Abstract
Lead (Pb) isotopes have been extensively employed in tracing sources of Pb and its transport pathways through the environment. However, Pb isotopic ratios in related geochemical reference materials are scarce. Here, we report high-precision Pb isotopic ratios measured by Nu Plasma II MC-ICP-MS using calibrated 205Tl/203Tl = 2.38865 (NIST SRM 997) for mass discrimination correction. The long-term external precision (2SD) for NIST SRM 981 of Pb, BCR-2, and BHVO-2 are 0.31‰ (n = 105), 0.42‰ (n = 11), and 0.25‰ (n = 5) for 208Pb/206Pb and 0.16‰, 0.53‰, and 0.07‰ for 206Pb/207Pb, both respectively, and their Pb isotopic ratios are in excellent agreement with the recommended values. Using this method, we report for the first time Pb isotopic compositions in shale SGR-1b (USGS); coal CLB-1 (USGS); stream sediments GSD-17, -21, and -23 (IGGE); soils GSS-12, -13, -14, -15, and -16 (IGGE); plants GSV-1, -2, and -3 (IGGE); and human hair GSH-1(IGGE).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lead is a non-essential heavy metal element toxic to human beings, which can be transported long distances. Excessive Pb uptake by children can significantly inhibit intelligence development, and Pb is considered one of the most important pollutants to human health by the Word Health Organization (WHO). The sources of lead in the environment generally are divided into natural and anthropogenic sources. Studies have shown that human activities such as mining, smelting, combustion of fossil fuels, and battery and cement production can release one to two times more Pb than natural sources into the environment. In particular, Pb released from coal combustion and non-ferrous metal smelting has been growing exponentially in China. Identifying the sources and transport pathways of Pb pollution in the environment is a hot topic that has attracted wide attention.
Lead has four isotopes in nature: 204Pb (1.42%), 206Pb (24.15%), 207Pb (22.08%), and 208Pb (52.35%). 206Pb, 207Pb, and 208Pb are the decay daughters of 238U, 235U, and 232Th, respectively. The superposition of common Pb and radiogenic Pb give Pb isotopes a unique “fingerprint.” Moreover, since Pb isotopic fractionation is very small during physical, chemical, and biological processes, Pb isotopes are widely used to trace the sources and pathways of Pb pollution, as well as to estimate Pb contributions from different geological or environmental end-members. Consequently, the accurate and precise measurement of Pb isotopes in environmental and geological samples is important for applying Pb isotopes as a tracer.
Double spike (DS) and thallium element doping (Tl ED) are commonly used to determine Pb isotopic composition. The external precision for 204Pb–207Pb DS is two to five times better than that of Tl ED. But with the development of multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS), the difference in analytic precision is narrowing. For 204Pb–207Pb DS, spiked (extracting 208Pb/206Pb ratios) and unspiked (using extracted 208Pb/206Pb ratios to correct mass fractionation) samples need to be simultaneously determined, making analytical efficiency relatively low. Hence, Tl ED is still the preferred approach for Pb isotope measurement. However, previous studies have shown that, although the mass of Pb and Tl are close, measured ratios of SRM 981 20xPb/204Pb (x = 208, 207, and 206) using 205Tl/203Tl=2.3871 (SRM 997 Tl) are always lower than the certificated values. Therefore, in order to obtain Pb isotope data with high accuracy and precision, 205Tl/203Tl ratios should be re-calibrated on different instruments. Meanwhile, Pb isotopic ratios in different geochemical reference materials (GRMs) matching different samples should be conducted to ensure the quality of Pb isotope data and to provide benchmarks for inter-laboratory calibration. We followed the guidelines by Rehkämper et al. (1998) to optimize the isotope ratios of SRM 997 Tl, and then determined the Pb isotopic composition of selected international and domestic GRMs.
2 Methods
NIST SRM-981 Pb and -997 Tl stock solutions (both 10 mg·L−1 in 5% HNO3, National Institute of Standards and Technology, or NIST, USA) were diluted and mixed to target Pb/Tl = 4:1 in 3% HNO3. BCR-2, BHVO-2, SGR-1b, and CLB-1 were purchased from the United States Geological Survey (USGS). GSD, GSS, and GSV series reference materials and human hair (GSH) were purchased from the Institute of Geophysical and Geochemical Exploration (IGGE), Chinese Academy of Geological Sciences (CAGS). All HNO3, HCl, and HF used in the experiment were twice distilled in an acid purification device (model DST-1000, Savillex, USA). Ultrapure HBr was purchased from Aladdin Corporation. Pipette tips and tubes were sequentially cleaned by 10% HNO3, 6 M HCL, and Milli-Q water.
