Abstract
When constrained by in vitro culture conditions, microspores from Opuntia ficus-indica (L.) Mill. (Barbary fig) anthers were forced to stall out their gametophytic pathway and switch towards androgenesis. Five microspore stages were characterized based on cellular architecture. A relationship was also established between anthers and bud flower features. Anthers were cultured on three culture media containing 2,4-dichlorophenoxyacetic acid, thidiazuron and gibberellic acid at 22°C for 4 wk, followed by heat shock treatment at 32°C or 42°C for 2 wk, and the stressed material was recultured at 22°C for 24 wk in the dark. When anthers were heat shocked at 32°C and 42°C, the cultivated anthers swelled and burst, followed by the microspores flowing onto the explant surface. Androgenesis occurred directly and indirectly at uni- and binucleate stages. The micromorphology of multicellular, proembryos, globular, torpedo and cotyledonary-like-shaped structures from dehisced anthers was confirmed by environmental scanning electron microscopy. Furthermore, at 42°C, microscopic analysis demonstrated that the non-responsive microspores achieved pollen maturation and more rarely, the emission of a pollen tube. However, 11.4 to 14.4% of the mature pollen grains were converted into pollen embryos. The established system may serve, for the first time, as a protocol to produce microspore embryos in O. ficus-indica. Nevertheless, more efforts are needed to complete the development of diplo-haploid plantlets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cactaceae is a family of dicotyledonous perennial plants with specialized features that are adapted for survival in arid and semi-arid regions in several zones including Middle East, North Africa, South America and the USA (Felker and Inglese 2003). Opuntia ficus-indica (L.) Mill., which belongs to the Cactaceae family, has several common names, including prickly pear, Barbary fig, nopal cactus or cactus pear (Mondragon and Bordelon 1996). Interest in this plant species is due to its ability to produce forage, fruit and vegetables (Russell and Felker 1987). This plant species is also used as part of an environmental strategy in arid areas to avoid the disastrous repercussions of prolonged and repetitive periods of drought and soil degradation caused by erosion and desertification (Nefzaoui et al. 2014).
Plant breeders have consistently tried to develop crop cultivars with improved yields, quality and tolerance to biotic and abiotic stresses (Shuro 2017). However, conventional techniques used by breeders are difficult to achieve for certain perennial plant species. Plant breeders have adopted advanced biotechnology tools to better manage time and efforts deployed for crop improvement (Germaná 2006). Biotechnological methods are usually applied with success, as these tools can facilitate a rapid production of plants with new traits (Angulo-Bejarano et al. 2019).
One of the most crucial biotechnological processes used in fruit breeding was haploid and double haploid production (Shuro 2017). Androgenesis is based on in vitro culture of the whole male organ, or isolated microspores in optimized culture conditions that are specific for each plant species (Seguí-Simarro and Nuez 2008). In cross-pollinated crops, which possess a long life cycle, the creation of homozygous lines takes 8 to 10 selfing generations using conventional methods (Germaná 2006). The time necessary to create new cultivars is decreased to 1 to 2 yr using the haploid technique (Prem et al. 2012).
In vitro development of microspore cells without fertilization is an alternative haplo-method for breeding purposes. From a practical point of view, this technique is considered as a method of choice in a large number of genetic studies (Testillano 2019). Microspores have the potential to deviate their gametophytic development pathway to pollen embryogenesis under definite in vitro culture conditions (Bélanger et al. 2018). To adopt haploid and double haploid pathways, microspores first de-differentiate their cells, reestablish competences (Germaná 2006) and finally regenerate into entire plants (Rivas-Sendra et al. 2017).
Double haploid protocols were already established for many plant families including Solanaceae (Corral-Martínez and Seguí-Simarro 2012), Cruciferae (Pilih et al. 2018) and Gramineae (Bélanger et al. 2018). However, the application of haploid production in several breeding programs is still hampered in some plant species such as O. ficus-indica because of recalcitrance of in vitro androgenesis (Germaná 2006).
Conventional breeding programs have already been investigated with O. ficus-indica to enhance various reproductive features such as nucellar embryony, cleistogamy, low seed germination and resistance to disease (Chessa and Nieddu 2002; Granata and Sidoti 2002). On the other hand, optimized in vitro culture protocols useful for biotechnology such as gene transfer (Felker et al. 2018; Angulo-Bejarano et al. 2019), somatic embryogenesis (Bouamama et al. 2011) and in vitro mass propagation (Zoghlami et al. 2012), were established to introduce improved characteristics such as fruit quality.
Some researchers have found that O. ficus-indica is unresponsive to haploid induction (Gonzalez-Melendi et al. 2005). The objective of the present study was to overcome this recalcitrance and produce embryos by inducing cell division and cell differentiation in microspores. The sporophytic pathway in Barbary fig was investigated by modulating numerous exogenous and endogenous factors, which included heat shock, the microspore developmental stage and phytohormones.
