Abstract
Cultivated caladiums (Caladium × hortulanum Birdsey) are popular ornamental plants. Although somaclonal variation occurs frequently in caladium during tissue culture, little research has been conducted on obtaining and detecting variants from long-term callus cultures. Herein, plants were regenerated from ‘Red Flash’ caladium calluses subcultured for approximately 40 mo, and 116 out of 520 established plants were grouped into 14 somaclonal variation types based on their morphological differences. Nuclear DNA content of six types (SVT1, SVT2, SVT4, SVT5, SVT8, and SVT10) varied from − 1.08% to 0.33% compared with the wild type, and these variants shared a similar chromosome number to the wild caladium (2n = 2x = 30). Three types (SVT3, SVT7, and SVT9) containing 2.82 to 5.42% less nuclear DNA content was the result of losing one or two chromosomes, and one type (SVT6) with significantly lower cellular DNA content was due to losing four chromosomes. Four types (SVT11–SVT14) contained 85.16 to 101.52% more DNA content and the SVT12 and the SVT13 had a double number of chromosomes (2n = 4x = 60), while the SVT11 and SVT14 had four more chromosomes and six less chromosomes as compared with a typical tetraploid, respectively. Correlation analysis suggested that leaf thickness, leaf index, and stomatal characteristics could be used as indicators of plant ploidy in caladium. A wide variation of pigment content was found among the variation types, and the content of chlorophyll, flavonoid, and anthocyanin had a significant positive correlation with the color parameters a* and b*. Leaf color variants created by prolonged in vitro callus cultures might hold great promise for caladium breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultivated caladium (Caladium × hortulanum Birdsey), a perennial herbaceous plant of the Araceae family, is an important foliage plant often planted in containers or landscape valued for its colorful and variable-shaped leaves (Wilfret 1993). In order to develop new cultivars with enhanced foliar characteristics, plant growth habit, petiole strength, tuber yield, light adaptability, disease and pest resistance, and chilling tolerance, sexual hybridization between elite cultivars, breeding lines, and/or species has been extensively carried out in caladium (Wilfret 1993; Deng 2012, 2018). However, this breeding system has been challenged by the loss of genetic diversity in breeding populations after decades of intensive hybridization and selection (Deng 2012, 2018). An efficient and reliable approach is therefore necessary with the aim to produce diverse variations for caladium breeding.
Plant tissue culture techniques offer an alternative of massive vegetative propagation and germplasm conservation of a variety of plant species within a limited space. However, a number of factors such as regeneration system, explant source, medium components, or duration and number of subcultures may induce variations of morphology, cytology, biochemistry, genetics, and epigenetics in regenerated plants (Bairu et al. 2011; Sarmah et al. 2017). This phenomenon of unexpected and random spontaneous variation during tissue culture is widely known as somaclonal variation. To detect true somaclonal variants, numerous methods such as morphological comparison, cytological analysis, physiological/biochemical detection, and molecular characterization have been extensively used in many plant species (Bairu et al. 2011; Sato et al. 2011; Sarmah et al. 2017; Żabicki et al. 2019) including caladium (Cao et al. 2016a). Although somaclonal variation has a negative effect on producing true-to-type plants in an in vitro culture system, it could be an important source of genetic variability for crop improvement (Krishna et al. 2016; Sarmah et al. 2017). In recent years, somaclonal variation has been successfully applied in ornamental plants to improve flower color, flowering period, leaf color, plant type, or other ornamental traits (Sato et al. 2011; Cao et al. 2016a; Sarmah et al. 2017).
Propagation of caladium has been realized through in vitro culture of meristematic tissues, leaf segments, or petiole segments (Ahmed et al. 2004; Thongpukdee et al. 2010; Cai and Deng 2016; Cao et al. 2016a; Zhang et al. 2019). Unexpectedly, it has been reported that somaclonal variation is common in the plants derived from in vitro culture of caladium, and observed variations include changes in leaf color characteristics, leaf shapes, petiole color, and petiole attachment (Ahmed et al. 2004; Thepsithar et al. 2010; Thongpukdee et al. 2010; Cao et al. 2016a). Furthermore, plant growth regulators used in the medium and explant types have been confirmed to show a remarkable influence on the occurrence of leaf color variants in caladium (Ahmed et al. 2004; Thongpukdee et al. 2010; Cao et al. 2016a). The mechanisms of somaclonal variation in caladium have been studied by Cao et al. (2016a) at the morphological, cytological, and molecular level, and the results suggested that chromosome losses and gains, chromosome doubling, and alterations at the DNA level might lead to the high frequency of somaclonal variants of ‘Red Flash’ caladium.
