Abstract
A highly efficient regeneration system of Anthurium andraeanum Linden was established using root segments as explants of four genotypes, Arizona, SWD, OYH, and Mississippi. The root explants were firstly incubated on callus induction media containing NH4NO3 level of 206 mg L−1 or 825 mg L−1, and the calluses were formed from both ends of the root segments after 1 mo of culture. Root-derived calluses were then transferred to shoot regeneration media with NH4NO3 level of 412 mg L−1 or 1650 mg L−1, and numbers of shoot were successfully produced from the surface of calluses after 2 mo of culture. Medium containing 206 mg L−1 NH4NO3 induced more callus compared to 825 mg L−1. In contrast, the number of shoots per callus was the same between the above two shoot regeneration media indicating that NH4NO3 level had little effect on shoot regeneration. Long-termin vitro maintenance of the callus-bearing shoot initials was possible without compromising shoot regeneration and subsequent rooting. Histological analysis showed that early cell division was initiated from the inner cortical parenchyma cells near the vascular tissue and from the vascular parenchyma cells adjacent to the xylem vessel. The primary callus mainly comprised inner parenchyma cells with scattered procambial-like cells and xylem elements and two different meristematic zones beneath epidermal layers. Transfer of callus to the regenerative medium caused meristemoid formation and subsequent shoot primordia production. Calcium oxalate crystals and tannin-like deposits were visible on the outer callus layer or within the cotyledon-like structure of shoot primordium throughout callus development. Starch grains were abundant in root explants, but disappeared in the profusely growing primary callus, and were mobilized in the shoot primordium, indicating energy requirements of these two developmental stages. Flow cytometry analysis demonstrated that ploidy levels were maintained during in vitro cultures, including long-term regenerated plants. The inter-simple sequence repeat analysis indicated genomic DNA stability of Arizona and OYH but occasional genetic variation in primary regenerates of SWD and Mississippi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthurium andraeanum Linden is one of the most valuable ornamental crops worldwide owing to its attractive inflorescence and exotic foliage. Traditionally, A. andraeanum is propagated in a greenhouse by offshoot or node cuttings, but the slow multiplication rate is the main constraint of this method for commercial propagation. In vitro propagation of A. andraeanum has been successful and widely applied for commercial purposes (Teixeira et al.2015). Various tissues, such as leaf lamina, leaf petiole, spathe, and nodal segment, have been used as explants for in vitro regeneration of A. andraeanum via organogenesis (directly by axillary bud outgrowth or callus-mediated adventitious bud initiation) or somatic embryogenesis (Pierik et al.1974; Pierik 1976; Kuehnle and Sugii 1991, Kuehnle et al.1992; Matsumoto et al.1996; Martin et al.2003; Gantait et al.2008; Pinheiro et al.2013; Cardoso and Habermann 2014; Jahan et al.2014). Compared with leaf lamina and petiole, roots were seldom used as starting material for in vitro propagation of A. andraeanum. Chen et al.(1997) briefly described shoot regeneration from root segments, providing no details about the factors affecting shoot regeneration. Wang et al.(2005) reported that half-strength Murashige and Skoog (MS; Murashige and Skoog 1962) medium supplemented with optimized combination of auxin and cytokinin was suitable for callus induction from root explants for only one genotype, whereas other genotypes responded poorly. Previous studies have reported that high levels of NH4NO3 hindered the response of leaf explants of A. scherzerianum(Geier 1986) and A. andraeanum(Yang et al.2008). It was probably that the response of A. andraeanum root explants (referenced above) was also negatively influenced by high NH4NO3 used in the medium.
In vitro roots are excellent explant sources because they are readily available and robust roots can be produced from stock cultures. The roots of A. andraeanum are amenable to Agrobacterium-mediated transformation (Chen and Kuehnle 1996, Chen et al.1997; Hosein et al.2012) and used for the establishment of a highly efficient regeneration systems.
During organogenesis, comprehensive analysis of anatomical changes is essential and necessary because it allows for identification of cell types that give rise to callus and helps to elucidate major events in the process of morphogenesis. Moreover, the histochemical detection of the mobilization and accumulation of starch facilitates the recognition of the stages of morphogenesis and regions of high energy demand (Varshney et al.2011; Rocha et al.2012). This information will provide a strong basis for establishing a proper propagation system. A histological inspection of the origin of somatic embryos from in vitro-grown lamina was conducted for A. andraeanum(Matsumoto et al.1996), but histological analysis of in vitro morphogenesis from various explants, especially root segments, has been relatively rarely documented (Bhattacharya et al.2015).
Shoot regeneration from callus, as well as in vitro subculture of over several years, is usually associated with the risks of cytological and genetic variation, resulting in non-uniform clonal progenies. Therefore, it is necessary and important to examine ploidy stability and genetic fidelity among clonal populations, especially for long-term sustained populations. Among several DNA-based molecular markers, the highly sensitive and reproducible inter-simple sequence repeats (ISSRs) have been used to test the genetic homogeneity of in vitro-derived plants in some species (Huang et al.2009; Lata et al.2009). ISSR analysis has been exploited to assess clonal fidelity and construct a genetic linkage map of Anthurium species by some researchers (Gantait et al.2008; Venkat et al.2014), and it can be potentially applied to test DNA genetic stability of the primary regenerative populations and long-term sustained populations of A. andraeanum.