Materials and Methods
Plant material
Flowers of a local Opuntia ficus-indica (Barbary fig) ‘Moore’ were collected from a farm in the Marja region ‘Grombalia’ of Tunisia at five different floral developmental stages (stage 1 to stage 5), based on morphological features. Correlations were established between flower bud length (FBL), anther color (AC), filament length (FL), and male gametophyte development (MGD). Flower classification was fixed as follows:
Stage 1: FBL: 2.5 mm; AC: green-yellowish; FL: 0.7 mm; MGD: microspore mother cell
Stage 2: FBL: 3 mm; AC: yellowish-translucent; FL: 1 mm; MGD: tetrad
Stage 3: FBL: 3.25 mm; AC: yellow-translucent; FL: 1.25 mm; MGD: uninucleate
Stage 4: FBL: 4.2 mm; AC: yellow; FL: 1.25 mm; MGD: binucleate
Stage 5: FBL: 5.3 mm; AC: dark yellow; FL: 2 mm; MGD: tricellular pollen
Induction phase
Flowers were washed with tap water, surface sterilized with 70% (v/v) ethanol for 3 min, and disinfected with 6% (v/v) of a commercial bleach (Javel Judy, 3.61% of NaClO; Groupe Judy, Dar Chaabane El Fehry, Nabeul, Tunisia) for 30 min. Flowers were then rinsed four to five times with sterilized distilled water. Anthers were detached aseptically from the whole flowers and cultured on a solidified CP medium (Chée and Pool 1987). Three phytohormone combinations were used: M1 = CP medium + 2 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D), M2 = CP medium + 2 mg L−1 2,4-D + 2 mg L−1 thidiazuron (TDZ), and M3 = CP medium + 2 mg L−1 2.4-D + 2 mg L−1 TDZ + 0.5 mg L−1 gibberellic acid (GA3), and all were supplemented with 500 mg L−1 glutamine and 30 g L−1 sucrose (Table 1). The pH of the media was adjusted to 5.8 with 1 M KOH, and then solidified with 0.3% (w/v) GelzanTM (CM Sigma-Aldrich® Chemie GmbH, Munich, Germany) before autoclaving at 102 kPa pressure for 24 min at 116°C. The sterilized media were poured into 6-cm Petri dishes (about 8 mL for each plate). Each treatment consisted of three replicates with 25 anthers per plate. Plated anthers were also subjected to heat shock at 32°C or 42°C, as follows: (treatment 1: 22°C for 4 wk, 32°C for 2 wk, and 22°C for 24 wk); (treatment 2: 22°C for 4 wk, 42°C for 2 wk, and 22°C for 24 wk); (treatment 3 was the control treatment: 22°C for 30 wk). The explants were sub-cultured every 4 to 5 wk onto freshly prepared induction medium, the plates were covered with aluminum foil and incubated in a phytotron (Plant Growth Chamber; Indiamart, Noida, India) under 16 h light (35 μmol m−2 s−1), 70% of humidity, and 8 h dark photoperiod. Phytohormones were from Sigma-Aldrich®.
ESEM analysis
Samples 0.5 cm in diameter derived from in vitro immature anthers, mature anthers, dehisced anthers, and anthers developing callus were mounted on aluminum stubs with double-sided adhesive tape and were inserted directly in an FEI QUANTA 200 environmental scanning electron microscope (ESEM; FELMI-ZFE, Graz, Austria), which was appropriate for biological material visualization, without a chemical fixation strategy. Secondary electron images were taken at 10 to 15 kV, as suggested in Borji et al. (2018).
Microspore visualization
Anthers were fixed in 3:1 ratio (v/v) of ethanol to glacial acetic acid solution and stored at − 20°C until analysis. Anthers were then squashed and stained in 0.5% (w/v) propionic-carmine on glass slides (Lauxen et al.2003) to investigate pollen viability and stages of development. Microspores were staged under a DM300 transmitted binocular light microscope (Leica Microsystems, Wetzlar, Germany).
Statistical analysis
The effects of the interaction between culture media and heat shock treatment on the appropriate microspore developmental stage were explored by analysis of variance (ANOVA). Three replicates per treatment (75 anthers) and at least 600 anthers were treated for each developmental stage. The entire set of experiments was repeated three times. Differences among means were tested by the Tukey test, which is a parametric test, at p < 0.05.
Results
Microspore characterization
To identify the appropriate developmental stage of microsporogenesis to produce haploids, the buds and flowers of Barbary fig ‘Moore’ were collected at different development periods according to their total length, color, and anther length. Based on both floral and male floral external characteristics, the anthers were categorized into five stages (stage 1 to stage 5). Overall, each floral stage was correlated with a pollen development stage.
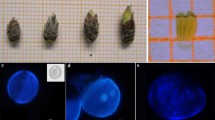
To target the adequate microspore developmental stage for pollen embryogenesis, a relationship was established between Barbary fig buds, anthers, and microspores. Freshly collected anthers showed various microgametogenesis stages. Both early and late unicellular microspores were illustrated (Fig. 1a, b). An early unicellular microspore is distinguished by an enlarged vacuole that restricts the movement and immobilizes the nucleus location. However, the vacuole volume decreases when microspores are late unicellular. Microspores had a visible cell layer at this stage known as the intine. The latter is surrounded by the exine, which is an inner cell layer that constitutes the outer pollen wall. Together, the intine and exine make up the pollen wall. In a regular gametophytic development, the bicellular stage is the result of an asymmetric division of the previous stage (unicellular). The latter assembled a miniscule generative cell and a voluminous vegetative cell in the cytoplasm (Fig. 1c). Next, the generative cell gave rise through a serial division, to the sperm cells next to each other, and bordered by the vegetative cytoplasm, which gave rise to the trinucleate or mature microspore, or mature pollen grain (Fig. 1d).
