measured using a Thermo Scientific MAT 253 dual inlet mass-spectrometer calibrated using VSMOW. The precision of the isotope measurements was estimated at ± 0.2‰ for O and ± 2‰ for H.
4 Results
4.1 Trace elements
The sediment samples were depleted in trace elements with the exception of Zr. The Zr range (651–1352 ppm) of the samples was higher than the Zr content of Post-Archean Australian Shale (PAAS) (210.00 ppm), upper continental crust (UCC) (190.00 ppm), and North American Shale Composite (NASC) (210.00 ppm). In contrast, Ba (56–157 ppm) was lower than PAAS (650.00 ppm), UCC (550.00 ppm), and NASC (636.00 ppm); V (38–90 ppm) was lower than PAAS (150 ppm), UCC (60 ppm), and NASC (130 ppm); and Sr (15.1–59.6 ppm) (Table 1) was lower than PAAS (200.00 ppm), UCC (143.00 ppm), and NASC (350.00 ppm). Average values of Co and Ni were 1.5 and 0.7 ppm, respectively. These concentrations are much lower than the corresponding values of 10 and 20 ppm, respectively, for Co and Ni contents of UCC. The average value of 53.1 ppm obtained for V is similar to an average of 60 ppm obtained by Taylor and McLennan (1985) for UCC. This indicates that although vanadium is considered to be associated with organic matter, it is hosted in the samples by detrital silicate minerals (Stow and Atkin 1987). All values for PAAS and UCC in this paragraph from Taylor and McLennan (1985); for NASC, from Gromet et al. (1984).
Other trace elements were generally lower than average crustal values. Zircon was significantly enriched in all samples, with values up to 1352 ppm. The Sr and Ba contents showed a general depletion in comparison with UCC values (Table 1, Fig. 5), averaging 27 and 89.41 ppm, respectively. Strontium and barium mostly reside in plagioclase and K-feldspar, respectively (Puchelt 1972). A depletion of Ba could be due to recrystallization of clays and progressive destruction of feldspars, probably due to preferential loss during weathering and erosion associated with hydration energy (Cullers et al. 1988; Liu et al. 2013).
The average Th value of 14.5 ppm of the samples was slightly higher than the corresponding UCC value of 10.7 ppm (Fig. 5).
The correlation coefficients of some of the trace elements relevant to the determination of their associations are presented in Table 2. The results show positive correlation between Ba and Co, Ba and Ni, and Ba and Sr. Positive correlations also exist between Ni and Sr, Ni and Co, and Th and La, suggesting that all these trace elements are associated with clay minerals. The prominent negative correlation between Zr and Co, as well as that of Zr and Ni, combined with higher Zr concentration, probably reflects the concentration of heavy minerals during recycling and sorting (Table 2).
The La/Sc (5.1–8.4), La/Th (2.1–3.9), Th/Sc (0.6–2.8), and Th/Co (10.4–20.6) values suggest a mixed source for the clay samples (Table 3) and when compared to ratios derived from felsic rocks, mafic rocks, UCC and PAAS, indicate that such ratios are within the range of felsic source rocks (Table 4). Th/U (3.2–6.4), Cu/Zn (0.3–12.6), and Cu + Mo/Zn (0.4–12.7) suggest an oxidizing depositional condition of the sediments within the Lower Benue Trough (Table 4). However, two samples (Al. 1.1 and Al. 1.2) returned high ratios, probably due to the high concentration of copper in these two samples (Table 4) as a result of copper’s association with organic matter. The UCC-normalized trace elements (Fig. 5) show elevated Hf and Zr, but low Sr and Rb values, consistent with felsic crustal rocks.
