Abstract
A bidirectional labeling method was established to distinguish the proportions of HCO3− and CO2 utilization pathways of microalgae in Lake Hongfeng. The method was based on microalgae cultured in a medium by adding equal concentrations of NaH13CO3 with different δ13C values simultaneously. The inorganic carbon sources were quantified according to the stable carbon isotope composition in the treated microalgae. The effects of extracellular carbonic anhydrase (CAex) on the HCO3− and CO2 utilization pathways were distinguished using acetazolamide, a potent membrane-impermeable carbonic anhydrase inhibitor. The results show utilization of the added HCO3− was only 8% of the total carbon sources in karst lake. The proportion of the HCO3− utilization pathway was 52% of total inorganic carbon assimilation. Therefore, in the natural water of the karst area, the microalgae used less bicarbonate that preexisted in the aqueous medium than CO2 derived from the atmosphere. CAex increased the utilization of inorganic carbon from the atmosphere. The microalgae with CAex had greater carbon sequestration capacity in this karst area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
High pH and high concentration of bicarbonate are two typical characteristics of karst lakes. The main component of karst is MgCa(CO3)2, a highly soluble rock. The proportion of CO2 in total dissolved inorganic carbon (DIC) is less than 1% in high pH conditions (Riebesell et al. 1993). Thus, the CO2 in aquatic media that can be directly utilized by photosynthesis in microalgae is limited (Talling 1976). Several microalgae have adapted by forming carbon-concentrating mechanisms (CCMs) to increase CO2 concentrations to meet their photosynthetic demands (Colman et al. 2002; Giordano et al. 2005). Another strategy is to utilize bicarbonate (Colman et al. 2002). Carbonic anhydrase (CA) may play the key role in these carbon assimilation systems.
CA (EC 4.2.1.1), a zinc-containing metalloenzyme, catalyzes the reversible interconversion between HCO3− and CO2. CA is one of the most important enzymes in physiological processes and significantly accelerates the photosynthetic assimilation of inorganic carbon (Ci) (Badger and Price 1994; Sültemeyer 1998). CA is widely distributed and multiple types exist in microalgae. One of the most important CAs is extracellular CA (CAex), which may be involved in CCMs and in HCO3− utilization (Williams and Turpin 1987; Badger and Price 1994; Elzenga et al. 2000; Mondal et al. 2016).
Stable carbon isotope (δ13C) analysis is an important tool to identify various Ci sources (Fry and Sherr 1984; Bade et al. 2006; Chen et al. 2009). Different Ci sources and assimilation mechanisms cause variations in δ13C fractionation. The HCO3− in the uncatalyzed pathway produces approximately 10‰ of δ13C fractionation (Mook et al. 1974), while HCO3− assimilation catalyzed by CAex produces only 1.1‰ of δ13C fractionation (Marlier and O’Leary 1984). An approximately 9‰ discrimination of carbon isotope has been found between the HCO3− catalyzed by CAex and that uncatalyzed (Wu et al. 2012).
Acetazolamide (AZ) is a potent membrane-impermeable CA inhibitor that selectively inhibits CAex activity (Moroney et al. 1985). The addition of AZ enables determination of the effect of CAex on Ci utilization.
Several studies have investigated mechanisms of Ci utilization in microalgal species (Axelsson et al. 1995; Moazami-Goudarzi and Colman 2011; Moulin et al. 2011; Smith-Harding et al. 2017). However, the conventional technique cannot quantify the proportions of DIC sources and their microalgal pathways in karst lakes (Xie and Wu 2017). This is the aim of this study.
To this end, microalgae from Lake Hongfeng were cultured in different concentrations of NaHCO3 and AZ. The proportion of Ci sources and pathways were determined by comparing their δ13C compositions under separate experiments adding two labeled δ13C bicarbonates. We then estimated the contribution of microalgal CAex to Ci sources and utilization pathways in the karst lake.
2 Materials and methods
2.1 Research site
Lake Hongfeng (106°19′ to 106°28′E, 26°26′ to 26°35′N) is in central Guizhou Province in the core of the southwest karst area of China. The concentration of HCO3− in Lake Hongfeng is 1.0–2.5 mmol/L, and the pH is 8.1 ± 0.4 (Wu et al. 2008).