Microgametogenesis in Opuntia ficus-indica (L.) Mill. (Barbary fig) at stages 3, 4 and 5 of flower development. (a) Microspore visualized at early uninucleate stage showing a large vacuole—vac, an excentrally located cytoplasm—cyt, and an exine—ex. (b) Late unicellular microspore characterized by the presence of an intine—int, dense cytoplasm, and vegetative cell. (c) Young bicellular microspore, showing a large vegetative—vac, a small generative—gc cell, and a dense cytoplasm enclosing numerous inclusions. (d) Tricellular or trinucleate pollen presenting a vegetative cell and two sperm cells—n and an operculum—op. Scale bar 10 μm. (e) Anther dehiscence and sporophytic microspore induction on the lateral anther walls. (f) Numerous microspores agglomerated—Agm together, and induction of multicellular and proembryos on the distal zone of the dehisced anther—Deh. ant.
Microspore embryogenesis
Each group of anthers was initially exposed to the same stressful in vitro culture environment. However, not all of the anthers followed the same pathway. In vitro culture of these anthers led to several states of the original material, based on the exogenous treatments employed and the physiological status of the male gametophyte.
This study confirmed that the cell physiological status tightly controls male gametophyte reprogramming. Most of the anthers collected from immature flowers at stage 1 and stage 2 did not undergo any modifications the first 8 wk, regardless of the treatment applied. Anther insensitivity impacted organs less than 3 mm in length, in which cultured microspores were predominantly at the pollen mother cell and tetrad stages. However, after 30 wk of culture, immature anthers became necrotic, regardless of the heat shock employed during culture.
For the control treatment fixed at 22°C, with anthers at stages 3 and 4, culture on M1, M2 and M3 did not result in any in vitro androgenic response but produced a large amount of homogenous callus. Phytohormones and the microspore stages significantly affected the percentage of callus formation. The best callogenic masses (52.2%) were recorded when uninucleate microspores (stage 3) were cultured on CP enriched with 2,4-D, TDZ and GA3 (M3), whereas binucleate microspores (stage 4) gave rise to 30% of callus using the same combination (Table 1).
One of the most significant events in this study was the reorientation of microspores at uni- and bicellular stages to the sporophytic pathway, when anthers had a heat shock treatment at 32°C (Table 1). After 8 to 10 wk of culture, responsive anthers were observed, and the androgenesis pathway took place. Swelling and bursting of in vitro cultivated anthers was followed by microspore exposure on anther surfaces. Under high temperature (32°C), the induction of the sporophytic pathway was achieved through two androgenesis routes. The main process was the direct development of embryo-like structures in the starting material. Anthers first dehisced and then revealed both isolated and agglomerated microspores (Fig. 1e, f).
The in vitro androgenesis pathway was supported by environmental scanning electron microscopy analysis (ESEM), in which the sexual organs burst, and exhibited pro-globular, globular, and post-globular structures. Two concomitant globular microspore embryos (G-ME) were detected with the ESEM micrograph. The first one (on the left) was covered with a well-developed extracellular matrix (ECM) and remained attached to the neighboring tissue by an attaching point (AP), which mimics the suspensor in somatic embryo structures. The other one (on the right) was converted to a callus mass (Fig. 2a). Additional embryogenic developmental stages were recorded (Fig. 2b, c). The post-globular embryo (PG-ME) is an intermediary stage characterized by an elongated shape, and the torpedo-like structure (T-ME) was marked by disproportionality between the two poles: a large proximal zone (pro) and a narrow distal zone (dis). The cotyledonary-like structure (C-ME) was distinguished by an embryogenic split (sp), and this stage can be described as an intussuscepted embryo folding. Furthermore, a well-defined cell wall was identified in the embryogenic developmental stages.
Opuntia ficus-indica (L.) Mill. (Barbary fig) microspore embryos visualized by environmental scanning electron microscope (ESEM) analysis. (a) Emergence of two concomitant microspores embryos (ME). On the left, globular—G-ME covered by an ECM matrix and partially attached to the neighboring tissue by attached-point—AP. On the right, ME covered with callus mass—MEc. (b) Cluster of ME at post-globular—PG-ME, torpedo—T-ME, and cotyledonary-like-shaped structures—C-ME. The C-ME is marked by the embryogenic split (intussuscepted embryos folding). (c) Torpedo-like structure with two distinct poles, a large proximal pole—pro, and a narrow distal pole—dis.
The second androgenic pathway that registered at 32°C was the induction of microspore embryos (ME) in the presence of proliferative callus masses. On the three-culture media M1, M2 and M3, anthers burst open, and callus entities were distinctly visible under light and ESEM microcopies (Fig. 3a, b). The ESEM analysis enabled observation of ME at the pro-embryo and globular stages, with an intervening callus phase (Fig. 3c, d). Furthermore, some features that implicated the embryogenic state were registered and included embryogenic split or intussuscepted folding, which marked the transition from the globular to torpedo stage at the apical part of the bipolar structure. The induction of an extracellular matrix (ECM) was also observed at this step. Certain zones of the mucilaginous masses developed into embryo-like structures, while filaments remained necrotic under light microscopy (Fig. 4a). A mature microspore-derived embryo was distinctly visualized by ESEM analysis, the latter was marked by an elongated and asymmetric shape confirming the presence of an apical pole and a root pole, and the androgenic structure was also characterized by the absence of a vascular connection with the mother tissue. Finally, the extracellular matrix covering the converted embryo was desquamating and a protoderme started to develop (Fig. 4b). Numerous ME converted into young cladodes after 24 wk of culture at 16 h of photoperiod on based medium without phytohormones; young plantlets are distinguishable by cylindrical shape and tender spines. White and globular androgenic embryos were also visualized at the basal end of the young cladodes (Fig. 4c). The neoformed plantlets required more efforts and time to reach maturity and to confirm their ploidy level.