4.2 Rare earth elements
The REE compositions of the kaolinitic sediments presented in Table 5 show that Ce was > 100 ppm in many of the samples. La, Nd, and Y were > 25 ppm, whereas Pr, Sc, Sm, Dy, and Gd values were between 4.0 and 10 ppm. Other REEs, notably Eu, Tb, Ho, Tm, and Lu were < 2.0 ppm. These REE values are generally lower than the values obtained by Okunlola and Idowu (2012) in the adjacent Bida Basin at the northwestern side of the Lower Benue Trough (Fig. 1). The investigated clay samples had fractionated REE patterns with (La/Yb)cn varying from 5.79 to 14.50 and (Gd/Yb)cn varying from 0.14 to 2.05 (Table 5). These characteristics indicate that the original source area was felsic and the negative Eu anomaly is regarded as evidence for a differentiated source, similar to granite (Taylor and McLennan 1985, 1985; McLennan 1989; McLennan et al. 1993) (Table 5). The REE data are comparable to PAAS values (Table 6). The Eu concentrations of the sediments were very low (Table 5), and the chondrite-normalized REE plot (Fig. 6) shows that the sediments were LREE-enriched, with a near-flat HREE pattern with very low to negative Eu and Tm anomalies. The two samples that showed high Ho composition suggest contamination, possibly from a mafic source. Overall, the REE compositions were consistent with a felsic source rock.
4.3 Hydrogen and oxygen isotopes
Hydrogen and oxygen isotopic compositions of the sediments are reported in the conventional delta (δ) in parts per thousand (‰) relative to VSMOW (Gofiantini 1984). The δD values of the clay fell between − 50.80 and − 64.40‰, and the δO18 values between 15.40 and 21.20‰ (Table 7). The oxygen and hydrogen fractionation factors between kaolinite and water were calculated using Eqs. 1 and 2 (Savin and Epstein 1970), and we used the δ18O = − 5‰ and δD = − 30‰ values of Friedman (1964) for local ground/meteoric water in equilibrium with kaolinite as an alternative way to calculate the fractionation factors for both oxygen and hydrogen isotopic compositions of the investigated clay deposits. The values obtained here are in good agreement with those of Savin and Epstein (1970), suggesting that the kaolinite in these samples was in oxygen/hydrogen isotopic equilibrium with local ground water.
Based on revisions of existing empirical data, Sheppard and Gilg (1996) proposed the following oxygen and hydrogen isotope fractionation equations between clay mineral (kaolinite) and water:
where 18α and Dα are the oxygen and hydrogen isotopic fractionation factors between kaolinite and water, respectively, and T is in Kelvin. The equation of oxygen isotope fractionation was employed to calculate the temperature of formation for these kaolinitic deposits (Table 7). The results show that temperatures of 54–91 °C were attained during the weathering and deposition of the kaolinitic sediments within the superficial level of the Earth. The δD and δ18O values are plotted in Fig. 7, with some scatter between the kaolinitic line of Savin and Epstein (1970), as modified by Sheppard and Gilg (1996), and the supergene/hypogene line (S/H) of Sheppard et al. (1969).
The δD and δ18O plot of the kaolinitic sediments of the Lower Benue Trough on the Savin and Epstein (1970) diagram as modified by Sheppard and Gilg (1996). The concentration of the data around the kaolinitic and the supergene/hypogene (S/H) lines of Sheppard et al. (1969) indicates formation or equilibration of kaolinite with meteoric waters
5 Discussion
The geochemical signatures of clastic sediments have often been used to ascertain provenance and tectonic setting (Taylor and Mclennan 1985; Cullers et al. 1987; Cullers 1995; Armstrong-Altrin et al. 2004). In particular, trace element and REE contents are particularly powerful indicators of origin and process. For example, Th and La, which are consistent with a more felsic source, and Sc and Cr, consistent with a mafic source, have been exploited to distinguish between felsic and mafic provenance by many authors (McLennan et al. 1980; McLennan 1989; Wronkiewicz and Condie 1990; McLennan and Taylor 1991). In addition, La/Sc, Th/Sc, and Th/Co ratios generally return significantly and radically different values in felsic and basic rocks (Wronkiewicz and Condie 1990; Cox et al. 1995; Cullers 1995). Some of the Th/Sc values for the Benue Trough sediments were greater than the UCC average value of 0.79, suggesting that the sediments may not have originated from the same source but rather from mixed sources (Tables 3 and 4).
Depletion of transition metals in the Benue Trough samples may be attributed to the abundance of felsic components in the source (Liu et al. 2013). The Th/Co, Th/Sc, and La/Sc ratios for clay samples from this study were compared with those of felsic and basic rock-derived sediments, UCC and PAAS values, and the Bida claystone (Tables 3 and 4), revealing that the chemistry of the Benue Trough rocks is consistent with a felsic source. In addition to these ratios, the higher LREE/HREE ratios and low Eu anomalies (Table 5) corroborate the felsic source-rock characteristics of the kaolinitic sediments. Chondrite-normalized REE distributions (Fig. 6) show that the deposits are LREE-enriched, with an almost flat HREE pattern, and very low to negative Eu and Tm anomalies.