Owing to its speed, efficiency, and reliability, flow cytometry (FCM) is regarded as one of the best methods to analyze DNA content and ploidy levels in plant cells. To date, DNA content and ploidy levels have been successfully estimated using this method in A. andraeanum and many other species (Loureiro et al.2007; Bliss and Suzuki 2012; Abhishek et al.2016). FCM has also been used to assess the ploidy stability of in vitro plantlets in species such as Solanum aculeatissimum, Centaurea ultreiae, and cherry (Mallón et al.2010; Ghimire et al.2012; Vujović et al.2012). Herein, FCM was used to rapidly detect ploidy levels among the initial and long-term sustained regenerates.
The aims of this study were as follows: (1) to establish a highly efficient in vitro regeneration system of A. andraeanum using root segments as explants after testing the influence of NH4NO3 content and other factors on callus induction and shoot regeneration; (2) to elucidate the cell origin of root-derived callus and examine major histological changes and starch reserve pattern changes during callus formation and shoot regeneration; and (3) to determine cytological and genetic stability among in vitro primary and long-term sustained regenerates.
Materials and Methods
Plant Material
Three diploid genotypes, Arizona, SWD, and Mississippi, and one tetraploid genotype, OYH, of A. andraeanum, were used in the experiments. Arizona, SWD, and Mississippi were collected from Bozon Agriculture Company (Beijing, China) and OYH was bred by our lab. Shoots were cultured on medium E, the composition of which was described below. After 2 mo, the adventitious roots produced from the base portions of the shoots were used as explants in the following experiments.
Media and Culture Conditions
Medium A was composed of half-strength MS macronutrients, full-strength MS micronutrients, 25 mg L−1 Na2Fe-EDTA, MS vitamins, and 30 g L−1 sucrose containing 2.2 μmol L−16-benzyladenine(6-BA) and 0.9 μmol L−1 2,4-dichlorooxyacetic acid (2,4-D). The composition of medium B was similar to that of medium A, except that the content of NO3NH4 in macronutrients was reduced to 206 mg L−1. Medium C consisted of full-strength MS macronutrients, full-strength MS micronutrients, 25 mg L−1 Na2Fe-EDTA, MS vitamins, and 30 g L−1 sucrose containing 4.4 μmol L−16-BA and 0.9 μmol L−1 2,4-D. Medium D differed from medium C in the amount of NO3NH4, which was reduced to 412 mg L−1. Media A and B were designated for callus induction, and media C and D were used for shoot differentiation. Medium E, used for rooting and composed of half-strength MS medium supplemented with 20 g L−1 sucrose and 0.9 μmol L−1 2,4-D. All media were adjusted to pH 5.5 before autoclaving and solidified with 0.7% Bacto agar. The chemicals used in the media and following experiments were purchased from Sangon Biotech (Shanghai, China) except otherwise specified.
After the removal of root tips, the roots were cut into segments approximately 1.0 cm long and divided into basal, middle, and proximal portions along the root axis. One hundred-milliliter Erlenmeyer flasks (Shuniu, Chengdou, China), each containing approximately 30 mL medium, were used for callus induction. Approximately 15 root segments were inoculated in each flask. The changes in root explants were examined every 3 or 4 d, and the total responses were recorded after 30 d following the initial culture.
The newly developed primary calluses at the ends of root segments were removed and transferred to Erlenmeyer flasks (five calluses per flask) containing approximately 40 mL medium for differentiation. After approximately one and a half month, the number of responding calluses and the number of regenerated shoots per responding callus were scored.
Robust shoots over 3 cm in length were removed from calluses and incubated in 40 mL medium E dispensed into a 100-mL glass box (Dahua, Xuzhou, China) for rooting. Meanwhile, the remaining calluses bearing several new tiny shoot initials were split and subcultured to medium C. Afterwards, the repeated subculture of callus was carried out with 2 mo interval.
All cultures were incubated at 25 ± 1°C under a 12-h photoperiod with a light intensity of 50 μmol m−2 s−1, supplied by cool-white fluorescent light. All experiments were carried out from the middle of March to the middle of July, 2017. The number of root segments for callus induction was at least 45 in each treatment, and the number of calluses for regeneration in each treatment was at least 30 for each treatment.
Histology
Root explants and calluses at different incubation periods were prepared for histological examination. The specimens were fixed in FAA solution (5% formalin, 5% glacial acetic acid, 90% ethanol), stained with hematoxylin (Sangon Biotech, Shanghai, China) for at least 1 wk, and rinsed with 1% (w/v) ammonia water (Sangon Biotech). The stained root explants or calluses were embedded in paraffin wax after dehydration with a graded ethanol-xylene series. Then, 5 μm sections were cut using a Leica RM 2015 microtome (Heidelberger, Germany) and de-waxed in xylene.
Serial sections were cut and stained with periodic acid Schiff (PAS) for starch detection, and in some cases, samples were counterstained with naphthol blue black (NBB) (Sangon Biotech) (Feder and O’Brine 1968). The sections were observed under an Olympus BX 51 compound microscope (Tokyo, Japan) with Nomarski optics. Images were captured using Olympus DP70 digital camera.