Indirect Opuntia ficus-indica (L.) Mill. (Barbary fig) microspore embryo (ME) induction after 32°C heat shock treatment. (a) Dehisced anthers exhibiting callus formation—cal, numerous microspore embryos—ME, and necrotic filament—NF. (b) Dehisced anthers visualized by environmental scanning electron microscope (ESEM) micrographs presenting mature pollen grain—MPG, and microsporangium callus—cal. (c, d) Polarized microspore embryos presenting an extracellular matrix. Microspore embryos are at globular and post-globular stages—G-MEs. Embryos are marked by an embryogenic split—S at the tip of the apical region of the bipolar structure. Deposition of an extracellular matrix constituting the future cell wall—ECM.
Indirect Opuntia ficus-indica (L.) Mill. (Barbary fig) microspore embryogenesis derived from dehisced anthers. (a) Numerous microspore embryos (ME) derived from a microsporangium callus—cal. (b) A mature microspore-derived embryo completely detached from the mother tissue and visualized by environmental scanning electron microscope (ESEM) analysis. (c) Numerous young cladodes (arrow) emerged on the callogenic material surface.
Regarding the interaction between media and the microspore stage when heat shock was applied at 32°C, the highest rate of indirect microspore embryogenesis induction was registered (24.4%), when uninucleate microspores were cultured on M3 medium (Table 1). Bicellular microspores cultured on M3 medium gave rise to 10% of ME that originated from embryogenic tissues. The other phytohormone combinations were also efficient for ME induction when the explants were heat shocked at 32°C. The association between 2,4-D and TDZ (M2) was more favorable for indirect induction of ME, than the application of 2,4-D as a unique auxin (M1). However, the synergistic effect between the auxin, cytokinin and gibberellic acid (M3) medium acted at a much higher rate than M1 and M2 culture media.
Two distinct events were recorded when anthers were heat shocked at 42°C. Stressed anthers cultured at stages 3 and 4 on the three culture media achieved their gametophytic pathways (Fig. 5a, b). Mature pollen grains were visualized by ESEM analysis through dehisced anthers (Fig. 5b’). The male gametophytes spilled along the filaments (Fig. 5c). However, heat shock negatively affected the filaments, which resulted in their necrosis.
Opuntia ficus-indica (L.) Mill. (Barbary fig) anther behavior after 42°C heat treatment. (a) Entire stamen—St showing a dehisced anther—Deh-ant and a necrotic filament—NF (front view). (b) Dehisced anthers lateral view. (b’) In vitro liberation of the microsporangium—Mspgm content. (c) Numerous mature pollen grains—MPG spill aggregate together along the filaments. (d) General view of MPG morphology and pollen tube emission—PTE on filament surface.
In parallel, the ESEM analysis allowed the study of the palynological characterization of Barbary fig pollen grain (PG). The general view of the PG was spheroidal and pantoporate, and the inner surface of the locules was large and presented convex membranes. The locules or germination pores contained small spherical bodies called orbicules. The PGs were covered with a tectum with no supratectate spinules. This PG wall presented perforate and elongated tectal perforations that corresponded to the mature and outer layer of exine. In addition, various mature pollen tubes were even emitted in vitro (Fig. 5d).
At 42°C, heat shock treatment was lethal for most of the male gametophytes. Anthers cultivated on M1 and M2 did not exhibit any indirect androgenic competence. However, direct pollen embryos were observed when explants were cultivated on M2 and M3. The best percentage (14.4%) of pollen grain (PE) was recorded with uninucleate microspores, while 11.1% of PE were registered with the binucleate stage (Table 1).
Mature pollen grains (MPG) emerged from dehisced anthers that were heat shocked at 42°C and cultured on M2 and M3 for 30 wk (Fig. 6), and some evolved into pollen embryos. The ESEM analysis enabled identification of some important features, which confirmed the deviation of MPG into PE. Pollen grains were surrounded by an ECM, which constituted a network around the transformed sexual organs. Intact MPG were also visualized. The ESEM analysis confirmed another interesting feature in the micromorphology of the MPG during the embryogenesis. The micrographs showed the installation of coating material around the sexual organs. Moreover, MPGs began to lose their original coating tissue (the exine). The micrograph showed a visible disorganized and desquamate exine on several PGs. Reworking was visualized on the desquamate PG, with elongated pollen embryos and a prominent split. This process evoked the embryogenic split, which is a striking feature found in somatic and zygotic embryos during their development (Fig. 6). Other specific features that confirmed the embryogenic state of MPG under the 42°C heat treatment were visualized by ESEM analysis (Fig. 7). A dehisced anther enabled observation of an MPG that was converted to a cordiform-shaped pollen embryo, or a heart-shaped pollen embryo (HPE; Fig. 7a, b). In addition, the loss of the cell wall was clearly visible, the tectum and the circular locules were mostly disintegrated, and some circular locules and few orbicules bodies were still detectable on the new coating tissue. The PG was strangely and closely attached to another pollen embryo (GPE), and the attachment point looked like a ring. Micromorphology of the lateral wall surfaces, around the PEs, was well organized and appeared to be ornamented with conical projections. Light microscopy revealed the conversion and the germination of PE into plantlet (Fig. 8a), when the neoformed entity was transferred on CP based medium without phytohormones at a 16-h photoperiod. From the cotyledonary leaves of the androgenic embryo emerged young cladodes (Fig. 8b, c). On the other hand, numerous recurrent embryos or secondary embryos emerged directly from the hypocotyl of the germinated pollen embryo. In addition, it was noted on the same figure that the apical end of the germinated pollen embryo did not exhibit any further development (Fig. 8d). Finally, a clear difference was demonstrated between a mature pollen grain, with the characteristics of a male gametophyte, which can be engaged in sexual reproduction, and another male gametophyte that embarks on a pollen embryogenesis route (Figs. 7 and 8). All the features described above strongly supported the haploid induction of pollen grains after maturation. Plant tissue culture such as haploid induction from O. ficus-indica is a technique which requires huge efforts and time to reach a final result.
