Abstract
We presented a two-stage combined bioreactor system consisting of a stir-tank and an airlift column, and challenged with Rhizoma zedoariae cell suspensions for β-elemene production. Two-stage culture was initiated when the cell concentration in both vessels was maintained at an appropriate density. The cells were proliferated in stirred-tank with the maximal growth rate of 0.17 d−1 to present enough cells for β-elemene synthesis. In the airlift column, continuous cell separation from culture medium was achieved by using a cell retention device based on centrifugal and gravity settling when the system was performed in perfusion mode. The results indicated that additives can efficiently promote the accumulation of β-elemene in R. zedoariae cells. In addition, the β-elemene content showed higher levels in cell lines of overexpressing 3-hydroxy-3-methylglutaryl coenzyme-A reductase, Farnesyldi phosphate synthase, and ST02C genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
β-Elemene is one of the sesquiterpenes used for the treatment of ovarian, breast, and lung cancers (Zhao et al. 2015). Presently, the raw material of β-elemene is mainly extracted from Rhizoma zedoariae, one of the Traditional Chinese Medicines, collected from farms (Zhou et al. 2016). Field cultivation is time-consuming and easily interfered by climate. Therefore, cell-suspension culture was considered as a promising way to produce this valuable pharmaceutical metabolite.
Much progress has been made in terpenoid synthesis (Fig. 1). Isopentenyl pyrophosphate (IPP) is a common precursor of terpenoid synthesis. 3-hydroxy-3-methylglutaryl coenzyme-A reductase (HMGR) and 1-Deoxy-D-xylulose 5-phosphate (DXS) are two rate-limiting enzymes of the mevalonic acid pathway (MVA) and mevalonate pathway (MEP) respectively for IPP biosynthesis (Liu et al. 2005; Aquil et al. 2009). Farnesyl-diphosphate synthase (FDS) catalyzed the synthesis of fluorinated polypropylene (FPP) to increase the metabolic flux towards sesquiterpenes (Ping et al. 2012). FPP can be converted into germacrene A with one-step synthesis, and germacrene A was further converted to β-elemene at high temperature without any biochemical catalysts(Leila et al. 2015).
Secondary metabolites are important defensive substances released by plants in response to adverse environmental stresses or attacking of pests and pathogens (Duan et al. 2013). Studies have shown that conditions suitable for plant cell proliferation often reduce the synthesis of secondary metabolites (Qi et al. 2014). Thus, it is a challenge to increase both biomass and target substance accumulation in plant cell suspensions in one bioreactor system.
Two-stage cultivation of plant cell refers to the culture procedures, which can be divided into two different stages according to the dyssynchrony of cell proliferation and secondary metabolites synthesis (Marisol et al. 2016). For a traditional two-stage run, cell proliferation and target substance synthesis usually proceed in different bioreactor systems, which increases the risk of contamination during cell transfer between bioreactors. Furthermore, it takes time for cells to adapt to a new growth environment.
When cell suspensions are cultivated in bioreactor, target metabolites released in the nutrient solution can be degraded or used to synthesize new compounds (Wang et al. 2010). Moreover, the synthesis of target substances can be disturbed due to feedback inhibition (Wang et al. 2015). Our early study (Wang and Qi 2009) also indicated that parts of in vitro plant cells were subjected to lethal browning due to oxidized phenolic compounds. Perfusion culture seems to have potential to decrease cell browning and feedback inhibition. Through constant medium replacement, the metabolites and other harmful substances are removed from the bioreactor, while additional nutrient materials are provided to cells.
For perfusion culture of plant suspension cells, continuous cell separation from the medium is also a challenge. In situ filtration has been applied to mammalian cell retention (De la Torre et al. 2010), but it has not been widely used in plant cells due to filter clogging at high cell densities. Gravitational sedimentation seems to be an alternative to retain cells in perfusion runs. But our studies (Wang and Qi, 2009; Wang et al. 2010) show that the cell retention efficiency based on the gravitational settling was less efficient at high cell density.
Retaining cells by centrifugal settling is likely to be effective especially for culture solutions with high viscosity (Christopher et al. 2009; Christopher et al. 2011); nevertheless, this is rarely used in plant cell culture. In this study, a combined bioreactor system consisting of a stir-tank and an airlift reaction column was used to conduct the two-stage perfusion cultivation of R. zedoariae suspension cells in one bioreactor system. Cell proliferation and β-elemene accumulation was achieved simultaneously in one bioreactor. The main components of the cell retention devices were a spiral-type centrifugal settling pipe and a gravity settling pipe with which we accomplished continuous cell separation from culture medium. In addition, the factors influencing cell retention, mass transfer, β-elemene synthesis, and cell growth were also investigated.
Materials and methods
Cell culture and maintenance
The tubers of R. zedoariae were sliced and then transfered to solid Murashige & Skoog medium to induce callus with 2 mg L−1 2,4-dichlorophenoxy acetic acid (2,4-D) and 0.2 mg L−1 6-benzylaminopurine (6-BA). The suspension cell lines were obtained when these calluses were transferred to liquid medium containing 28 g L−1sucrose, 0.5 mg L−1 6-BA, 1.0 mg L−1 naphthylacetic acid (NAA), 1.0 mg L−1 2,4-D, and kept at a rotational speed of 110 g. The cell cultures were sub-cultured each 2 wk with shaking at 120 g. All cultures were kept at 25°C in the dark.