Hallberg (1976) used Cu/Zn and (Cu + Mo)/Zn ratios as redox determinants. He stated that high Cu/Zn and (Cu + Mo)/Zn ratios indicate reducing depositional conditions, while low Cu/Zn and (Cu + Mo)/Zn ratios suggest oxidizing conditions. In this study, almost all Cu/Zn ratios for the sediments are within the range of 0.2–3.6, except for Al. 1.1 (12.6) and Al. 1.2 (6.8) (Table 3), consistent with oxidizing depositional conditions. The U/Th ratio may also be used as a redox indicator, with a higher ratio found in organic-rich mudstones (Jones and Manning 1994). A U/Th ratio < 1.25 suggests oxic depositional conditions, whereas values > 1.25 suggest suboxic to anoxic conditions (Nath et al. 1997). The samples analyzed in this study have consistently very low U/Th ratios of 0.2–0.3, suggesting deposition of the sediments in an oxic environment.
Jones and Manning (1994) also suggested that Ni/Co ratios < 5 indicate oxic environments, whereas ratios > 5 indicate suboxic and anoxic environments. All the analyzed samples in this study had Ni/Co ratios of < 1 (Table 3), further confirming that the Lower Benue Trough kaolinitic sediments were deposited under oxic chemical conditions.
The clay mineral in these investigated deposits, kaolinite, can form in a wide range of environments near the Earth’s surface during weathering, diagenesis, or hydrothermal processes (Murray and Janssen 1984; Savin and Lee 1988). In order to know the possible conditions of kaolinite formation, it is important to use other tools to refine information on genesis and potential paleoclimate. According to Savin and Lee (1988), the isotopic composition of clay minerals (e.g., kaolinite) may reflect geologic conditions during their formation, provided that the minerals did not undergo changes in isotopic composition after deposition. For example, the O isotope composition of kaolinites of sedimentary origin usually varies from +19 to +23‰, and kaolinite from residual deposits has 18O values between +15 and +19‰ (Murray and Janssen 1984). In the present study, the 18O of kaolinite in the Benue Trough measured +15.4 to +21.2‰. This range is consistent with a residual material derived from chemical weathering of felsic rock, which was subsequently transported by water over a very short distance (colluvial deposit) (+15.4 to +19‰), or a relatively long distance (alluvial deposit) and deposited in a sedimentary basin (+19 to +21.2‰), where the sediments experienced longer interaction with meteoric water enriched in δD and δ18O, resulting in varied δD and δ16O values due to the generally high temperature of formation (54–91 °C) (Table 7). The values recorded in Table 7 are characteristic of kaolinite formed under hot and humid climatic conditions (Hassanipak and Eslinger 1985; Mizota and Longstaffe 1996). From the above-mentioned data, the paleoclimate was probably much hotter. Kaolinites formed during weathering at high latitudes or altitudes, or at a large distance from the coast, where meteoric water is more depleted in δD and δ18O, generally have relatively low temperature of formation (Hassanipak and Eslinger 1985; Mizota and Longstaffe 1996). The δD and δ18O values of the kaolinitic sediments of the Benue Trough measured in the current study suggest that the Cretaceous clay deposits formed under climatic conditions much hotter than the present day, yielding the calculated temperature shown in Table 7. The current isotopic data were compared with published data (Table 8), which clearly illustrate the contrast between high δ18O clay minerals formed at equatorial latitudes, and the higher latitudes around Australia with lower δD and δ18O values.