Flow Cytometric Analyses and Determination of Chromosome Numbers
Approximately 100 mg fresh leaves from each tested specimen were chopped in 1 mL Tris-MgCl2 buffer solution (0.2 mol L−1 Tris-HCl, 4 mmol L−1 MgCl2·6H2O, 1% polyvinylpyrrolidone 10, 0.5% (v/v) Triton X-100, pH 7.5) with a sharp razor blade for about 1 min. The mixture was then filtered through a 50-μm nylon filter (Sangon Biotech) to eliminate large cell debris. The nuclei suspension was treated with 1 μL RNase (10 mg mL−1) (Takara, Otsu, Japan) to remove RNA and simultaneously stained with 1 μL propidium iodide (PI; 10 mg mL−1) (Sangon Biotech) for approximately 10 min. PI fluorescence was detected with a BD FACSVerse flow cytometer (Beckton, Dickinson, Co., Franklin Lakes, NJ) using a 600-nm dichroic longpass and 575 nm bandpass filters. Routinely, the peaks of fluorescent intensity of 2C DNA were adjusted to near 50 channel; 5000 to 10,000 nuclei were measured per sample and evaluated using BD FACSuite software (BD Biosciences, Franklin Lakes, NJ).
From 10:00 to 11:00 in the morning, rapidly growing, 0.5-cm-long young root tips were pretreated with 0.002 mol L−18-hydroxyquinine (Sangon Biotech) solution for 3 to 4 h at room temperature, fixed in the Carnoy’s fixative (25% glacial acetic acid, 75% ethanol) for 24 h, and stored in 70% ethanol at 4°C until use. The fixed root tips were washed three times with distilled water, then hydrolyzed in 1 M HCl at 60°C for 8 to 10 min, followed by rinsing with distilled water three times and staining with carbol fuchsin solution (Sangon Biotech) for 10 min. The stained root tips were placed onto a glass slide, squashed gently to disperse chromosomes evenly, and then observed under an Olympus BX51 microscope.
Inter-Simple Sequence Repeats (ISSR) Analysis
Twenty plantlets were randomly selected from a batch of primary regenerates and from long-term sustained regenerates (subculture over 3 yr). Each genotype comprised three populations (mother plant providing the root explants, primary and secondary regenerates), with a total of 12 populations in the four genotypes, used for ISSR analysis.
Total DNA was extracted from fresh leaves of each population according to the method of Ziegenhagen and Scholz (1993). The integrity of DNA was verified by electrophoresis on 1.0% agarose gel (Sangon Biotech) and staining with ethidium bromide (Sangon Biotech) after removal of RNA, and DNA was quantified by spectrophotometry. A final DNA concentration of 5 μg L−1 was used in ISSR analysis after dilution.
DNA amplification reactions were carried out in 25 μL reaction mixtures comprising 20 ng template DNA, 1.5 U Taq DNA polymerase (Takara), 1.6 mmol L−1 MgCl2, 1.5 mmol L−1 dNTPs, 0.8 μmol L−1 primer, and 1× reaction buffer (75 mmol L−1Tris-HCl (pH 8.8), and 0.01% DMSO) (Takara). The optimized PCR procedure was as follows: an initial step at 94°C for 5 min, followed by 40 cycles of 94°C for 1 min, 51.8 to 54.1°C (primer AW79146 at 53.1°C, AW79148 and AW 79149 at 51.8°C, AW79155 at 53.9°C, and AW79159 at 54.1°C) for 1 min, and 72°C for 2 min and a final step of 72°C for 5 min. PCR products were separated on a 2.0% (m/v) agarose gel in 0.5× TAE buffer. The DNA bands were examined under UV light after staining with ethidium bromide (0.001%) and analyzed using SIM BIO-1D software (Gold SIM, Beijing, China). The size of the amplification products was estimated using a DL 2000 molecular size marker (Takara). Only distinct, reproducible, and well-resolved DNA bands ranging from 150 to 2000 bp were considered in the analysis. These bands were recorded as present (1) or absent (0) for each reaction in all samples tested.
The calculation of the distance matrix and clustering analysis of all data sets were performed using DPS Version 655 (Tang and Zhang 2013). Genetic similarities among different samples were measured using Jaccard’s similarity coefficient (Jaccard 1908) implemented in the SIMQUAL module. Based on similarity coefficients, a dendrogram was constructed using the unweighted pair group method with arithmetic average (UPGMA) and the sequential, hierarchical, and nested clustering (SHAN) routine in DPS Version 655.
Results
Callus Induction and Shoot Regeneration
The explants from the thick and robust roots produced a compact pale-yellowish callus after approximately 1 mo culture (Fig. 1A). The responses were similar between the basal, central, and proximal root segments, irrespective of the medium used. Therefore, the mixtures of root segments taken from different portions were used as explants in the following experiments.
Root segments of each genotype cultured on different media (medium A or B) responded differently and were divided into two groups: Arizona, OYH, and Mississippi, which were more affected by NH4NO3 level, and SWD, which was not affected by NH4NO3 levels in the medium. Callus initiation of the first group was hindered by higher levels of NH4NO3. For instance, the sign of callus initials of Arizona, consisting of swelling at the ends of root segments, were visible 10 d after incubation on medium B with 206 mg L−1 NH4NO3. In contrast, the response of those cultured on medium A with 825 mg L−1 NH4NO3 was delayed and root swellings were observed after14 d of culture. Twenty-one days were needed for the production of visible calluses from explants on medium B, whereas 24 d were required for calluses formation from those on medium A. Only 38.2% of explants on medium A produced calluses, significantly less than those on medium B. In addition, most of the calluses on medium A exhibited slow growth with a diameter no more than 2 mm. The percentage of responding root segments of Mississippi on medium A was less than that obtained on medium B although callus initiation commenced at about the same time in both media. In contrast, SWD was not affected by the NH4NO3 levels; callus initiation and visible callus formation occurred at similar times in root explants cultured on either medium A or B, and up to 85% of the roots responded to culture medium with the percentage of responding roots being negligibly different between medium A and B (Table 1).