A number of early reports suggested that increasing subculture numbers and duration could increase the somaclonal variation rate, especially long-term subcultured callus and cell suspension cultures (Bairu et al. 2011; Sarmah et al. 2017). Although leaf color variation occurred at a relatively high frequency in caladium, production and characterization of somaclonal variants by in vitro culture of long-term subcultured callus are not available. Thus, the objectives of this study were to (1) evaluate the types and frequencies of leaf color variants regenerated from ‘Red Flash’ caladium callus after subculturing over 40 mo; (2) characterize the morphological features, stomatal parameters, nuclear DNA content, chromosome number, and pigment contents of the established variation plants; and (3) further analyze the correlation among leaf traits, stomata characteristics and nuclear DNA content, and the correlation between the pigment content and the color parameters. Results of this research should be helpful for further leaf color breeding in caladium, other aroids and ornamental plants.
Materials and methods
Plant establishment and visual screening of leaf color variants
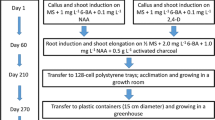
‘Red Flash’ caladium callus was induced previously according to Cai and Deng (2016), and subcultured twice a month on Murashige and Skoog (MS) (Murashige and Skoog 1962) basal medium containing 2 mg L−1 TDZ (Thidiazuron) and 2 mg L−1 NAA (1-Naphthaleneacetic acid). After approximately 20 cycles of culture, the callus was transferred onto MS medium containing 1 mg L−1 NAA and 0.1 mg L−1 6-BA (6-Benzyladenine) for plant regeneration. MS basal salts, TDZ, NAA, and BA were all obtained from PhytoTechnology Laboratories (Shawnee Mission, KS). Callus induction and subculture were conducted in the dark at 25 ± 1 °C, and plant regeneration was performed at 25 ± 1 °C and 14 h/10 h (light/dark) cycles under cool white fluorescent lamps of about 44 μmol m−2 s−1. For acclimatization and transplantation, well-rooted plantlets were taken from the culture medium and carefully washed under tap water to remove the residual medium, and then transplanted individually to cell trays (32 cells per tray, 6 × 6 × 11 cm per cell) filled with a commercial potting mix (Nursery Soil Mixture, Jiangsu, Jiangsu Peilei Biotechnology CO., LTD., Xuzhou, China) and cultured in an artificial climate chamber at an ambient temperature between 26 °C and 28 °C, a light level of approximately 66 μmol m−2 s−1 and 70 to 75% relative humidity. Three mo after transplantation, all plants were visually examined every week and any plants exhibiting obviously different morphological traits from the wild type were flagged and monitored closely. When the plants were 5-mo-old, all potential variants and 4 wild plants were transplanted into plastic pots (15 cm in diameter and 17.5 cm in height) for further growth. All the plants were cultured under the similar conditions as described above. One year after transplantation to the pots, the plants with stable morphological difference from the wild caladium were used for the following study.
Morphological characterization and color measurement
Plant height, leaf length, and leaf width of the variant and wild plants were measured by a stainless steel ruler. Leaf thickness was measured at three points on the edge of each leaf by an electronic vernier caliper, and petiole diameter was taken in the middle part of each petiole. Leaf color characteristics were measured by a portable colorimeter with a C light source (CR-10, Konica Minolta, Osaka, Japan) according to the instruction of the manufacturer. Prior to color measurement, the colorimeter was calibrated against a white standard calibration plate to ensure reliability. At least three mature leaves per plant were used for morphological analysis and leaf color measurement.
Comparison of stomatal characteristics
Stomata length, width, and density were measured according to Cai et al. (2015). Briefly, nail polish imprints were taken from the abaxial surface of the mature leaves and placed on glass cover slips, and then photographed under a light microscope (Panthera L, Motic, Xiamen, Fujian, China). Ten independent counts were made on each plant.
Analysis of nuclear DNA content
Nuclear DNA content in the variants and the wild plants was measured by a CytoFLEX flow cytometer (Beckman Coulter, Indianapolis, IN), and Oryza sativa ssp. Japonica ‘Nipponbare’ (0.90 pg/2C) (Marie and Brown 1993) was used as the internal standard. Approximately 35 mg of fresh leaves (avoiding the main veins) were rapidly chopped with a sharp single-sided blade in a Petri dish containing 300 μL of cold woody plant buffer (WPB) developed by Loureiro et al. (2007), and then filtered through a 40-μm nylon mesh into a sample loading tube. Thereafter, 300 μL of the stock solution (50 μg mL−1) containing propidium iodide (PI) and RNase A (Yuan Ye Bio-Technology Co. Ltd., Shanghai, China) were added to the sample tube and kept in the dark for 30 min, and then shaken gently for 5 s before sampling on the flow cytometer. At least three runs were carried out for each sample, and at least 3000 nuclei were counted in each run. Nuclear DNA content of caladium samples = nuclear DNA content of ‘Nipponbare’ rice × (mean fluorescence value of caladium samples ÷ mean fluorescence value of ‘Nipponbare’ rice).