A few samples in the δD and δ18O diagram (Fig. 7) fell between the kaolinitic line of Savin and Epstein (1970) as modified by Sheppard and Gilg (1996) and the supergene/hypogene line (S/H) of Sheppard et al. (1969), suggesting formation or equilibration of kaolinite with meteoric waters at surficial temperatures. The distribution of the data does not support a hydrothermal origin (S/H line of Sheppard et al. 1969) because hydrothermal deposits are characterized by much lower δ18O values (Sheppard et al. 1969; Gazis et al. 1987). The slight deviations of some samples from the kaolinitic line suggest post-depositional modification of the isotopic composition of these clay deposits, perhaps due to the interaction between earlier-formed kaolinite and downward percolating meteoric water (Longstaffe and Ayalon 1990). The similarity in the δD and δ18O values of the Cretaceous deposits suggests that though the clay samples were taken from different localities within the Benue Trough, they formed under almost the same climatic conditions (Table 7). The small variation in δD values observed in the investigated clay deposits (Fig. 7) suggests that the δD values either do not reflect differences in pore-fluid composition, or that they were subsequently affected by processes that minimized any initial differences between the colluvial and alluvial deposits. The variation in values could be related to considerable amounts of quartz, which might cause a significant effect of non-clay minerals on the isotopic composition of the clay deposits. However, the isotopic composition of the water in contact with the clay mineral during formation, the clay-water fractionation factors, the extent to which clay-water isotopic equilibrium was established, and the temperature at which fractionation occurred cannot be ruled out as contributing factors (Savin and Epstein 1970). The δD values for some samples (Fig. 7) suggest that the clay mineral is in hydrogen isotopic equilibrium with the present formation water, as supported by the δ18O values. Such a re-equilibration of hydrogen isotopes appears to have a significant effect at temperatures < 100 °C (Table 7). At higher temperatures (~ 200 °C), H+-exchange, rather than OH− replacement, would be the controlling mechanism (Longstaffe and Ayalon 1990).
6 Conclusions
Geochemical data, such as La/Sc, Th/Sc, and Th/Co ratios, show that the Cretaceous kaolinitic sediments (Ajali/Mamu and Enugu/Nkporo Formations) were derived from felsic source rocks rather than mafic source rocks. This conclusion is also broadly supported by the REE data. The hydrogen and oxygen isotopic compositions of the investigated clay deposits are very similar, suggesting their formation under similar hot and humid climatic conditions. The geochemical data suggest the deposition of the kaolinitic sediments under hot and humid climatic conditions closer to the equator than the present location. The minor scatter of the stable isotope data between the kaolinitic and supergene/hypogene lines suggests equilibration of kaolinite with meteoric waters at surficial temperatures rather than hydrothermal origin.
The data from trace elements, REEs, and O2 and H2 isotopes on the kaolinitic-rich sediment were very useful as they provide information on the origin, source, and depositional process, including the altitude of deposition and ancient climatic conditions.
References
Akande SO, Hoffknecht A, Erdtmann BD (1992) Environment of ore formation and anchizonal metamorphism in Pb-Zn-Ba-F deposits of the Benue Trough, Nigeria. Geolgie en Miijnbouw 71:131–144
Armstrong-Altrin JS, Lee YI, Verma SP, Ramasamy S (2004) Geochemistry of sandstones from the Upper Miocene Kudankulam Formation, southern India: implications for provenance, weathering, and tectonic setting. J Sediment Res 74:285–297
Baioumy H (2013) Hydrogen and oxygen isotopic compositions of sedimentary kaolin deposits, Egypt: Paleoclimatic implications. Applied Geochemistry, pp 182–188
Bigeleisen J, Perlman ML, Prosser HC (1952) Conversion of hydrogenic materials to hydrogen for isotopic analysis. Anal Chem 24:1356–1357