Arizona and SWD were selected to examine shoot regeneration. After subculturing on medium C or D, calluses increased in size and became pale green. Between 20 and 25 d after transfer, several green nodule spots emerged on the callus surface and further differentiated into shoot buds 2 mo later (Fig. 1B, C). In most cases, the production of shoots occurred as several new tiny shoot buds that initiated close to the existing large shoots.
The level of NH4NO3 did not influence shoot production from callus grown on medium C; the shoot length ranged from 1.5 to 3.5 cm long and, on average, 6.4 and 4.6 shoots per callus for Arizona and SWD, respectively, were regenerated. Plant regeneration did not differ from callus grown on either media C or D (Table 2).
After the first transfer, the calluses were repeatedly transferred to medium C at two-mo intervals. The shoots more than 3 cm long were removed for rooting, and the remaining callus-bearing tiny shoots were divided and transferred to fresh medium at each subculture.
During early subculture, the number of shoots regenerated increased until the third subculture, when it plateaued to approximate 12 shoots per callus (Table 2). The competence for callus regeneration remained constant through 3 yr of successive subculture, and no depression in shoot formation was observed; nearly all shoots had regular growth and appeared robust.
The shoots rooted well after being transferred onto medium E. First roots appeared within 2 wk, and 100% of shoots produced fresh and robust roots after 1 mo, with at least five roots per plantlet (Fig. 1D). The rooting ability remained constant in long-term sustained cultures.
Histology
Histological studies of root explants incubated on medium B revealed that the first cell divisions occurred in the inner cortex parenchyma cells close to vascular tissue, together with vascular parenchyma cells, and within 5 to 7 d of culture. (Fig. 2A, B). Concurrent periclinal cell divisions were observed simultaneously in the epidermis of root explants (Fig. 2B).
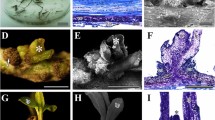
Histology of Anthurium andraeanum Linden callus formation from root segments. (A, B) Transverse (A) and longitudinal (B) sections of root explant showing the initial division of parenchyma cells oriented in the inner cortex region or parenchyma cells adjacent to xylem within vascular tissue (arrow); the initial division of epidermal cells were also observed (triangle). (C) Section showing extensive cell proliferation within root explant and profusely periclinal division of epidermal cells (triangle). (D, E) Section of new primary callus showing procambial-like cell groups (arrow) and extensive cell division at the intervening region between root explant and callus (triangle). (F) Callus showing cells with densely stained irregular-shaped nuclei (outer rectangle, yellow color) and cells with large round nuclei (inner rectangle, red color). (G) Section of callus showing xylem elements (arrow). (H) The root explant bearing newly growing callus stained by periodic acid Schiff, showing heavy starch accumulation in parenchyma cells of the root explant (rectangle, green color), and mobilization of starch grains in callus. (I) Primary callus showing cells devoid of starch grains. Note: G Nomarski image and the others were ordinary bright field images. Bar: 50 μm (A, B, E, F, G, and I) and 100 μm (C, D, and H).
Over time, extensive cell division occurred from inside to the outside of the cortex tissue, and cell divisions in the epidermis continued (Fig. 2C), which led to the connection of these two proliferating zones and eventually gave rise to the formation of compact callus observed macroscopically at the cut ends of the root explant (Fig. 2D).
The primary callus consisted of several distinct regions. The inner region of callus was mainly comprised of large parenchyma cells containing small nuclei at the edge of the cell wall and scattered procambial-like and the xylem elements. These procambial-like cells were narrow and thin shaped with elongated nuclei, and the xylem elements might derived from disruption of original root xylem vessels (Fig. 2D, E, G). The outer layer was composed of epidermal-like cells devoid of cytoplasmic content (Fig. 2D). The first meristematic zone beneath the epidermal layer was formed by a group of tiny cells with densely stained irregular-shaped nuclei, possibly as a result of amitosis. Internal to the first meristematic zone was the second meristematic zone composed of small dense cytoplasmic cells with large round nuclei (Fig. 2F). Cells undergoing extensive proliferation were visible in the intervening region of the callus and root explants (Fig. 2D).
Within 15 to 20 d after callus was transferred to the regenerative medium, many small meristematic centers emerged within the region from about the 10th to 20th cell layer beneath the epidermis (Fig. 3A). Over time, these small meristematic centers grew toward the periphery of the callus by crushing the outer cell layers of the callus and finally developing into meristemoids, which were accompanied by newly formed tracheary elements (Fig. 3B, C). At the meristemoid formation stage, several small pro-meristemoids were visible in the inner region close to meristemoids (Fig. 3D). In the deeper region of callus, embryo-like structure was observed occasionally (Fig. 3E), but further development was not detected, although root-like structures infrequently emerged in the inner part of callus. Over time, meristemoid developed into bud primordium and further differentiated into adventitious shoot buds composed of a meristematic dome flanked by leaf primordia, which were surrounded by a cotyledon-like structure (Fig. 3F). Large idioblasts with calcium oxalate crystals (raphides) or tannin-like deposits appeared within a cotyledon-like structure throughout all developmental stages (Fig. 3G). Protoxylem tissue was visible within the cotyledon-like structure (Fig. 3H). At the edge of mother callus, daughter callus was also observed (Fig. 3I).