Chromosome counting
Chromosome observation was performed according to the method described by Cao et al. (2016a) with minor modification. Vigorously growing root tips (about 1-cm long) were collected and instantly immersed in 0.002 M 8-hydroxyquinoline solution, and maintained in this solution for at least 4 h in darkness. Pretreated root tips were rinsed thoroughly under running water for 10 min and then fixed in freshly prepared Carnoy’s fixative solution (3 methanol: 1 glacial acetic acid, v/v) at 4 °C for 24 h. After fixation, the roots were washed with running water for 10 min, and then hydrolyzed with 1-N HCl solution in a water bath at 60 °C for 5 min. Thereafter, the macerated root tips were rinsed with deionized water three times and then transferred onto a glass microscope slide, root cap isolated with a scalpel and stained in a drop of carbol fuchsin stain (Solarbio, Beijing, China) for 10 min. Finally, the root cap tissue was covered with a cover slide and pressed lightly to spread the stained cells, and cells with darkly stained and well-spread chromosomes were photographed at 1000 times magnification under a bright field microscope (Panthera L, Motic, China).
Pigment content
Content of the total chlorophyll, flavonoids, and anthocyanins in the interveinal areas and leaf margins of the mature leaves was non-invasively estimated by a portable Dualex 4 sensor (ForceA, Orsay, France) following the instruction of the manufacturer, and shown in units of μg cm−2 according to Cerovic et al. (2012). Three leaves were measured for each plant, and the experiment was repeated twice.
Data analysis
Data were analyzed statistically by the SPSS 23.0 software package. Significant differences of all measured parameters were analyzed by one-way analysis of variance (ANOVA), and correlations among the different parameters were analyzed using Pearson’s correlation. The data were expressed as mean value ± standard deviation.
Results
Detection of somaclonal variation and variant types
A total of 520 plants regenerated from the long-term cultured callus were established in artificial climate chambers. The plants were closely observed 3 mo after transplantation to identify morphological variants and this procedure was repeated again 1 yr after transplantation to the plastic pots to ensure stable morphological changes of selected plants. Finally, 116 normal growing plants were identified as somatic variants for they showed a remarkable level of morphological difference compared with the wild type during the course of plant development, with the variation rate as high as 22.31% (Table 1). Based on visual screening, morphological analysis, and color measurements, these plants could be classified into 14 somaclonal variant types, i.e., SVT1 through SVT14 as shown in Figs. 1 and 2, Tables 1 and 2.
Typical leaves of the wild type and 14 somaclonal variation types (SVT1–SVT14) of ‘Red Flash’ caladium (Caladium × hortulanum Birdsey) regenerated from long-term subcultured callus. The photos of the leaves were taken 1 year after the regenerated plants were established in plastic pots filled with a commercial potting mix and grown in an artificial climate chamber. Scale bar 5 cm.