2.2 Microalgae incubation
The microalgal samples were obtained from Lake Hongfeng. All samples were incubated at 25.0 ± 1.0 °C under a 150 μmol m−2 s−1 light intensity with a 12/12 h day/night cycle. The pH was adjusted to 8.10 by adding NaOH. AZ was obtained from Sigma-Aldrich Co. (St. Louis, USA).
The microalgae were grown in the lake water in Erlenmeyer flasks after filtration through a 45-µm glass microfiber filter. Treatments are listed in Table 1. The cultures were treated for 5 days.
2.3 Measurement of the microalgal growth
Microalgal protein was analyzed using the method of Coomassie Brilliant Blue (Sedmak and Grossberg 1977). A volume of 5–10 ml of microalgae was centrifuged, and then resoluted. The optical density was tested using a spectrophotometer (Labtech UV-2000, Boston, USA) at 595 nm (OD595). The protein content is expressed as ug/L based on the aqueous medium.
2.4 Measurement of δ13C in microalgae
The microalgae materials were freeze-dried and then converted to CO2 at 850 °C in a quartz tube with copper oxide to provide oxygen for combustion. The extracted CO2 from the samples was purified as follows.
Water and oxygen were removed from the gas stream using two traps. The first was an alcohol–liquid nitrogen mixture to separate the water vapor, and the second was liquid nitrogen to condense the CO2. After this double distillation, the isolated CO2 was collected into a sample tube. The CO2 sample was analyzed with an isotope ratio mass spectrometer (Finnigan MAT 252, Bremen, Germany). All isotopic compositions (δ13C) are expressed as per mille (‰) and compared with the Pee Dee Belemnite (PDB) standard [see Eq. (1)]. The analytical precision was ± 0.1‰.
where Rsample and Rstandard are the ratios of heavy to light isotope (13C/12C) of the sample and the standard, respectively.
2.5 Distinguishing the different carbon sources and metabolic pathways
This study chose an open system to simulate natural conditions. In this type of system, Ci in the liquid medium and the atmosphere is in dynamic balance. We created a bidirectional labeling method that simultaneously cultured microalgae in NaHCO3 of different δ13C to address this problem.
There is an assumption that the proportion of added Ci utilization is the same under the same concentration of HCO3− at the same time regardless of which labeled HCO3− was added. This is the theoretical basis of the bidirectional labeling method.
In general, algae can utilize DIC from the atmosphere and added HCO3−. Therefore, the δ13C of the algae was fit for the bivariate isotope-mixture model that can be expressed as:
where δTi is the δ13C value of the algae cultured in the same concentration of HCO3− with different δ13C values; fBi is the proportion of the utilization of DIC from the added HCO3− in the total carbon sources used by the microalgae; and δAi and δBi are the δ13C values of the algae after using DIC from atmospheric CO2 or from the added HCO3−, respectively, as their sole carbon sources.
In this experiment, the microalgae could utilize both CO2 and HCO3− as carbon sources. Approximately 9‰ carbon isotope discrimination has been observed between CO2 and HCO3− utilization pathways (Wu et al. 2012). Therefore, δAi and δBi can be expressed as follows:
where fbi is the proportion of the HCO3− pathway; δa is the δ13C value of the algae after using DIC in the sole form of the CO2 utilization pathway from the atmospheric source; and δai is the δ13C value of the algae after using DIC in the sole form of the CO2 utilization pathway of the added HCO3− source.