Bird MI, Chivas AR (1988) Stable-isotope evidence for low-temperature kaolinitic weathering and post-formational hydrogen-isotope exchange in Permian kaolinites. Chem Geol Isot Geosci Sect 72:249–265
Boynton WV (1984) Geochemistry of the rare earth elements: meteorite studies. In: Henderson P (ed) Rare earth element geochemistry. Elsevier, pp. 63–114
Clayton RN, Mayeda TK (1963) The use of bromine pentafluoride in the extraction of oxygen from oxides and silicates for isotopic analysis. Geochim Cosmochim Acta 27:43–52
Cox R, Lowe DR, Cullers RL (1995) The influence of sediment recycling and basement composition on evolution of mudrock chemistry in the south-western United States. Geochim Cosmochim Acta 59:2919–2940
Craig H (1961) Standard for reporting concentrations of deuterium and oxygen-18 in natural waters. Science 133:1833–1834
Cullers RL (1995) The controls on the major and trace element evolution of shales, siltstones and sandstones of Ordovician to Tertiary age in the Wet Mountain region, Colorado, USA. Chem Geol 123:107–131
Cullers RL, Barrett T, Carlson R, Robinson B (1987) Rare mineralogic changes in Holocene soil and stream sediment: a case study in the wet mountains region, Colorado, USA. Chem Geol 63:275–297
Cullers RL, Basu A, Suttner L (1988) Geochemical signature of provenance in sand-size material in soils and stream sediments near the Tobacco Root batholith, Montana, USA. Chem Geol 70:335–348
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436–468
Friedman I (1964) The variation of the deuterium content of natural waters in the hydrological cycle. Rev Geophys 2:177–224
Gazis C, Taylor HP Jr, Hon K, Tsvetkov A (1987) Late Cretaceous lateritic-derived sedimentary deposits (oolitic ironstone, kaolin deposits, bauxite) in Upper Egypt. Berl Geowiss Abh 75:727–758
Gilg HA (2000) D/H evidence for the timing of kaolinization in Northeast Bavaria, Germany. Chem Geol 170:5–18
Gofiantini R (1984) Report on the advisory group meeting on stable isotope reference samples for geochemical and hydrological investigations. Rep. Dir. Gen. IAEA, Vienna, 77
Gromet LP, Dymek RF, Haskin LA, Korotev RL (1984) The ‘North American Shale Composite’: its compilation, major and trace element characteristics. Geochim et Cosmochim Acta 48:2469–2482
Hallberg RO (1976) A geochemical method for investigation of palaeo redox conditions in sediments. Ambio Special Rep 4:139–147
Hassanipak AA, Eslinger EV (1985) Mineralogy, crystallinity, 180/160 and D/H of Georgia kaolins. Clays Clay Miner 33:99–106
Idakwo SO, Barnabas GY, Alege ST, Alege KE (2013) Paleoclimate reconstruction during Mamu Formations (Cretaceous) based on clay mineral distribution in Northern Anambra Basin, Nigeria. Int J Sci Tech 2:879–885
Jones B, Manning DC (1994) Comparison of geochemical indices used for the interpretation of paleo-redox conditions in ancient mudstones. Chem Geol 111:111–129
Lawrence JR, Taylor HP Jr (1971) Deuterium and oxygen-18 correlation. Claystone Geochim Cosmochim Acta 36:1377–1393
Lawrence JR, Taylor HP Jr (1972) Hydrogen and oxygen isotope systematics in weathering profiles. Geochim Cosmochim Acta 36:1371–1393
Levinson AA (1974) Introduction to exploration geochemistry. Applied Publishing, Illinos, p 612
Liu B, Wang Y, Su X, Zheng H (2013) Elemental geochemistry of northern slope sediments from the South China Sea: implications for provenance and source area weathering since Early Miocene. Chem Erde 73:61–74
Longstaffe FJ, Ayalon A (1990) Hydrogen-isotope geochemistry of diagenetic clay minerals from Cretaceous sandstones, Alberta, Canada: evidence for exchange. Appl Geochem 5:657–668
McLennan SM (1989) Rare earth elements in sedimentary rocks: influence of provenance and sedimentary processes. Geochem Mineral Rare-Earth Elem Rev Mineral 21:169–200
McLennan SM, Taylor SR (1991) Sedimentary rocks and crustal evolution: tectonic setting and secular trends. J Geol 99:1–21
McLennan SM, Nance WB, Taylor SR (1980) Rare earth element-thorium correlations in sedimentary rocks, and the composition of the continental crust. Geochim Cosmochim Acta 44:1833–1839
McLennan SM, Hemming S, McDaniel DK, Hanson GN (1993) Geochemical approaches to sedimentation, provenance, and tectonics. In: Johnson MJ, Basu A (eds) Processes controlling the composition of clastic sediments, Special paper, Geological Society of America, vol 284, 21–40