Histology of Anthurium andraeanum Linden shoot regeneration from root-derived callus. (A) Callus presenting small meristematic cell groups (arrow) oriented in the region from the approximate 10th cell layer to 20th cell layer beneath epidermis. (B) Meristemoids at the periphery of callus. (C) High magnification of meristemoid showing tracheid-like elements (arrow). (D) Callus showing small pro-meristemoid (arrow) internal to the larger meristemoid. (E) Embryo-like structure in the deeper layers of callus. (F) Callus showing shoot bud with apical meristem (apm, dot), leaf primordia (lp, triangle), and cotyledon-like structure (cls, arrow). (G) Cotyledon-like structure (cls) showing calcium oxalate crystals (raphides) (arrow) and tannin-like deposits (triangle). (H) Shoot bud showing xylem vessel (arrow). (I) Daughter callus growing out from mother callus. (J) Callus showing abundant starch accumulation. (K) Callus showing meristematic cell groups and adjacent parenchyma cells devoid of starch grains. (L) Bud primordium showing cells devoid of starch grains and the nuclei stained by naphthol blue black. Bar: 100 μm (A, B, F, H, I, and J); 50 μm (C–E, G, H, K, and L).
Starch grains accumulated abundantly in the parenchyma tissue of root explants; however, once cells began to divide, such starch reserves dissolved rapidly. In the newly growing callus, the starch deposits were too scarce to be detectable (Fig. 2H, I). However, as callus enlarged and parenchymal cells differentiated, the intensive starch accumulation resumed (Fig. 3J), much of which was observed in parenchyma cells, except in those adjacent to meristematic cell groups (Fig. 3K). The meristematic cells within differentiating shoot buds were also devoid of starch reserves (Fig. 3L), and the nuclei were darkly stained by NBB, indicating active cell protein synthesis corresponding to cell division (Fig. 3L).
FCM Analysis
To evaluate the organogenic pathway using roots as explants, the primary and long-term sustained regenerates were chosen and used for ploidy detection through analysis of the leaf nuclear suspension. The DNA histograms with high resolution were obtained, and the values of fluorescence channel of 2C DNA among different primary regenerates for Arizona and long sustained cultures showed little variation (average value of fluorescence channel ± SD, 58.20 ± 2.50), with the coefficient of variation for 2C peak of less than 3% in each sample (Fig. 4A, B). Chromosome count analysis in representative samples confirmed the presence of 30 chromosomes for Arizona (Fig. 4C). Similar FCM results were obtained for SWD and Mississippi. The fluorescence channel intensity of OYH, the genotype with double the chromosome number of Arizona, was twice as high as that of Arizona (data not shown).
ISSR Analysis
Of the 16 tested ISSR primers, five primers produced clear and distinct amplification bands after the ISSR reaction with a mixture of genomic DNA; those primers were used for further analysis of genetic variation among 12 populations. The five primers amplified a total of 52 bands with an average of 10.4 scoreable bands per primer. The molecular weight of all the scored amplified products ranged from 150 to 2000 bp (Fig. 5). Primer AW79146 yielded the most bands (14), while primer AW79159 produced the least bands (8).
Electrophoretic gel separation of the amplification products obtained by primer AW79148 from the population of mother plants (lanes 1, 4, 7, and 10), in vitro primary regenerates (lanes 2, 5, 8, and 11), and long-term sustained regenerates (lanes 3, 6, 9, and 12) of Anthurium andraeanum Linden. Genotypes: lanes 1–3, SWD; lanes 4–6, Arizona; lanes 7–9, OYH; and lanes 10–12, Mississippi. Lane 13, negative control. M, molecular marker DL 2000.
Based on similarity coefficients generated from ISSR data, cluster analysis was performed for 12 populations from the four genotypes, including mother plantlets, primary regenerative populations, and long-term sustained populations. The populations were clustered into four major groups at 67% similarity (Fig. 6). The three populations of Arizona and OYH genotypes each were grouped with a similarity of over 92%, indicating genetic fidelity was persevered after callus induction and long-term maintenance. For the other two genotype groups, the similarities between primary regenerative and extended sustained populations exceeded 92%. However, those between the mother plant and the primary regenerative population ranged from 85 to 87%, indicating that genetic variation might occasionally occur during callus induction or shoot regeneration. Therefore, more attention should be paid to detect the off-type shoots among the primary regenerative population.
Unweighted pair group method with arithmetic average (UPGMA) dendrogram of the genetic relationship of 12 populations of Anthurium andraeanum Linden constructed using similarity coefficients based on inter-simple sequence repeats (ISSR) marker analysis. (A–D) Genotypes of SWD, Arizona, OYH, and Mississippi, respectively. 1, 4, 7, and 10 and 2, 5, 8, and 11 represent populations of in vitro primary regenerates and long-term sustained regenerates, respectively, and 3, 6, 9, and 12 are mother plant.
Discussion
As observed for leaf segment explants of A. scherzerianum(Geier 1986) and leaf explants of A. andraeanum(Yang et al.2008), we found that low level of NH4NO3 was beneficial to callus formation from root explants. Medium with 206 mg L−1 NH4NO3, the callus response from root explants improved, number of days required for callus initiation decreased, and the percentage of root segments forming calluses increased significantly. The beneficial influence of low NH4NO3 levels on callus induction was also observed in several other genotypes besides those reported herein (Zhang et al., personal communication). Moreover, it was observed that NH4NO3 was not necessary as successful callus induction was obtained from root explants incubated on half-strength modified MS medium or Nitsch medium without NH4NO3 (Zhang et al., personal communication).