BCR-2 and BHVO-2, 60 mg, were weighed into 15-mL PFA beakers, and digested using 3:1 and 2:1 HNO3 + HF. The residue was dissolved by aqua regia until the solution was clear, and then dried down and dissolved in 10% HNO3 as the stock solution. Stream sediments, soils, shales, and coal samples were weighed at 0.1 g ± 5% in 30-mL Teflon bombs. After adding 2.5 mL HNO3 + 0.8 mL HF, the bombs were placed in a 160–185°C oven for 18 h, and then 2 mL H2O2 was added to decompose the organic matter. The solutions were evaporated to incipient dryness and heated an additional 12 h after adding 3 mL HNO3 for the second digestion. Then the samples were transferred to 15-mL PFA beakers and 1 mL H2O2 + 0.5 mL HF was added to thoroughly dissolve the siliceous and organic matter. Finally, the samples were dissolved in 1 mL of 10% HNO3 as the stock solution.
All chemical pre-treatments were conducted in a class 100 clean hood in the Isotope Geochemistry Laboratory, China University of Geosciences (Beijing). Pb was purified using Bio-Rad AG1-8× (200–400 mesh) resin, filled in customized 0.3 mL FEB columns. Detailed procedures are described in Li et al. (2015). Briefly, samples containing 300 ng Pb were dissolved in 1 mol·L−1 HBr after evaporating to dryness, and then Pb was separated from the sample matrix using 1.5 mL of 1 mol·L−1 HBr and 0.6 mL of 2 mol·L−1 HCl. Pb was eluted by 6 mol·L−1 HCl and collected. This procedure has nearly 100% recovery for Pb. The purified samples were dissolved in 3% HNO3 for isotope measurement.
Lead isotope analysis was carried out with Nu Plasma II MC-ICP-MS at the State Key Lab of Environmental Geochemistry, Institute of Geochemistry. The signals of Pb, Tl, and 202Hg were monitored by Faraday cups H2(208), H1(207), Ax(206), L1(205), L2(204), L3(203), and L4(202). Samples were introduced into the plasma via an Ariduss II desolvating sample introducing system equipped with an ESI Teflon aspire nebulizer (100 μl·mL−1) and Cetac AXR110 antosampler. The typical signal of 208Pb for 100 ng·mL−1 is 17 V or higher to ensure 204Pb is greater than 0.2 V.
3 Results and discussions
3.1 Optimization of 205Tl/203Tl value
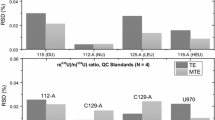
Rehkämper et al. (1998) suggested the same exponential factor (f) between Pb and Tl when using 205Tl/203Tl of SRM 997 to correct for mass fractionation. Using 208Pb/206Pb = 2.16770 (SRM 981) as the normalized value reported in recent literature, we recalibrated SRM 997 205Tl/203Tl to be 2.38865. This value is within the range presented by Rehkämper, and similar to that obtained by other researchers assuming different f values for Pb and Tl (e.g. f Pb =0.9746f Tl, White et al. 2000). Taking 205Tl/203Tl = 2.38865 as the standard value, the fractionation factors of 205/203Tl and 208/206Pb display good linear correlation during the 1 year measurement of SRM 981 Pb. We obtained Pb isotopic ratios (2SD, n = 135) of 208Pb/206Pb = 2.16764 ± 0.00031, 207Pb/206Pb = 0.91471 ± 0.00016, 206Pb/204Pb = 16.9432±0.0058, 207Pb/204Pb = 15.4987 ± 0.0023, and 208Pb/204Pb = 36.7285 ± 0.0112, which are identical to the latest values reported by Taylor (2015), and the precision is twice as good as the previous ED method.
3.2 Influence of procedural blank
A procedural Pb blank can affect Pb isotopic ratios. Lead concentrations in blank solutions were generally less than 0.35 ng·mL−1 in our experiments. To guarantee data quality, we diluted SRM 981 Pb with procedural blank solution to be 100–1000 ng·mL−1 of standard solution, and tested the effects of concentrated 0.5–2 ng·mL−1 Pb blank to Pb isotopes of SRM 981. We found no impact when [Pbblank]/[Pbsample] was ≤1/500. When [Pbblank]/[Pbsample] ≈ 1/100, measured SRM 981 ratios showed small deviations, especially 206Pb/204Pb and 208Pb/204Pb.
3.3 Lead isotopic composition in reference materials
BCR-2 (n = 11) and BHVO-2 (n = 5) were used to check the accuracy and precision of our procedure. Ratios of 206Pb/204Pb, 207Pb/204Pb, and 208Pb/204Pb were 18.7613 ± 69, 15.6294 ± 51, and 38.7483 ± 173 for BCR-2 and 18.6210 ± 23, 15.5385 ± 25, and 38.2366 ± 90 for BHVO-2, respectively. These values are identical to those reported by Baker et al. (2004) (Table 1). For shale SGR-1b (n = 11) and coal CLB-1(n = 3), determined 208Pb/206Pb and 206Pb/207Pb values were 1.98702 ± 67 and 2.06690 ± 192, and 1.25273 ± 53 and 1.19458 ± 90, respectively. 208Pb/206Pb and 206Pb/207Pb for SGR-1b and CLB-1 are reported for the first time; the 206Pb/207Pb value for CLB-1 was within the range of 1.21 ± 0.2 for US coals reported by Somoano et al. (2009).