Based on Eqs. (3) and (4), Eq. (2) can be expressed as:
Equation (5) can be simplified to:
For the two labeled types of NaHCO3 in our experiments, Eq. (6) can be rewritten as:
There are some important facts that we should note in Eqs. (7) and (8). The first is that the proportion of added Ci utilization is the same at the same concentration of HCO3− regardless of which labeled NaHCO3 was added. Therefore, fB1 = fB2 = fB. The second is that the proportion of the HCO3− pathway is the same at the same concentration of HCO3− regardless of the labeled δ13C of NaHCO3. Therefore, fb1 = fb2 = fb. Although δa1 and δa2 cannot be obtained, the difference between them is simply the difference between δ13C values of the first and second labeled NaHCO3 in the medium. Therefore, the (δa1 − δa2) can be replaced with (δC1 − δC2). Therefore, fB can be expressed as follows:
where δC1 and δC2 are the δ13C values of the first and second labeled NaHCO3 in the medium, respectively. From Eq. (9), it can be concluded that the proportion of the utilization of DIC from the added HCO3− in the total carbon sources used by the microalgae (fB) was dependent only on the δ13C values of the algae harvested and the labeled NaHCO3 added, regardless of DIC form and origin.
The (δai − δa) in Eq. (6) can be replaced with (δCi − δC0). A new equation can then be formulated as:
Therefore, Eq. (6) can be rewritten as:
The proportion of the HCO3− pathway (fb) can then be calculated as:
When fbi = 0, Eq. (12) can be rewritten as:
From Eqs. (9), (12), and (13), the proportion of the HCO3− pathway by the microalgae (fb) can be calculated. It was dependent only on the δ13C values of the algae harvested and the labeled NaHCO3 added.
To analyze the complete picture of Ci utilization, the bidirectional labeling method (NaH13CO3 with different δ13C values added) can solve the difficulties of the time course of parameters (concentrations and isotopic data in incubation).
2.6 Statistical analysis
All experiments were conducted in triplicate. Data are expressed as mean ± standard error.
3 Results
3.1 Microalgae biomass
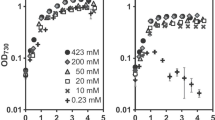
The content of protein in the treated microalgae increased in parallel with the NaHCO3 added (Table 2). However, it decreased with increasing concentrations of AZ added. Under the same concentration of NaHCO3, microalgae growth was severely restricted by AZ. Compared to the control, the average effect of AZ on the microalgal protein content was 69% at 1.0 mmol/L AZ and 35% at 10.0 mmol/L AZ. Among all treatments, the maximal growth rate treatment (20.0 mmol/L NaHCO3 without AZ) was 4.12-fold higher than the minimal treatment (1.0 mmol/L NaHCO3 with 10.0 mmol/L AZ).
3.2 Stable carbon isotope composition of the microalgae
The δ13C of the DIC was − 11.0‰ ± 0.4‰ which is positive relative to the δ13C of the added NaHCO3 (− 17.4‰ or − 28.4‰). In the end, the δ13C value of the microalgae decreased as the amount of NaHCO3 increased; it also decreased as AZ increased (for the same concentration of NaHCO3) (Table 3). The δ13C of the microalgae cultured in the treatment was affected by the added NaHCO3 with different δ13C values. In general, the more negative the NaH13CO3 added, the more negative the δ13C of the microalgae harvested for the same concentration of NaHCO3 added.
3.3 Variation in carbon sources during different concentrations of NaHCO3 and acetazolamide
Based on Eq. (9), we calculated the proportion of the utilization of DIC from the added NaHCO3 (fB). The fB increased with increasing NaHCO3 concentration whether AZ was present or not (Table 4). In the treatment at 20.0 mmol/L NaHCO3, fB increased with increasing concentration of AZ added.
3.4 Variation in carbon pathways under different concentrations of NaHCO3 and acetazolamide
Based on Eq. (12), the proportion of the HCO3− pathway (fb) in microalgae was calculated. It decreased with increasing NaHCO3 concentration in the treatment without AZ (Table 5). The fb also decreased with increasing AZ concentration at the same concentration of added NaHCO3. In the treatment with 10.0 mmol/L AZ, fb values were all very small (fb ≤ 0.12).
4 Discussion
4.1 The effect of bicarbonate and acetazolamide on microalgae growth and carbon isotopes
With increasing HCO3− added to the culture medium, growth of the microalgae increased. It had already been widely confirmed that HCO3− can stimulate algal growth (Wu et al. 2012; White et al. 2013; Xie and Wu 2017). However, the growth of the treated microalgae decreased sharply with the increase in AZ added to the culture medium since AZ is a CA inhibitor that selectively inhibits CAex activity (Moroney et al. 1985). With AZ, the CAex that catalyzes the reversible interconversion between HCO3− and CO2 was inhibited. Thus, microalgae growth was delayed. The result is that microalgae biomass decreased significantly with addition of AZ.