It has been thought that the physiological conditions of a plant largely influence the response of the explant. The position of leaves collected as explants and their age had a notable effect on callus formation of Anthurium(Geier 1986; Bejoy et al.2008). Unlike leaf explants, the responses of root segments collected from different root sections were similar. However, the response of the robust roots sampled in hot summer season (the maximum outdoor temperature in most of the days from mid-May to late August was above 30°C) was poor in terms of time required for callus initiation, the reason of which might be that in vitro stocks, providing root explants, still preserved seasonal physiological fluctuations despite being cultured under constant 25°C.
Histological techniques are a useful tool in the study of plant morphogenesis and are widely applied in the in vitro tissue culture (Varshney et al.2011; Rocha et al.2012). A systematic and detailed report on the histological description of a series of events in the process of in vitro organogenesis from root explants of A. andraeanum is presented here: the division of parenchyma cells leading to callus initiation, the emergence of procambial-like cells and xylem elements within callus, the formation of meristemoid, regeneration of shoots, and cycles of starch accumulation and mobilization during in vitro morphogenesis. These data will contribute to a better understanding of the regeneration system of A. andraeanum and will increase our knowledge of the root explant application in genetic transformation of A. andraeanum.
Starch is considered a primary source of energy for cell growth and proliferation. It has been reported that starch accumulates in the regenerative tissues of Humulus lupulus before shoot formation (Paris and Pais 2000). Starch accumulation followed by consumption also occurs during the somatic embryogenesis processes in Eucalyptus (Pinto et al.2010). Consistent with these studies, the cycle of starch accumulation/mobilization in the process of A. andraeanum morphogenesis was observed, indicating a crucial role of starch in the organogenesis of A. andraeanum.
Calcium oxalate crystals (raphides), which are present in the embryo’s cotyledon and the inner integument of the ovule, represent a unique characteristic of A. andraeanum zygotic embryos (Matsumoto and Kuehnle 1998). Raphides were also observed within somatic embryos (Matsumoto et al.1996). We observed the deposition of calcium oxalate crystals within the enlarged callus and cotyledon-like tissue, suggesting that the presence of calcium oxalate crystals is associated with the morphogenesis potential of A. andraeanum.
In vitro plant cultures might show changes in ploidy level and DNA variation, much of which are caused by growth regulators usually added to the medium, oxidative stress during explant preparation, and environmental factors during culture. FCM analysis herein demonstrated that the original ploidy characteristics were preserved among in vitro primary and long-term sustained cultures derived from root explants. Similar observations were reported for the field-grownA. andraeanum raised by in vitro culture of shoot tips (Gantait and Sinniah 2011) and in other species such as Scutellaria baicalensis(Alan et al.2007), Centaurea ultreiae(Mallón et al.2010), and Gentiana pannonica(Fiuk et al.2010).
To date, several DNA markers, including ISSR, have been developed and used to identify germplasm and genetic diversity of Anthurium and to construct genetic linkage maps (Arachchi et al. 2001; Neto et al.2014; Venkat et al.2014). Herein, ISSR molecular markers were used to detect genetic DNA fidelity among populations of plantlets regenerated from root-derived callus. The stability of genetic DNA was conserved after long-term culture, as indicated by the similarity indices (0.92 to 1.00) among primary cultures and long-term cultures of Arizona and OYH. This was consistent with the report by Gantait et al.(2008) who showed that the genetic identity of both ex vitro hardened clones and in vitro sustained clones propagated via axillary bud was similar to that of their mother plants. Genetic uniformity of plants regenerated via the protocorm-like body pathway was revealed in A. andraeanum ‘CanCan’ and ‘Fantasia’ after assessment with the random amplified polymorphic DNA (RAPD)(Gantait et al.2012; Bhattacharya et al.2016). The similarity indices between primary cultures and long-term sustained cultures of SWD and Mississippi were above 90%, but those between mother plant and primary cultures were relatively low, suggesting those two genotypes might be more prone to genetic variation at the stage of callus formation and shoot differentiation. This was consistent with the observation that susceptibility to genetic variation differed among various genotypes of the same species (Bairu et al.2011).
In conclusion, a highly efficient regeneration system of A. andraeanum has been established using root segment as explants after decreasing the content of NH4NO3 in the medium used for callus formation. Its efficiency was verified by histological and histochemical investigations of callus origin and morphogenesis, and by confirming the ploidy stability of primary regenerates and long-term sustained regenerates. However, when using this established protocol for mass multiplication, occasional somaclonal variation should be of greater concern among primary regenerates because some genotypes might be more susceptible to DNA variation at the stage of callus formation. Moreover, micropropagation of A. andraeanum should be conducted in warm, but not hot, seasons when in vitro roots are used as explants.