Remarkable differences were found in the morphological characteristics (Figs. 1 and 2, and Table 1) and color parameters (Table 2) among the different types of variant plants. The plant height of the wild caladium plants was the highest, and each heart-shaped leaf was characterized with red main veins, white and red-purple spots between veins, and yellow-green margins. Leaves of the SVT1, SVT2, and SVT3 were drastically different from the wild type, with green main veins and leaf margins, and the plant number of the three variant types accounted for 66.38% (77/116) of all the variant plants. It seemed that the three types shared most of the leaf coloration patterns, while the results of color measurements showed significant difference among them. In the CIE L*, a*, and b* color representation system, the L* value is defined as an indicator of lightness on a numerical scale ranging from 0 (black) to 100 (white). The color parameters a* and b* vary from − 60 to 60, and negative a* represents green and positive a* indicates red, whereas negative b* indicates blue and positive b* is for yellow (Lancaster et al. 1997). The leaf margins, interveinal areas, leaf spots, and main veins of the SVT2 and SVT3 were all characterized by a negative value for parameter a*, indicating that the leaves of the SVT2 and SVT3 have a green color, which was consistent with the visual observation results. The main veins of the rest of the variant types all exhibited a red color, while their leaf color patterns were obviously different from each other. The leaves of the SVT4 were significantly smaller with light-green and curly leaf margins when compared with the wild type, and their leaf number per plant reached the highest among the variants. The SVT5 had small leaf spots and light-green leaf margins, and their leaf index (2.01) was the greatest among the variants and wild plants. Moreover, the parameters L* of all parts in the SVT5 were relatively high, which suggested that the leaves of the SVT5 had a higher color brightness. The leaves of the SVT6 were almost red except for the yellow-green leaf margins and small leaf spots, as was described evidently by parameters L*, a*, and b*. The SVT7 was characterized by medium plant height, leaf length and width, red interveinal areas, and lack of leaf spots, and their leaf thickness (0.21 mm) was significantly less than other plants. A large number of small and red-purple spots were observed in the leaves of the STV8, and they had broad leaves (20.4 cm in length and 12.1 cm in width) with rugose leaf blades. The leaves of the SVT9 were characterized with pink interveinal areas, fewer spots and wavy leaf margins, and the mean leaf number of the SVT9 reached 7.3, which was significantly higher compared with most of the variants. The leaves of the SVT10 had deep red main veins and dark green leaf margins, as could be explained by a much higher a* value in the main veins (39.71) and a lower b* value measured from the leaf margins (15.67). Unlike the wild type and the other types of variants, the SVT11–SVT14 all shared rounder and thicker leaves. Especially for the STV13, the mean leaf thickness reached 0.42 mm, significantly higher than those of the other variants and the wild type. Among the four variant types, the SVT12 had the largest leaves with slender spots, but they grew very slowly and the plant height (15.6 cm) was the least among all the analyzed plants.
Stomatal characteristics
Stomatal characteristics of the variants and the wild type were presented in Fig. 3 and Table 3. For the SVT11–SVT14, it was observed that these plants had significantly larger stomatal guard cell length and significantly reduced stomatal density as compared with the other variation types and the wild-type plants. The largest size of stomata was observed in the SVT11, and it was 39.8% longer and 15.3% wider than that of the wild type. The minimum stomatal density was also observed in the SVT11, which was only 36.5% of that of the wild caladium. Wide differences of stomatal characteristics were also observed between the other types and the wild caladium, especially for the stomatal guard cell width. The stomatal guard cell length of the wild type and the other variation types varied from 19.0 μm to 22.1 μm, while it varied from 10.8 μm to 17.6 μm for the mean stomatal guard cell width. The highest stomatal density was observed in the SVT2 with significantly smaller stomata compared with the wild type.
Nuclear DNA content
As shown in Fig. 4, the wild type and all the variation types showed one main peak of relative fluorescence intensity, indicating that they were not chimeras or mixoploids. The 2C peak of the diploid wild caladium was situated at a value of about 100 in the histogram, and the variants including the SVT1–SVT10 all had a main peak at the diploid area, suggesting they appeared to be diploids. The 2C peak of the SVT11–SVT14 was at about 200, which was double that of the wild type, indicating that they were potential tetraploids.
Extensive nuclear DNA content variation from 7.77 pg per 2C (SVT6) to 18.60 pg per 2C (SVT11) was observed among the 14 variation types, which was equivalent to 15.82% reduction and 101.52% increase compared with the wild type, respectively (Table 3). The nuclear DNA content in six variation types (SVT1, SVT2, SVT4, SVT5, SVT8, and SVT10) was very similar to that of the wild-type and nuclear DNA content change compared with the wild type varied from − 1.08% to 0.33%. Three variant types including the SVT3, SVT7, and SVT9 had 2.82%, 5.42%, and 5.31% lower DNA content compared with the wild caladium, respectively. The 2C DNA content in the SVT6 plants was only 7.77 pg, significantly lower than those in other types of variants and the wild type. Surprisingly, the SVT11, SVT12, SVT13, and SVT14 showed increased DNA content by 101.52%, 93.72%, 93.28%, and 85.16% compared with the wild type, respectively.
Correlation analysis among leaf traits, stomatal characteristics, and nuclear DNA content
A correlation analysis was performed to further understand the relationship among the leaf traits, stomatal characteristics, and nuclear DNA content of the variants and the wild type. Significant correlations were found among all the analyzed variables (Table 4). The nuclear DNA content was positively correlated with leaf thickness and stomatal guard cell length and width, with a correlation coefficient of 0.942, 0.919, and 0.776, respectively, while it was negatively correlated with leaf index (− 0.795) and stomatal density (− 0.929). These results indicated that the leaf traits (leaf thickness and leaf index) and the stomatal characteristics (guard cell length and width, and stomatal density) could be used as an indicator of plant ploidy in caladium plants.