Simultaneously, the stable carbon isotope composition in algae reflects the utilization of DIC (Chen et al. 2009). In this study, the more negative the added NaH13CO3, the more negative the δ13C detected in microalgae for the same concentration of added NaHCO3. Stable carbon isotope composition in the microalgae and HCO3− concentration in the medium were negatively correlated, and especially so when NaH13CO3 (− 28.4‰) was added. This demonstrates that the utilization of DIC can alter the δ13C value in microalgae. Moreover, the stable carbon isotope composition without AZ was significantly different from that with AZ. The δ13C value in microalgae with AZ was more negatively altered than that without AZ. The δ13C in microalgae was the most negative in the presence of 10.0 mmol/L AZ. This suggests that CAex can alter the utilization of DIC, and accelerate the rapid interconversion between HCO3− and CO2, because the slow (uncatalyzed) interconversion of CO2 would bring approximately 10‰ stable carbon isotope fractionation (Mook et al. 1974).
4.2 The effect of bicarbonate and acetazolamide on microalgae carbon utilization
The proportion of the added HCO3− increased with the level of additional HCO3− (Table 4). However, the proportion of the HCO3− pathway decreased in parallel with the increase in additional HCO3− (Table 5), as CAex was also inhibited by high concentrations of added NaHCO3 (Wu et al. 2012). In addition, the proportion of the HCO3− pathway decreased with increasing AZ. These results demonstrate that CAex boosted the proportion of the HCO3− pathway.
The pH of karst lakes in southwest China is generally approximately 8.1. CO2 in the water is limited—usually less than 1% of total DIC (Riebesell et al. 1993). Under these conditions, bicarbonate is the main form of DIC (Fig. 1). However, the rate of direct bicarbonate utilization by anion exchange is slow in microalgae (Fig. 1). Compared with the direct utilization of CO2 and bicarbonate by microalgae without CAex, the major pathway converting CO2 from bicarbonate is rapidly catalyzed by CAex (Fig. 1). CAex accelerated photosynthetic Ci assimilation, promoted the conversion of bicarbonate to CO2 for algal physiological needs, and constantly assimilated CO2 from the atmosphere into the water. Ultimately, we found that the algae used atmospheric CO2 as the main DIC source via the bicarbonate pathway under the catalysis of CAex (Fig. 1).
In the natural water of karst areas, microalgae have adjusted their Ci metabolism strategy to adapt to the environment. This study found that microalgal utilization of the bicarbonate that preexisted in the water at the karst area was very small whether AZ was added or not. In Lake Hongfeng, the dominant family is Chlorophyceae, which has high CA activity (Wu et al. 2008). When AZ was added in the medium, CAex activity and growth of the dominant microalgae species were inhibited. As 2.5 mmol/L NaHCO3 was added—which is similar to natural conditions in karst areas—both growth and carbon sequestration capacity of the microalgae were largely suppressed by 10.0 mmol/L AZ (37% compared to that without AZ). This shows that microalgae with CAex have greater carbon sequestration capacity in karst lakes.
5 Conclusion
The bidirectional labeling method presented in this study is an effective way to quantify the proportions of Ci sources and their utilization pathways in microalgae. It can help delineate the mechanism of Ci utilization in microalgae under different conditions. In the natural water of karst areas, microalgae used less bicarbonate preexisting in the aqueous medium than CO2 derived from the atmosphere. CAex generally increased the utilization of Ci from the atmosphere. The microalgae with CAex had greater carbon sequestration capacity in the lake.