Dehisced Opuntia ficus-indica (L.) Mill. (Barbary fig) anther after 42°C heat treatment visualized by environmental scanning electron microscope (ESEM) analysis. Numerous mature pollen grains—PGs redirected to sporophytic pathway inside and outside the anther walls—AW. The earliest stages of pollen embryos—PE were surrounded by an extracellular matrix—ECM. Intact PG are also visualized.
Environmental scanning electron microscope (ESEM) analysis of reoriented Opuntia ficus-indica (L.) Mill. (Barbary fig) mature pollen grains—PG after heat shock at 42°C. (a) Dehisced anther—Deh-ant surrounded area containing numerous mature pollen grains—MPG (bold narrows) and numerous pollen embryos—PE (white narrows). Emergence of a heart-shaped pollen embryos—HPE (red narrow) from the Deh-ant. (b) Micrograph highlighting the details of the HPE fused to a globular-like shape—GPE (right), with a desquamate tectum—tec. (c) Entire late mature PG from in vitro anther dehiscence showing a spheroid PG surrounded by a perforate tectum, which contains about 20 to 22 locules. Locule inner surface lost the major orbicule bodies. (d) Details of torpedo pollen embryos—TPE on anther wall—AW surface, note the embryogenic split—Sp at the tip of TPE.
Conversion of a germinated Opuntia ficus-indica (L.) Mill. (Barbary fig) pollen embryo into in vitro plantlet after heat shock at 42°C. (a) Germinated pollen embryo (GPE) induced on CP based medium (Chée and Pool 1987) without phytohormones under 16 h of photoperiod. The GPE present cotyledons—Cot, hypocotyl—Hyp, and roots—Rt. (b) From the apical meristem of the GPE emerge young cladode—Yg clad. (c) From the deteriorate cotyledons emerge a cluster of embryos—Clu emb. In parallel the primary young plantlet continues to grow—Yg clad. (d) Numerous recurrent embryos—Rec embr emerged directly from the hypocotyl of the GPE.
Discussion
This research study describes the optimization of earlier stages of microspore embryogenesis in a North African Barbary fig cultivar. Haploid production is a routine technique used in breeding programs for numerous herbaceous plant species including cereals (Dziurka et al. 2019) and Brassicaceae (Pilih et al. 2018), due to their high response towards androgenesis. But the execution of the technique in fruit crop species, especially in Opuntia species, is still very limited, due to the low induction rate, difficulty in plant recovery, and the highly genotype-dependence of the process. These challenges limit the progression of microspores from the gametophytic to the sporophytic pathway (Germaná et al. 2011). Therefore, a microspore embryogenesis protocol for O. ficus-indica was developed.
To expand probabilities of successful haploid induction in O. ficus-indica, the work was initiated with the assembly of some phenological criteria. The correlation between morphological characteristics of flowers led to five main classes of flowering (stage 1 to stage 5). This correlation was already established in many plant species including prickly pear (O. ficus-indica; Gonzalez-Melendi et al. 2005), apricot (Prunus armeniaca) (Germanà et al. 2011) and grapevine (Vitis vinifera) (Bouamama et al. 2007). The progression of microspore cellular organization was summarized in 5 steps: mother pollen grain; the early and late unicellular stages, which are characterized by a large vacuole; the bicellular stage that has generative and vegetative cells; and the mature pollen grain that contains two sperm cells. Most of the studies completed on microspore ontogeny indicated that microspores completed serial cell divisions with an exact ordered sequence, which finally led to mature pollen grain formation, and contained both vegetative and generative cells, and two sperm cells, which were engaged in specific roles of the microgametogenesis pathway, as described by Goralski et al. (2005).
The effects of three culture media enriched with 2,4-D, TDZ and GA3, and heat shock treatments of 32°C and 42°C compared to a control treatment of 22°C, were studied on anthers cultured from five cytological events of the male gametophyte. Overall, it was concluded that the 32°C and 42°C heat treatments favored microspore embryogenesis on the three culture media. However, in the control treatment, the anthers did not show any androgenic response. Winiarczyk and Gębura (2017) confirmed that under certain stressful in vitro culture conditions, microspore deviation can occur from a gametophytic pathway to a sporophytic one. They also confirmed that haploid embryos are induced according to cell totipotency. Such cellular competence recognized in the male gametophyte is thought to be an avoidance form to escape apoptosis that is inflicted by stressful conditions (Bonet et al. 1998).