Chromosome counts
To further verify the ploidy level of the established variants, chromosomes of the wild caladium and 14 types of somaclonal variation were counted as shown in Fig. 5 and Table 3. At least 20 cells with well-spread chromosomes were observed for the other variation types and wild type except the SVT12 and SVT13, and 5–8 cells with well-extended chromosomes were photographed as the two variation types grew slowly, thus not providing enough actively growing roots. The wild ‘Red Flash’ had 30 chromosomes as previously reported by Cao et al. (2016a). The chromosome numbers of the six variation types including SVT1, SVT2, SVT4, SVT5, SVT8, and SVT10 were similar to the wild type (2n = 2x = 30), which was consistent with their nuclear DNA content. Aneuploidy was observed in 4 variation types, i.e., the SVT3, SVT6, SVT7, and SVT9. SVT3 had a 2.82% decrease in nuclear DNA due to the loss of one chromosome (2n = 2x – 1 = 29) and the two variants, SVT7 and SVT9, containing 5.31 and 5.42% had less nuclear DNA due to the loss of two chromosomes (2n = 2x – 2 = 28), respectively. SVT6 lost four chromosomes (2n = 2x – 4 = 26), which was confirmed by a significantly lower nuclear DNA content as compared with the wild type. Among the four variation types that contained 85.16 to 101.52% more nuclear DNA content, both of the SVT12 and the SVT13 had a double number of chromosomes (2n = 4x = 60) as compared with the wild type, while the SVT11 had four more chromosomes than expected (2n = 4x + 4 = 64) and the chromosome number of the SVT14 was six less (2n = 4x – 6 = 54) than that of a typical tetraploid.
Micrographs of somatic chromosomes in the root tips of the wild type and seven variation types of ‘Red Flash’ caladium (Caladium × hortulanum Birdsey) regenerated from long-term subcultured callus. Photographs were taken under a bright field microscope at × 100 magnification. (a) SVT6 (2n = 2x – 4 = 26), (b) SVT7 (2n = 2x – 2 = 28), (c) SVT3 (2n = 2x – 1 = 29), (d) wild type (2n = 2x = 30), (e) SVT1 (2n = 2x = 30), (f) SVT14 (2n = 4x – 6 = 54), (g) SVT13 (2n = 4x = 60), (h) SVT11 (2n = 4x + 4 = 64). Scale bars 10 μm.
Content of chlorophyll, flavonoid, and anthocyanin
A wide variation of pigment content was measured by Dualex 4 in the interveinal areas and leaf margins of the variants and wild plants (Table 5). Significantly higher chlorophyll content was detected in the interveinal areas of the three variation types with the green main vein (SVT1 through SVT3), while their flavonoid and anthocyanin content was opposite, with significantly lower levels compared with the wild caladium and most of the other variants. The variation of the anthocyanin content in the interveinal areas of all the analyzed variant types and the wild type showed a similar trend to that of the flavonoid content. The chlorophyll content in the leaf margins of all analyzed plants were correspondingly higher than that in the interveinal areas, and the variation ranges of both the anthocyanin and flavonoid content in the leaf margins were much smaller as compared with the interveinal areas. The highest chlorophyll content in leaf margins was recorded in the SVT14 with a dark green leaf edge. The yellow-green leaf margins of the SVT6 characterized by red interveinal areas and main veins which contained the maximum content of both flavonoid (1.11 μg cm−2) and anthocyanin (0.68 μg cm−2), and the lowest content of chlorophyll (9.12 μg cm−2). From the above analysis, it could be seen that the pigment content in the interveinal areas and leaf margins played an important role in regulation of the leaf color of caladium.
Correlations between the pigment content and the color parameters
Results of correlation analysis between the pigment content and the color parameters in the interveinal areas of the wild type and the variants were shown in Table 6. The content of chlorophyll, flavonoid, and anthocyanin was all significantly correlated with a* and b* and had no significant correlation with L*. The chlorophyll content was shown to have a negative and positive correlation with a* and b*, respectively. Both the flavonoid content and the anthocyanin content were positively correlated with a* and negatively correlated with b*. Moreover, an extremely significantly negative correlation was found between the chlorophyll content and the flavonoid or anthocyanin content, while the flavonoid content had an extremely significantly positive correlation with the anthocyanin content. Similar correlation results were also found in the leaf margins (data not shown).