Mizota C, Longstaffe FJ (1996) Origin of Cretaceous and Oligocene kaolinites from the Iwaizumi Clay deposit, Iwate, Northeastern Japan. Clay Clays Miner 44:408–416
Murray HH, Janssen J (1984) Oxygen isotopes-indicators of kaolinite genesis. In: Proceedings of 27th international geological congress, non metallic ores, vol 15, pp 287–303
Nath BN, Bau M, Ramalingeswara Rao B, Rao CM (1997) Trace and rare earth elemental variation in Arabian Sea sediments through a transect across the oxygen minimum zone. Geochim Cosmochim Acta 61:2375–2388
Obaje NG (2009) Geology and mineral resources of Nigeria. Springer, Berlin
Odoma AN, Obaje NG, Omada JI, Idakwo SO, Erbacher J (2015) Mineralogical, chemical composition and distribution of rare earth elements in clay-rich sediments from southeastern Nigeria. J Afr Earth Sci 102:50–60
Okunlola OA, Idowu O (2012) The geochemistry of claystone-shale deposits from the Maastritchian Patti formation, Southern Bida basin, Nigeria. Earth Sci Res J 16:57–67
O’Neil JR, Kharaka YK (1976) Hydrogen and oxygen isotope exchange reactions between clay minerals and water. Geochim Cosmochim Acta 40:241–246
Petters SW (1982) Central West African Cretaceous-Tertiary benthic foraminifera and stratigraphy. Palaeontographical Sonder – Abduck aus Palaeontographica, vol 179, pp 1–104
Puchelt H (1972) Barium. Handbook of geochemistry (Wedepohl KH et al, eds), B1 56O2, Springer, Berlin
Reyment RA (1965) Aspect of the geology of Nigeria. University Ibadan Press, Nigeria
Savin SM, Epstein S (1970) The oxygen and hydrogen isotope geochemistry of claystone minerals. Geochim Cosmochim Acta 34:25–42
Savin SM, Hsieh JCC (1998) The hydrogen and oxygen isotope geochemistry of pedogenic clay minerals: principles and theoretical background. Geoderma 82:227–253
Savin SM, Lee M (1988) Isotopic studies of phyllosilicates. In: Bailey SW (ed) Hydrous phyllosilicates (exclusive of Micas), Reviews in Mineralogy Paper 19, pp 189–223
Sheppard SMF, Gilg HA (1996) Stable isotope geochemistry of clay minerals. Clay Miner 31:1–21
Sheppard SMF, Neilsen RL, Taylor HP Jr (1969) Oxygen and hydrogen isotope ratios of clay minerals from porphyry copper deposits. Econ Geol 64:755–777
Singh M, Sharma M, Tobschall HL (2005) Weathering of the Ganga alluvial plain, northern India: implications from fluvial geochemistry of the Gomati River. Appl Geochem 20:1–21
Stow DAV, Atkin BP (1987) Sediment facies and geochemistry of Upper Jurassic mudrocks in the central North Sea area. In: Brooks J, Glennie K (eds) Petroleum geology of North West Europe, Graham and Trotman, London, 797–808
Taylor SR, McLennan SM (1985) The continental crust: its composition and evolution. Blackwell, Oxford, p 312
Wronkiewicz DJ, Condie KC (1990) Geochemistry and mineralogy of sediments from the Ventersdorp and Transvaal Supergroups, South Africa: cratonic evolution during the Early Proterozoic. Geochim Cosmochim Acta 54:343–354
Yurtsever Y, Gat JR (1981) Atmospheric waters. In: Gat JR, Gonfiantini R (eds) Stable isotope hydrology: Deuterium and oxygen-18 in the water cycle. International Atomic Energy Agency, Vienna, pp 103–142
Zaborski PM (1998) A review of the cretaceous system in Nigeria. Afr Geosci Rev 5:385–483
Zhou T, Dobos SK (1994) Stable isotope geochemistry of kaolinite from the white section, Black Ridge, Clermont, Central Queensland: implications for the age and origin of the white section. Clays Clay Miner 42:269–275
Acknowledgements
The authors appreciate the assistance of Matthew Lilley, Benjamin Underwood, and Peter Sauer of the Department of Geological Sciences, Indiana University, Bloomington, Indiana, USA for their assistance with the oxygen extraction and with oxygen and hydrogen isotope analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bolarinwa, A.T., Idakwo, S.O. & Bish, D.L. Rare-earth and trace elements and hydrogen and oxygen isotopic compositions of Cretaceous kaolinitic sediments from the Lower Benue Trough, Nigeria: provenance and paleoclimatic significance. Acta Geochim 38, 350–363 (2019). https://doi.org/10.1007/s11631-019-00328-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-019-00328-y