In vitro anther behavior is controlled by both the physiological and cytological state of microspores. The present results indicated that immature anthers cultured at stage 1 and 2, which corresponded to mother pollen grain and tetrad stages, respectively, did not undergo any sporophytic pathway. Gonzalez-Melendi et al. (2005) confirmed that O. ficus-indica microspore cultivation at the tetrad stage produced primary callus, but neoformed entities did not evolve into further organized structures.
In the present study, the only responsive anthers were limited to unicellular and bicellular stages that corresponded to stage 3 and stage 4. Soriano et al. (2013) and Chong-Pérez et al. (2018) reported that the most amenable pollen stages for haploid formation were localized to the uninucleate stage and early bicellular stage.
Using microscopic tools, the micromorphological events related to the first microspore embryogenesis stages were described in this study. Heat-shocked microspores at the uninucleate and binucleate stages switched their gametophytic pathway and gave rise outside and inside the dehisced anthers to multicellular entities directly and indirectly through a callus intervening phase. The cellular architectural organization of multicellular structures was followed microscopically. In the same manner, the earlier stages of microspore embryogenesis were studied in Prunus armeniaca. Germaná et al. (2011) demonstrated how in vitro cultured microspores stalled out the gametophytic developmental pathway and underwent a symmetrical division. During the division, the microspores progressively lost cytoplasmic vacuoles and multicellular structures were neoformed. At this stage, the authors indicated that multicellular entities remained ringed by the exine, which is the pollen wall. Germaná et al. (2011) noted that the protective cell wall of competent anthers was destroyed and revealed callogenic and globular entities. The existence of a thick inner wall situated directly below the exine has been designated as a typical characteristic of the multicellular entities developed after embryogenesis induction, which forms an eventual marker of the sporophytic route (Germaná 2006).
On the other hand, it was demonstrated that the microspores that were unresponsive, which did not participate in the androgenic process, did achieve their gametophytic pathway. In this context, Prem et al. (2012) reported that the insensitive Brassica napus microspores achieved their in vitro gametophytic development leading to mature pollen.
The present experiments confirmed that heat shock exposure was mandatory for the deviation from normal fertile pollen to pollen embryogenesis. The 32°C heat treatment was a successful trigger mechanism to reprogram microspores towards androgenesis in Barbary fig. The effectiveness of heat shock treatments on pollen embryogenesis has been implicated in many reports. Previous studies on the application of heat shock between 32 and 35°C have been reported on cultured anthers (Bárány et al. 2010; Pintos et al. 2013; Heidari-Zafreh et al. 2019). Furthermore, several studies demonstrated that important cellular consequences accompany the redirection from the gametophytic to the sporophytic route. It was reported that heat treatment caused a cytoskeletal remodeling through the microtubules and actin filament configurations, because they are considered to be dynamic organs (Dubas et al. 2010). The microtubules operate and turn the division plane from asymmetric to the symmetric position. Other cellular events marked the installation of a new regeneration mode, which included vacuolization changes and architectural rearrangements that affected cell wall formation (Hause et al. 1993).
During in vitro pollen development, it was demonstrated that any nutritional deficiency or any environmental perturbation negatively affected pollen development and viability (Prem et al. 2012). The present results showed that the 42°C heat treatment used as a stressing factor positively affected anther contents and efficiently reoriented mature pollen grains to pollen embryos. Normally, microspore embryogenesis ability is focused on PMI and is attributed to the ability of the immature microspore to divide when exposed to exogenous stresses. However, younger (mother pollen grain) and older (pollen grain) stages cannot re-orient the division phase (Soriano et al. 2013). The present results suggest that mature pollen grain (older stages) could be converted into pollen embryos when exogenous constraining conditions were assembled.
Finally, the palynological characterization of Barbary fig was a focus area using ESEM analysis, with the knowledge that pollen morphology had been studied for some Italian and South American Opuntia cultivars (ElBehi et al. 2015; Miesen et al. 2015). However, there was not any reference on pollen grain morphology for North African Barbary fig cultivars. This study focused on the morphology and the ornamentation of the exine. The PG was characterized by a spherical and apolar shape. The reticulated tectum was adorned with a global distribution of the apertures. In this case, the type of aperture was designated as pantoporate, and the PG was considered as a non-equatorial pore. Germination pores presented orbicules bodies, which were dispersed at late mature PG stage. Prieu et al. (2017) confirmed that apertures are very important in the wall structures, as they are implicated in the pollen tube germination and environmental exchanges. In another study, Gotelli et al. (2009) described the general morphology in four Pterocactus (Cactaceae). The PG described in the present study had a similar diameter compared to the four mature PG described in Pterocactus. While in O. ficus-indica ‘Moore’, the pores were more abundant than cactus species described by Gotelli et al. (2009). The results in the present study are in accordance with ElBehi et al. (2015). The authors confirmed that tectum of ‘Gialla’, ‘Bianca’, and ‘Rossa’ cactus pear (O. ficus-indica) was cross-linked by 20 to 24 circular locules, which was a number comparable to those found in O. ficus-indica ‘Moore’ pollen grains.