Discussion
This study comprehensively analyzed morphological, cytological, and physiological characteristics of somaclonal variation from long-term subcultured callus of ‘Red Flash’ caladium. Out of 520 established plants, 116 showed stable morphological differences in the leaf color patterns from the wild type, and they could be grouped into 14 somaclonal variation types including three types (SVT1 through SVT3) having green veins, four types (SVT11 through SVT14) showing rounder and thicker leaves, two types (SVT8 and STV9) exhibiting rugose leaf blades, and one type (SVT7) lacking of leaf spots (Table 1, Fig. 1, and Fig. 2). The variation types found in this study were more abundant as compared with the study on somaclonal variation from leaf culture of ‘Red Flash’ caladium by Cao et al. (2016a), where 24 out of 208 plants were identified as somaclonal variants and grouped into 10 types. Increasing subculture times and subculture duration enhances the occurrence of somaclonal variation, especially cell suspension and callus cultures (Bairu et al. 2011; Sarmah et al. 2017). Results of this study also found that using the long-term subcultured callus as initial explants greatly increased the rate and types of somaclonal variation in caladium.
Phenotypic and genetic changes which resulted from somaclonal variation can be detected by morphological, cytological, physiological/biochemical, and molecular methods (Bairu et al. 2011; Sato et al. 2011; Sarmah et al. 2017; Żabicki et al. 2019). Leaf color is an important phenotypic trait in ornamental plants, and the Royal Horticultural Society (RHS) Color Chart has been often used to describe leaf color changes in caladium (Cai et al. 2015; Cao et al. 2016a). In this study, leaf color changes of the variants and wild type were measured by a portable colorimeter CR-10 in a simpler and quicker way compared with the conventional RHS color chart. Flow cytometry analysis and chromosome counts were usually conducted to have a better understanding of nuclear DNA content variations and chromosome aberrations in somaclonal variants, respectively. Previous reports suggested that stomatal length and frequency could be used as a measure of plant ploidy level (Beck et al. 2003). In this study, significant correlations were found among leaf traits, stomatal characteristics, and nuclear DNA content and suggested that the leaf thickness, leaf index, stomatal guard cell length and width, and stomatal density could be used as indicators of plant ploidy level in caladium (Table 4). Cytological instability has been demonstrated in cells grown in vitro (Bairu et al. 2011), and prolonged period of tissue culture will increase the frequency of gross chromosomal aberrations (Kumar and Mathur 2004). In this study, cytogenetic analysis also revealed that chromosomal loss, gain, or doubling occurred in the regenerated plants consisting of six diploids, two tetraploids, and six aneuploids (including diploid and tetraploid aneuploids). Chromosome numbers of most of the variant types were generally consistent with the measured nuclear DNA content, while the DNA content of several variation types, especially the SVT12 and SVT13 with 60 chromosomes, was not doubled compared with the wild type (Fig. 4 and Table 3). These results suggested that chromosome structural change(s) such as chromosome segment deletion might participate in the formation of the related variants, and karyotype analysis and in situ hybridization technique should be adopted to confirm this phenomenon in the future.
Chlorophyll, carotenoid, and flavonoid (mainly anthocyanin) are three main pigments in leaves, flowers, and fruits in higher plants. Flavonoid, especially anthocyanin accumulation, is a major pigment responsible for formation of red leaves (Feild et al. 2001), and changes of leaf coloration is generally considered to be associated with changed pigment composition and content (Li et al. 2018). In this study, the content of chlorophyll, flavonoid, and anthocyanin in the leaves of the caladium variants was evaluated by a portable Dualex 4 sensor, a device used in non-destructive estimation of chlorophyll, flavonoid, and anthocyanin content (Table 5). The measured content was all significantly correlated with a* and b*, two color parameters determining color differences from green to yellow and shades of red, respectively, indicating that the Dualex 4 sensor was reliable and efficient in evaluating the pigment content in caladium. The non-destructive method of pigment content analysis has also been applied in citrus (Hussain et al. 2012), chili pepper (Rodríguez-Calzada et al. 2019), and several other dicots or monocots (Cerovic et al. 2012). Significantly higher chlorophyll content was found in the interveinal areas of the three variation types with green main veins (SVT2 through SVT4), and the leaves of the three types were almost green, as could be explained by the higher chlorophyll content versus the lower flavonoid and anthocyanin in the leaves.