References
Abhishek S, Sreetama B, Maumita B (2016) Novel nuclei isolation buffer for flow cytometric genome size estimation of Zingiberaceae: a comparison with common isolation buffers. Ann Bot 118:1057–1070. https://doi.org/10.1093/aob/mcw173
Alan AR, Zeng H, Assani A, Shi WL, Mcrae HE, Murch SJ, Saxena PK (2007) Assessment of genetic stability of the germplasm lines of medicinal plant Scutellaria baicalensis Georgi (Huang-qin) in long-term, in vitro maintained cultures. Plant Cell Rep 26:1345–1355. https://doi.org/10.1007/s00299-007-0332-9
Arachchi D, Henny R, Guy C, Li QB (2001) DNA fingerprinting to identify nine Anthurium pot plant cultivars and examine their genetic relationship. Hortscience 36(4): 758-760. https://10.21273/`HORTSCI.36.4.758
Bairu MW, Aremu AO, Staden JV (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Regul 63:147–173. https://doi.org/10.1007/s10725-010-9554-x
Bejoy M, Sumitha VR, Anish NP (2008) Foliar regeneration in Anthurium andraeanum Hort. cv Agnihothri. Biotechnology 7:134–138
Bhattacharya C, Dam A, Karmakar J, Bandyopadhyay TK (2015) Efficient organogenesis from the induced meristemoid of Anthurium andraeanum Linden cv. Tinora. Plant Sci Today 2:82–86. https://doi.org/10.14719/pst.2015.2.2.110
Bhattacharya C, Dam A, Karmakar J, Bandyopadhyay TK (2016) Direct somatic embryogenesis and genetic homogeneity assessment of regenerated plants of Anthurium andraeanum Linden cv. Fantasia. In Vitro Cell Dev Biol - Plant 52:512–519. https://doi.org/10.1007/s11627-016-9763-8
Bliss BJ, Suzuki JY (2012) Genome size in Anthurium evaluated in the context of karyotypes and phenotypes. AoB Plants 2012:pls006. https://doi.org/10.1093/aobpla/pls006
Cardoso JC, Habermann G (2014) Adventitious shoot induction from leaf segments in Anthurium andraeanumis affected by age of explant, leaf orientation and plant growth regulator. Hortic Environ Biotechnol 55:56–62. https://doi.org/10.1007/s13580-014-0022-9
Chen FC, Kuehnle AR (1996) Obtaining transgenic Anthurium through Agrobacterium-mediated transformation of etiolated internodes. J Amer Soc Hortic Sci 121:47–51
Chen FC, Kuehnle AR, Sugii N (1997)Anthurium roots for micropropagation and Agrobacterium tumefaciens-mediated gene transfer. Plant Cell Tiss Org Cult 49:71–74
Feder N, O’Brine TP (1968) Plant microtechnique: some principles and new methods. Am J Bot 55:123–142
Fiuk A, Bednarek PT, Rybczyński JJ (2010) Flow cytometry, HPLC-RP, and metAFLP analyses to assess genetic variability in somatic embryo-derived plantlets of Gentiana pannonica Scop. Plant Mol Biol Rep 28:413–420. https://doi.org/10.1007/s11105-009-0167-3
Gantait S, Mandal N, Bhattacharyya S, Das PK (2008) In vitro mass multiplication with pure genetic identity in Anthurium andreanum Lind. Plant Tiss Org Cult Biotech 18:113–122. https://doi.org/10.3329/ptcb.v18i2.3361
Gantait S, Sinniah UR (2011) Morphology, flow cytometry and molecular assessment of ex-vitro grown micropropagated Anthurium in comparison with seed germinated plants. Afr J Biotechnol 10:13991–13998. https://doi.org/10.5897/AJB11.1855
Gantait S, Sinniah UR, Mandal N, Das PK (2012) Direct induction of protocorm-like bodies from shoot tips, plantlet formation, and clonal fidelity analysis in Anthurium andreanum cv. CanCan. Plant Growth Regul 67:257–270. https://doi.org/10.1007/s10725-012-9684-4
Geier T (1986) Factors affecting plant regeneration from leaf segments of Anthurium scherzerianum Schott (Araceae) cultured in vitro. Plant Cell Tiss Org Cult 6:115–125
Ghimire BK, Chang YY, Chung IM (2012) Direct shoot organogenesis and assessment of genetic stability in regenerants of Solanum aculeatissimum Jacq. Plant Cell Tiss Org Cult 108:455–464. https://doi.org/10.1007/s11240-011-0057-x
Hosein FN, Lennon AM, Umaharan P (2012) Optimization of an Agrobacterium-mediated transient assay for gene expression studies in Anthurium andraeanum. J Amer Soc Hortic Sci 137:263–272. https://doi.org/10.21273/JASHS.137.4.263
Huang WJ, Ning GG, Liu GF, Bao MZ (2009) Determination of genetic stability of long-term micropropagated plantlets of Platanus acerifolia using ISSR markers. Biol Plant 53:159–163. https://doi.org/10.1007/s10535-009-0025-z
Jaccard P (1908) Nouvelles recherches sur la distribution florale.- Bul.Soc. Vaudoise. SciNat. 44:223–270
Jahan MT, Islam MR, Khan R, Mamun ANK, Hakim L (2014)In vitro clonal propagation of Anthurium (Anthurium andraeanum L.) using callus culture. Plant Tiss Org Cult Biotech 19:61–69. https://doi.org/10.3329/ptcb.v19i1.4961
Kuehnle AR, Chen FC, Sugii N (1992) Somatic embryogenesis and plant regeneration in Anthurium andraeanum hybrids. Plant Cell Rep 11:438–442
Kuehnle AR, Sugii N (1991) Callus induction and plantlet regeneration in tissue cultures of Hawaiian Anthuriums. Hortscience 26:919–921