This paper presents the first report on O. ficus-indica pollen embryogenesis. The aim was to alleviate the recalcitrance of microspores when this important plant breeding method is used and to develop an efficient and reliable pollen embryogenesis protocol. Therefore, the application of endogenous and exogenous factors, which included culture media and heat shock at several microspore stages, facilitated development of a protocol for the earlier stages of microspore or pollen embryogenesis for the first time in Barbary fig ‘Moore’. In fact, 2,4-D, thidiazuron and GA3 exogenously employed in the based-culture media seemed to be the most reliable combination, and the synergistic effect of auxin, cytokinin and gibberellin enhanced the sporophytic route in Barbary fig. Both unicellular and bicellular microspores were favorable to induce the androgenesis process. The stressed microspores, at 32°C, were at an optimum metabolic state and therefore exhibited indirect and direct embryogenic responses. Moreover, at 42°C, pollen embryos were obtained from mature pollen grains exclusively, without any intervening callus phase. These events were reported for the first time in O. ficus-indica. Pollen embryos derived from mature pollen grain also seemed to rarely occur. An ESEM analysis was also used to describe palynology characteristics of mature pollen grain in Barbary fig.
In vitro culture conditions that included heat shock treatments, phytohormones and cytological developmental stages were essential to lead microspores towards pollen embryogenesis. However, this procedure needs to be completed by achieving double haploid plantlet production.
References
Angulo-Bejarano PI, Sharma A, Paredes-Lopez O (2019) Factors affecting genetic transformation by particle bombardment of the prickly pear cactus (O. ficus-indica). 3 Biotech 9:98. https://doi.org/10.1007/s13205-019-1627-6
Bárány I, Fadón B, Risueno MC, Testillano PS (2010) Microspore reprogramming to embryogenesis induces changes in cell wall and starch accumulation dynamics associated with proliferation and differentiation events. Plant Signal Behav 5:341–345
Bélanger S, Marchand S, Jacques PE, Meyers B, Belzile F (2018) Differential expression profiling of microspores during the early stages of isolated microspore culture using the responsive barley cultivar Gobernadora. G3 8:1603–1614
Bonet FJ, Azhaid L, Olmedilla A (1998) Pollen embryogenesis: atavism or totipotency? Protoplasma 202:115–121
Borji M, Bouamama-Gzara B, Farhat C, Teyssier C, Ben Amar A, Mliki A, Zekri S, Ghorbel A (2018) Micromorphology, structural and ultrastructural changes during somatic embryogenesis of a Tunisian oat variety (Avena sativa L. var ‘Meliane’). Plant Cell Tissue Organ Cult 132:329–342. https://doi.org/10.1007/s11240-017-1333-1
Bouamama B, Ben Salem-Fnayou A, Ben Jouira H, Ghorbel A, Mliki A (2007) Influence of the flower stage and culture medium on the induction of somatic embryogenesis from anther culture in Tunisian grapevine cultivars. J Int Sci Vigne Vin 41:1–8
Bouamama B, Ben Salem A, Zoghlami N, Zemni H, Mliki A, Ghorbel A (2011) Somatic embryogenesis and plantlet regeneration from immature anthers in Opuntia ficus-indica. J Hortic Sci Biotechnol 86:313–318
Chée R, Pool RM (1987) Improved inorganic media constituents for in vitro shoot multiplication of Vitis. Sci Hortic 23:85–96
Chessa I, Nieddu G (2002) Investigations on variability in the genus Opuntia as fruit crop for genetic improvement. Acta Hortic 575:345–353
Chong-Pérez B, Carrasco B, Silva H, Herrera F, Quiroz K, Garcia-Gonzales R (2018) Regeneration of highland papaya (Vasconcellea pubescens) from anther culture. Appl Plant Sci 6:1–7
Corral-Martínez P, Seguí-Simarro JM (2012) Efficient production of callus-derived doubled haploids through isolated microspore culture in eggplant (Solanum melongena L). Euphytica 187:47–61
Dubas E, Wedzony M, Petrovska B, Salaj J, Iwona Z (2010) Cell structural reorganization during induction of androgenesis in isolated microspore cultures of triticale (x triticosecale wittm). Acta Biol Cracov Ser Bot 52:73–86
Dziurka K, Dziurka M, Warchoł M, Czyczyło I, Marcińska I, Noga A, Kapłoniak K, Skrzypek E (2019) Endogenous phytohormone profile during oat (Avena sativa L.) haploid embryo development. In Vitro Cell Dev Biol Plant 55:221–229. https://doi.org/10.1007/s11627-019-09967-5
ElBehi AW, Orlandi F, Bonofiglio T, Inglese P, Sortino G, Liguori G, Romano B, Fornaciari M (2015) Pollen morphology and reproductive performances in Opuntia ficus-indica (L.) Mill. Acta Hortic 1067:217–223. https://doi.org/10.17660/ActaHortic.2015.1067.30
Felker P, Inglese P (2003) Short-term and long-term research needs for Opuntia ficus-indica (L.) Mill. utilization in arid areas. J Prof Assoc Cactus Dev 5:131–151
Felker P, Bunch R, Tine JA, Russo GR, Gould J, Arnold M, Wang F, Rong Y, Wright M (2018) Stable transformation of Opuntia ficus-indica callus cultures as evidenced by fluorescence of the tandem dimer Tomato gene. J Prof Assoc Cactus Dev 20:34–51