Wild ‘Red Flash’ caladium is a fancy heart-shaped leaf cultivar characterized with red main veins, red-purple spots, and non-rugose leaf. In this study, green main veins and absence of spots and rugose leaves were presented in the established plants (Fig. 2). The color of caladium main veins is considered to be controlled by three alleles Vr (red), Vw (white), and Vg (green) at a single locus (V), and the dominance order is Vr > Vw > Vg (Deng and Harbaugh 2006). Wild ‘Red Flash’ caladium has a genotype of VrVg (Deng and Harbaugh 2006). In this study, green main veins were observed in the SVT2 and SVT4 plants without changing in chromosome number, and in the SVT3 with reduced chromosome, as might be due to the loss of Vr allele or the mutation of Vr to Vg. Leaf spots are controlled by a single locus with two alleles (S and s), and the non-spotted caladium has a homozygous recessive genotype ss (Deng et al. 2008). The SVT7 lost two chromosomes and had no leaf spots, as might result from a mutation from SS to ss or Ss to ss. Rugose leaves are an intriguing trait in plants and can increase their ornamental value. Rugose or non-rugose phenotype is determined by two alleles at one locus in caladium, i.e., dominant allele RLF and recessive allele rlf controlling rugose and non-rugose, respectively (Cao et al. 2016b). In this study, both the SVT8 and the SVT9 expressed large irregular depression or elevated areas ridge on both sides of their leaf blades, as indicated that a mutation from rlf to RLF might occur during the callus induction, callus preservation, or plantlet regeneration. These speculations still need more robust evidence to be supported.
Somaclonal variation provides great promise for crop improvement and has greatest potential for foliage plants as the value of these plants largely depends on their various leaf colors or leaf shapes (Sarmah et al. 2017; Deng 2018). In this study, several somaclonal variation events showed improved esthetic values such as nearly 100% red leaves (SVT6 and SVT7), increased leaf number and relatively short and compact plant architecture (SVT9), and rugose leaf blades (SVT8 and SVT9) (Table 1 and Fig. 2). Chromosome doubling was also found among the plants exhibiting somaclonal variation, the SVT11–SVT14 (Table 3 and Fig. 5).In addition, although several variant types characterized with improved ornamental values were aneuploids (SVT6 and SVT7; Table 3 and Fig. 5), they could maintain their genetic identity by asexual propagation using tubers. Aneuploids can lead to changes in global gene expression, gene structure, and phenotype in organisms (Huettel et al. 2008), thus providing a powerful tool for plant genetic study and chromosome engineering in caladium.
Conclusions
The results of this study showed that somaclonal variation occurred frequently when long-term subcultured calluses were used as the initial materials in micropropagation of ‘Red Flash’ caladium. Several cytological changes including chromosome loss, chromosome gain, and polyploidization seemed to be involved in the origin of somaclonal variation. The chlorophyll, flavonoid, and anthocyanin content in leaves had a great influence on the leaf color patterns of the variants and the wild caladium. A number of the variants with improved ornamental characteristics should be valuable for future caladium cultivar development and genetic studies.
References
Ahmed EU, Hayashi T, Yazawa S (2004) Auxins increase the occurrence of leaf-colour variants in caladium regenerated from leaf explants. Sci Hortic 100:153–159
Bairu MW, Aremu AO, Van Staden J (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Regul 63:147–173
Beck SL, Dunlop RW, Fossey A (2003) Stomatal length and frequency as a measure of ploidy level in black wattle, Acacia mearnsii (de wild). Bot J Linn Soc 141:177–181
Cai XD, Cao Z, Xu SX, Deng Z (2015) Induction, regeneration and characterization of tetraploids and variants in ‘tapestry’ caladium. Plant Cell Tiss Org Cult 120:689–700
Cai XD, Deng Z (2016) Thidiazuron promotes callus induction and proliferation in Caladium x Hortulanum Birdsey UF-4609. Propag Ornam Plant 16:90–97
Cao Z, Sui SZ, Cai XD, Yang Q, Deng Z (2016a) Somaclonal variation in ‘Red Flash’ caladium: morphological, cytological and molecular characterization. Plant Cell Tiss Org Cult 126:269–279
Cao Z, Sui SZ, Yang Q, Deng Z (2016b) Inheritance of rugose leaf in caladium and genetic relationships with leaf shape, main vein color, and leaf spotting. J Am Soc Hortic Sci 141:527–534
Cerovic ZG, Masdoumier G, Ghozlen NB, Latouche G (2012) A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol Plant 146:251–260