References
Axelsson L, Ryberg H, Beer S (1995) Two modes of bicarbonate utilization in the marine green macroalga ulva lactuca. Plant Cell Environ 18:439–445
Bade DL, Pace ML, Cole JJ, Carpenter SR (2006) Can algal photosynthetic inorganic carbon isotope fractionation be predicted in lakes using existing models? Aquat Sci 68:142–153
Badger MR, Price GD (1994) The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Biol 45:369–392
Chen Z, Cheng HM, Chen XW (2009) Effect of Cl− on photosynthetic bicarbonate uptake in two cyanobacteria Microcystis aeruginosa and Synechocystis PCC6803. Chin Sci Bull 54:1197–1203
Colman B, Huertas IE, Bhatti S, Dason JS (2002) The diversity of inorganic carbon acquisition mechanisms in eukaryotic microalgae. Funct Plant Biol 29:261–270
Elzenga JTM, Prins HBA, Stefels J (2000) The role of extracellular carbonic anhydrase activity in inorganic carbon utilization of phaeocystis globosa (prymnesiophyceae): A comparison with other marine algae using the isotopic disequilibrium technique. Limnol Oceanogr 45:372–380
Fry B, Sherr EB (1984) δ13C measurements as indicators of carbon flow on marine and freshwater ecosystems. Contrib Mar Sci 27:13–47
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Marlier JF, O’Leary MH (1984) Carbon kinetic isotope effects on the hydration of carbon dioxide and the dehydration of bicarbonate ion. J Am Chem Soc 106:5054–5057
Moazami-Goudarzi M, Colman B (2011) Inorganic carbon acquisition in two green marine stichococcus species. Plant Cell Environ 34:1465–1472
Mondal M, Khanra S, Tiwari ON, Gayen K, Halder GN (2016) Role of carbonic anhydrase on the way to biological carbon capture through microalgae-a mini review. Environ Progress Sustain Energy 35(6):1605–1615
Mook WG, Bommerson JC, Staverman WH (1974) Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth Planet Sci Lett 22(2):169–1769
Moroney JV, Husic HD, Tolbert N (1985) Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol 79:177–183
Moulin P, Andría JR, Axelsson L, Mercado JM (2011) Different mechanisms of inorganic carbon acquisition in red macroalgae (Rhodophyta) revealed by the use of TRIS buffer. Aquat Bot 95:31–38
Riebesell U, Wolf-Gladrow DA, Smetacek V (1993) Carbon dioxide limitation of marine phytoplankton growth rates. Nature 361:249–251
Sedmak JJ, Grossberg SE (1977) A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem 79:544–552
Smith-Harding TJ, Beardall J, Mitchell JG (2017) The role of external carbonic anhydrase in photosynthesis during growth of the marine diatom Chaetoceros muelleri. J Phycol 53(6):1159–1170
Sültemeyer D (1998) Carbonic anhydrase in eukaryotic algae: characterization, regulation, and possible function during photosynthesis. Can J Bot 76:962–972
Talling JF (1976) The depletion of carbon dioxide from lake water by phytoplankton. J Ecol 64(1):79–121
White DA, Pagarette A, Rooks P, Ali ST (2013) The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J Appl Phycol 25(1):153–165
Williams TG, Turpin DH (1987) The role of external carbonic anhydrase in inorganic carbon acquisition by chlamydomonas reinhardii at alkaline pH. Plant Physiol 83:92–96
Wu YY, Li PP, Wang BL, Liu CQ, He M, Chen CH (2008) Composition and activity of external carbonic anhydrase of microalgae from karst lakes in China. Phycol Res 56:76–82
Wu YY, Xu Y, Li HT, Xing DK (2012) Effect of acetazolamide on stable carbon isotope fractionation in chlamydomonas reinhardtii and chlorella vulgaris. Chin Sci Bull 57:786–789
Xie TX, Wu YY (2017) The biokarst system and its carbon sinks in response to pH changes: a simulation experiment with microalgae. Geochem Geophys Geosyst 18:827–843
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (U1612441), Foundation of Guizhou Province ([2014] 2131) and Doctor Foundation of Guizhou Normal University (0514014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Wu, Y. & Zhao, L. Effects of carbon anhydrase on utilization of bicarbonate in microalgae: a case study in Lake Hongfeng. Acta Geochim 37, 519–525 (2018). https://doi.org/10.1007/s11631-018-0277-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-018-0277-4