Germaná MA (2006) Doubled haploid production in fruit crops. Plant Cell Tissue Organ Cult 86:131–146
Germaná MA, Chiancone B, Padoan D, Bárány I, Risueño MC, Testillano P (2011) First stages of microspore reprogramming to embryogenesis through anther culture in Prunus armeniaca L. Environ Exp Bot 71:152–157
Gonzalez-Melendi P, Germanà MA, Levy Guarda N, Chiancone B, Carmen Risueño M (2005) Correlation of sequential floral and male gametophyte development and preliminary results on anther culture in Opuntia ficus-indica. Acta Physiol Plant 27:687–694
Goralski G, Rosier F, Matthys-Rochon F, Przywara L (2005) Cytological features of various microspore derivatives appearing during culture of isolated maize microspores. Acta Biol Cracov Ser Bot 47:75–83
Gotelli M M, Scambato A Pollen development and morphology in four species of Pterocactus (Cactaceae), Galati B, Kiesling R (2009) . Ann Bot Fenn 46:409-415
Granata G, Sidoti A (2002) Survey of diseases discovered on Opuntia ficus-indica in producer countries. Acta Hortic 581:231–237
Hause B, Hause G, Pechan P, van Lammeren A (1993) Cytoskeletal changes and induction of embryogenesis in microspore and pollen cultures of Brassica napus L. Cell Biol Int 17:153–168
Heidari-Zefreh AA, Shariatpanahi ME, Mousavi A, Kalatejari S (2019) Enhancement of microspore embryogenesis induction and plantlet regeneration of sweet pepper (Capsicum annuum L.) using putrescine and ascorbic acid. Protoplasma 256:13–24. https://doi.org/10.1007/s00709-018-1268-3
Lauxen M, Kaltchuk-Santos E, Hu C, Callegari-Jacques SM, Bodanese-Zanettini MH (2003) Association between floral bud size and developmental stage in soybean microspores. Braz Arch Biol Technol 46:515–520
Miesen F, De Porras ME, Maldonado A (2015) Pollen morphology of Cactaceae in Northern Chile. Gayana Bot 72:258–271
Mondragon JC, Bordelon BB (1996) Cactus pear (Opuntia spp. Cactaceae) breeding for fruit production. J Prof Assoc Cactus Dev 1:19–35
Nefzaoui A, Louhaichi M, Ben Salem H (2014) Cactus as a tool to mitigate drought and to combat desertification. J Arid Land Stud 13:121–124
Pilih KR, Potokar UK, Bohanec B (2018) Improvements of doubled haploid production protocol for white cabbage (Brassica oleracea var. capitata L.). Folia Hort 30:57–66
Pintos B, Sanchez N, Bueno MA, Navarro RM, Jorrin J, Manzanera JA, Gomez-Garay A (2013) Induction of Quercus ilex L. haploid and double haploid embryos from anther cultures by temperature-stress. Silvae Genet 62:210–218
Prem D, Solís MT, Bárány I, Rodríguez-Sanz H, Risueño M, Testillano PS (2012) A new microspore embryogenesis system under low temperature which mimics zygotic embryogenesis initials, expresses auxin and efficiently regenerates doubled-haploid plants in Brassica napus. BMC Plant Biol 12:127. https://doi.org/10.1186/1471-2229-12-127
Prieu C, Sauquet H, Gouyon PH, Albert B (2017) More than sixty origins of pantoporate pollen in angiosperms. Am J Bot 104:1837–1845
Rivas-Sendra A, Calabuig-Serna A, Seguí-Simarro JM (2017) Dynamics of calcium during in vitro microspore embryogenesis and in vivo microspore development in Brassica napus and Solanum melongena. Front Plant Sci 8:1–14
Russell CE, Felker P (1987) The prickly pears (Opuntia spp., Cactaceae): a source of human and animal food in semiarid regions. Econ Bot 41:433–445
Seguí-Simarro JM, Nuez F (2008) How microspores transform into haploid embryos: changes associated with embryogenesis induction and microspore-derived embryogenesis. Physiol Plant 134:1–12
Shuro AR (2017) Review paper on approaches in developing inbred lines in cross-pollinated crops. Biochem Mol Biol 2:40–45
Soriano M, Li H, Boutilier K (2013) Microspore embryogenesis: establishment of embryo identity and pattern in culture. Plant Reprod 26:181–196
Testillano PS (2019) Microspore embryogenesis: targeting the determinant factors of stress-induced cell reprogramming for crop improvement. J Exp Bot 70:2965–2978. https://doi.org/10.1093/jxb/ery464
Winiarczyk K, Gębura J (2017) Formation of a unique structure during microsporogenesis in Tinantia anomala anthers. Protoplasma 254:785–790
Zoghlami N, Bouamama B, Khammassi M, Ghorbel A (2012) Genetic stability of long-term micropropagated Opuntia ficus-indica (L.) Mill. plantlets as assessed by molecular tools: perspectives for in vitro conservation. Ind Crop Prod 36:59–64
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Neftali Ochoa-Alejo
Rights and permissions
About this article
Cite this article
Bouamama-Gzara, B., Zemni, H., Zoghlami, N. et al. Behavior of Opuntia ficus-indica (L.) Mill. Heat-Stressed Microspores Under In Vitro Culture Conditions as Evidenced by Microscopic Analysis. In Vitro Cell.Dev.Biol.-Plant 56, 122–133 (2020). https://doi.org/10.1007/s11627-019-10032-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-019-10032-4