Deng Z (2012) Caladium genetics and breeding: recent advances. Floric Ornam Biotechnol 6:53–61
Deng Z (2018) Caladium. In: Van Huylenbroeck J (ed) Ornamental crops, Handbook of plant breeding, vol 11. Springer, Cham, pp 273–299
Deng Z, Goktepe F, Harbaugh BK (2008) Inheritance of leaf spots and their genetic relationships with leaf shape and vein color in caladium. J Am Soc Hortic Sci 133:78–83
Deng Z, Harbaugh BK (2006) Independent inheritance of leaf shape and main vein color in caladium. J Am Soc Hortic Sci 131:53–58
Feild TS, Lee DW, Holbrook NM (2001) Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiol 127:566–574
Huettel B, Kreil DP, Matzke M, Matzke AJ (2008) Effects of aneuploidy on genome structure, expression, and interphase organization in Arabidopsis thaliana. PLoS Genet 4(10):e1000226
Hussain S, Curk F, Dhuique-Mayer C, Urban L, Ollitrault P, Luro F, Morillon R (2012) Autotetraploid trifoliate orange (Poncirus trifoliata) rootstocks do not impact clementine quality but reduce fruit yields and highly modify rootstock/scion physiology. Sci Hortic 134:100–107
Krishna H, Alizadeh M, Singh D, Singh U, Chauhan N, Eftekhari M, Sadh RK (2016) Somaclonal variations and their applications in horticultural crops improvement. 3. Biotech 6:54
Kumar PS, Mathur VL (2004) Chromosomal instability in callus culture of Pisum sativum. Plant Cell Tiss Org Cult 78:267–271
Lancaster JE, Lister CE, Reay PF, Triggs CM (1997) Influence of pigment composition on skin color in a wide range of fruit and vegetables. J Am Soc Hortic Sci 122:594–598
Li WX, Yang SB, Lu ZG, He ZC, Ye YL, Zhao BB, Wang L, Jin B (2018) Cytological, physiological, and transcriptomic analyses of golden leaf coloration in Ginkgo biloba L. Hortic Res 5:12
Loureiro J, Rodriguez E, Doležel J, Santos C (2007) Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann Bot 100:875–888
Marie D, Brown SC (1993) A cytometric exercise in plant DNA histograms with 2C values for 70 species. Biol Cell 78:41–51
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Rodríguez-Calzada T, Qian M, Strid Å, Neugart S, Schreiner M, Torres-Pacheco I, Guevara-González RG (2019) Effect of UV-B radiation on morphology, phenolic compound production, gene expression, and subsequent drought stress responses in chili pepper (Capsicum annuum L.). Plant Physiol Biochem 134:94–102
Sarmah D, Sutradhar M, Singh BK (2017) Somaclonal variation and its application in ornamentals plants. Int J Pure App Biosci 5:396–406
Sato M, Kawabe T, Hosokawa M, Tatsuzawa F, Doi M (2011) Tissue culture-induced flower-color changes in Saintpaulia caused by excision of the transposon inserted in the flavonoid 3′, 5′ hydroxylase (F3′5′H) promoter. Plant Cell Rep 30:929–939
Thepsithar C, Thongpukdee A, Chiensil P (2010) Micropropagation of Caladium bicolor (Ait.) vent. ‘Thep Songil’ and incidence of somaclonal variants. Acta Hortic 855:273–280
Thongpukdee A, Thepsithar C, Chiensil P (2010) Somaclonal variation of Caladium bicolor (Ait.) vent. ‘Jao Ying’ after in vitro culture propagation. Acta Hortic 855:281–288
Wilfret GJ (1993) Caladium. In: de Hertogh A, le Nard M (eds) The physiology of flower bulbs. Elsevier Amsterdam, pp 239–247
Żabicki P, Sliwinska E, Mitka J, Sutkowska A, Tuleja M, Migdałek G, Żabicka J, Słomka A, Kwiatkowska M, Kuta E (2019) Does somaclonal variation play advantageous role in conservation practice of endangered species?: comprehensive genetic studies of in vitro propagated plantlets of Viola stagnina Kit. (Violaceae). Plant Cell Tiss Org Cult 136:339–352
Zhang YS, Gu SJ, Chen JJ, Cai XD (2019) Effects of different nutrient solutions on the acclimatization of in vitro Caladium plantlets using a simplified hydroponic system. Sains Malays 48:1627–1633
Acknowledgments
We are grateful to Dr. Zhanao Deng (Gulf Coast Research and Education Center, University of Florida, Wimauma, Florida, United States of America) for providing the ‘Red Flash’ caladium.
Funding
This study was funded in part by the Scientific Research Project of Hubei Education Department of China (No. B2018024).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editor: Neftali Ochoa-Alejo
Rights and permissions
About this article
Cite this article
Chen, JJ., Zhang, YS., Duan, JX. et al. Morphological, cytological, and pigment analysis of leaf color variants regenerated from long-term subcultured caladium callus. In Vitro Cell.Dev.Biol.-Plant 57, 60–71 (2021). https://doi.org/10.1007/s11627-020-10106-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-020-10106-8