The Pb isotopes in GSD-17, -21, -23; GSS-12, -13, -15, -16; GSV-1, -2, and -3; and GSH-1 are listed in Table 1. GSD-17, -21, and -23 are reference materials for stream sediment, individually collected from mining areas of Pb–Zn and Cu–Ag ore deposits in Heilongjiang, Xinjiang, and Jiangxi Provinces of China. The 206Pb/204Pb and 207Pb/204Pb ratios in GSD-17 are quite different from those in GSD-21 and -23, but very similar to those from Pb–Zn deposits. GSD-21 and -23 had similar Pb isotopic composition even though they were taken from different locations.
GSS-12, -13, -14, -15, and -16 are soil samples and can also be viewed as environmental reference materials (ERMs), and were collected from the north Xinjiang, the North China Plain, the Sichuan Basin, the Yangtze River Plain and the Pearl River Delta, respectively. 208Pb/206Pb and 207Pb/206Pb ratios in these samples suggest that GSS-12, -16, -13, and -15 have similar Pb isotopic compositions, in contrast to GSS-14. These results demonstrate that samples from different areas could have similar Pb ratios, raising cautions when utilizing Pb isotopes to trace the source and transport of Pb in the environment.
GSV-1 and GSV-2 are ERMs for shrub branches and leaves. Their 208Pb/206Pb and 207Pb/206Pb values were quite close, only differing by 0.358‰ and 0.587‰, respectively, both within the analytical standard deviation (2SD). This suggests that they may share the same Pb source even though they were collected in different areas of Qinghai Province. GSV-3 is derived from poplar leaves, taken from Langfang City of Hebei Province, and its 208Pb/206Pb and 207Pb/206Pb ratios were notably different from GSV-1 and -2. The difference may be related to the Pb source and/or Pb isotopic fractionation within plants. GSH-1 is an ERM for human hair, also collected from Langfang City, and had a similar Pb isotopic composition to GSV-3, indicating that Pb in the human body may be related to Pb in local residents’ vegetables.
4 Conclusions
Utilizing the optimized 205Tl/203Tl = 2.38865 of SRM 997 Tl to correct instrumental mass fractionation, measured SRM 981 Pb, BCR-2, and BHVO-2 values were consistent with published values. Using this method, we measured Pb isotopic compositions in selected new reference materials, including SGR-1b, CLB-1, and 11 other reference materials from IGGE. These data can be used for inter-laboratory comparison and quality assurance when measuring related geological or environmental samples.
References
Baker J, Peate D, Waight T, Meyzen C (2004) Pb isotopic analysis of standards and samples using a 207Pb–204Pb double spike and thallium to correct for mass bias with a double-focusing MC-ICP-MS. Chem Geol 211:275–303
Collerson KD, Kamber BS, Schoenberg R (2002) Applications of accurate, high-precision Pb isotope ratio measurement by multi-collector ICP-MS. Chem Geol 188:65–83
Li SZ, Ma JX, Zhu XK, Tang SH (2015) Pb isotopic fractionation during the ion exchange process and the modification of methods for isotope determination by MC-ICP-MS. Acta Petrologica et Mineralogica 34(5):785–792
Rehkämper M, Halliday AN (1998) Accuracy and long-term reproducibility of lead isotopic measurements by multiple-collector inductively coupled plasma mass spectrometry using an external method for correction of mass discrimination. Int J Mass Spectrom 181(1998):123–133
Somandao DM, Kylander ME, Anton MAL, Ruiz IS, Tarazona MRM, Ferrat M, Kober B, Weiss DJ (2009) Stable lead isotope compositions in selected coals from around the world and implications for present day aerosol source tracing. Environ Sci Technol 43:1078–1085
Taylor RN, Ishizuka O, Michalik A, Miltona JA, Croudacea IW (2015) Evaluating the precision of Pb isotope measurement by mass spectrometry. J Anal At Spectrom 30:198–213
White WM, Albarède W, Télouk P (2000) High-precision analysis of Pb isotope ratios by multi-collector ICP-MS. Chem Geol 167:257–270
Woodhead JD, Hergt JM (2000) Pb-isotope analyses of USGS reference materials. Geostand Newsl 24:33–38
Acknowledgements
This work was supported by National Key Basic Research Program of China (2014CB238903), the National Natural Science Foundation of China (Nos. 41473028, 41673017, U1612441) and the Opening Fund of State Key Laboratory of Environmental Geochemistry (SKLEG2015201).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. Accurate and precise determination of lead isotopic composition in selected geochemical reference materials.
Additional information
11th International Symposium on Geochemistry of the Earth’s Surface.
Rights and permissions
About this article
Cite this article
Wu, G., Zhu, JM., Tan, D. et al. Accurate and precise determination of lead isotope composition in selected geochemical reference materials. Acta Geochim 36, 421–425 (2017). https://doi.org/10.1007/s11631-017-0181-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-017-0181-3