Lata H, Chandra S, Techen N, Khan I, Elsohly M (2009) Assessment of the genetic stability of micropropagated plants of Cannabis sativa by ISSR markers. Planta Med 76:97–100. https://doi.org/10.1055/s-0029-1185945
Loureiro J, Rodriguez E, Dolezel J, Santos C (2007) Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann Bot 100:875–888. https://doi.org/10.1093/aob/mcm152
Mallón R, Rodríguez-Oubiña J, González ML (2010)In vitro propagation of the endangered plant Centaurea ultreiae: assessment of genetic stability by cytological studies, flow cytometry and RAPD analysis. Plant Cell Tiss Org Cult 101:31–39. https://doi.org/10.1007/s11240-009-9659-y
Martin KP, Joseph D, Madasser J, Philip VJ (2003) Direct shoot regeneration from lamina explants of two commercial cut flower cultivars of Anthurium andraeanum Hort. In Vitro Cell Dev Biol - Plant 39:500–504
Matsumoto TK, Kuehnle AR (1998) Zygotic embryogenesis in Anthurium (Araceae). Am J Bot 85:1560–1568
Matsumoto TK, Webb DT, Kuehnle AR (1996) Histology and origin of somatic embryos derived from Anthurium andraeanum Linden ex André Lamina. J Amer Soc Hortic Sci 121:404–407. https://doi.org/10.21273/JASHS.121.3.404
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Neto JDS, Soares TCB, Motta LB, Cabral PDS, Silva JA (2014) Molecular characterization of Anthurium genotypes by using DNA fingerprinting and SPAR markers. Genet Mol Res 13:4766–4775. https://doi.org/10.4238/2014.July.2.6
Paris AM, Pais MS (2000) Organogenesis from internode-derived nodules of Humulus lupulus var. Nugget (Cannabinaceae): histological studies and changes in the starch content. Am J Bot 87:971–979. https://doi.org/10.2307/2656996
Pierik RLM (1976)Anthurium andraeanum plantlets produced from callus tissues cultivated in vitro. Physiol Plant 37:80–82. https://doi.org/10.1111/j.1399-3054.1976.tb01876.x
Pierik RLM, Steegmans HHM, Van Der Meys JAJ (1974) Plantlet formation in callus tissues of Anthurium andraeanum Lind. Sci Hortic 2:193–198
Pinheiro MVM, Martins FB, Cruz ACFD, Carvalho ACPPD, Ventrella MC, Otoni WC (2013) Maturation of Anthurium andraeanum cv. Eidibel somatic embryos from nodal segments. In Vitro Cell Dev Biol - Plant 49:304–312. https://doi.org/10.1007/s11627-013-9522-z
Pinto G, Silva S, Neves L, Araújo C, Santos CO (2010) Histocytological changes and reserve accumulation during somatic embryogenesis in Eucalyptus globulus. Trees 24:763–769. https://doi.org/10.1007/s00468-010-0446-5
Rocha DI, Vieira LM, Tanaka FAO, Silva LCD, Otoni WC (2012) Somatic embryogenesis of a wild passion fruit species Passiflora cincinnata Masters: histocytological and histochemical evidences. Protoplasma 249:747–758
Tang QY, Zhang CX (2013) Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci 20:254–260
Teixeira DSJA, Dobránszki J, Winarto B, Zeng SJ (2015)Anthurium in vitro: a review. Sci Hortic 186:266–298. https://doi.org/10.1016/j.scienta.2014.11.024
Varshney A, Sangapillai R, Patil MS, Johnson TS (2011) Histological evidence of morphogenesis from various explants of Jatropha curcas L. Trees 25:689–694. https://doi.org/10.1007/s00468-011-0546-x
Venkat SK, Bommisetty P, Patil MS, Reddy L, Chennareddy A (2014) The genetic linkage maps of Anthurium species based on RAPD, ISSR and SRAP markers. Sci Hortic 178:132–137. https://doi.org/10.1016/j.scienta.2014.08.017
Vujović T, Cerović R, Ružić D (2012) Ploidy level stability of adventitious shoots of sour cherry ‘aanski Rubin’ and Gisela 5 cherry rootstock. Plant Cell Tiss Org Cult 111:323–333. https://doi.org/10.1007/s11240-012-0197-7
Wang GL, Chen C, Li ZX, Li W (2005) Establishment of rapid propagation system of aerial root segments of Anthurium andraeanum Linden in vitro. Plant Physiol Commun 41:297–301 (in Chinese)
Yang X, Hou Z, Ji J (2008) Effect of culture medium and temperature on the ratio of callus of Anthurium leaf. J Shenyang Agri Univ 39:15–18. https://doi.org/10.1007/s10499-007-9164-4 (in Chinese)
Ziegenhagen B, Scholz PG (1993) A procedure for mini-preparations of genomic DNA from needles of silver fir (Abies alba mill.). Plant Mol Biol Rep 11:117–121. https://doi.org/10.1007/BF02670469
Funding
The Natural Science Foundation of Tangshan Normal College (Grant No. 09A01) supported this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Editor: Ming Cheng
Rights and permissions
About this article
Cite this article
Zhang, H., Wang, G., Qiao, Y. et al. Plant regeneration from root segments of Anthurium andraeanum and assessment of genetic fidelity of in vitro regenerates. In Vitro Cell.Dev.Biol.-Plant 57, 954–964 (2021). https://doi.org/10.1007/s11627-021-10172-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-021-10172-6