Bioreactor configuration and perfusion culture
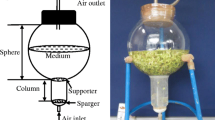
A schematic representation of the bioreactor system is shown in Fig. 2. This bioreactor system includes a stir-tank and airlift bioreactor with the total working volume of 2 L. The stirred tank and airlift column were connected by a glass pipe. The medium inlet in the airlift column was higher than liquid surface, which made the two reaction vessels relatively independent of each other. The stir-tank bioreactor with a working volume of 1 L was used in the first stage for cell growth and division. The connected pipe also served as a gravitational settling device for primary cells retention when the system was run in perfusion mode. The external loop air-lift reactor with a working volume of 1 L was used in the second stage for secondary metabolites accumulation. An important component of this system is the cell retention device that positioned in the air-lift reactor includes a spiral-type centrifugal settling pipe and a gravity settling pipe with the diameter of 2 cm, through which we achieved continuous cell separation from culture medium. A peristaltic liquid pump controlled the perfusion medium feed rate, and a microporous glass flake embedded in the pipe acted as a gas sparger. The liquid was rotated clockwise once gas was ventilated into the airlift reactor via the gas sparger located in the riser region. A microprocessor was used to monitor dissolved oxygen (DO) levels and pH.
Schematic representation of the two-stage bioreactor system. 1, valve. 2, upstream zone. 3, air sparger. 4, sampling port. 5, spiral-type centrifugal settling pipe. 6, liquid inlet of downstream zone. 7, outlet of discarded medium. 8, air filter. 9, pH probe. 10, dissolved oxygen probe.11, gravity settling pipe. 12, inlet of fresh medium.
Bioreactor cultivation was also conducted using the same culture media ingredients in Erlenmeyer flasks. Cell suspensions were transferred from shake flasks into the bioreactor and inoculated at an initial cell density of 5 g L−1 dry weight. The perfusion rates were 5–20% (v/v) every day and the aeration rates were set at 0.1–0.5 air volume/culture volume/min (v/v/m). A batch mode was performed as well under the same conditions as those in the perfusion mode with no exchange of culture medium.
Determination of cell growth rate and cell retention efficiency
In order to remove the residual sugar and other impurities from cells, the cell samples from bioreactor were filtered on filter paper under vacuum and then washed in distilled water three times. The filtered cells were weighed in pre-weighted dishes to obtain their fresh weight (FW) and the dry weight (DW) after drying at 40°C for 3 d. The average cell growth rate was calculated as the formula as follows:
(Maximum dry cell weight-Initial dry cell weight) / (Initial dry cell weight × ∆t)
where ∆t refers to the cultivation period during which the maximum dry cell mass was obtained (Wang and Qi 2009). The cell retention efficiency referred to the cell loss rate that was extracted from the discarded medium.
Determination of the volumetric oxygen mass transfer coefficient (kLa)
The kLa was measured by the dynamic gassing-in and gassing-out method in distilled water (Wang et al. 2015) at different aeration and stirring rates. O2 gas was degassed by feeding N2 gas that fed at the same flow rate as air. The value of kLa was defined as follows:
where c* is the oxygen solubility in the liquid (mgL−1); and c is the oxygen concentration in the liquid (water) at time t (mg L−1).
Determinating the content of β-elemene
Dry cells were ground to fine powder in a mortar, and then the powder was filtered through a 40-mesh sieve. Ether was added to the filtered powder and extracted with sonication for 90 mins. HPLC analysis was performed on an Agilent1100 apparatus equipped with a chromatographic column of Kromasil C18 (4.6 mm × 250 mm × 5 μm). The temperature of column was 25°C and the mobile phase consisted of acetonitrile-water (65:35, v/v).The detection wavelength was 214 nm and the flow rate was 1.00 mL min−1. The injection volume was 10 μl. β-Elemene were detected and quantified by comparison with authentic standard curves and retention times. The standard sample was 0.1 mg mL−1 β-elemene solution (Sigma, Shanghai division). The regression equation of standard curves were β-elemene of Y = 2240.1 x + 21.907, r = 0.9999 respectively.
Quantitative PCR for HMGR, DXS, and FDS gene expression
Quantitative PCR (qPCR) was performed with a qPCR kit (TaKaRa, Shanghai, China) with SYBR Green PCR Master Mix. The results showed that methyl jasmonic acid induced β-elemene biosynthesis. Total RNA was extracted from common cells (control) and high β-elemene content cell lines (treated by jasmonic acid). The primer sequences for qPCR were showed in Table 1.
For quantitative PCR reaction, the total volume was 20 μL and the reaction was as follows: The cDNA was denatured at 94°C for 2 min followed by 45 cycles of amplification (15 s at 94°C; 15 s at 55°C; 30 s at 68°C) and actin was used as a reference gene. Fold increase/RQ was calculated using the formula RQ = 2−ΔΔCt, where ΔCt = average Ct (gene of interest)–average Ct (Actin), and ΔΔCt = ΔCt − ΔCt (control of the experiment; cDNA of common cell suspensions). All experiments were designed with three biological replicates.
Cloning of HMGR, Germacrene A synthetase (GAS), and FDS genes
The complete sequences of HMGR and FDS genes were obtained in transcriptome database of R. zedoariae, and they were cloned by one-step RT-PCR kit (TaKaRa, Shanghai, China). Hyun and David (2012) characterized a terpene synthase named ST02C in ginger. The assay for ST02C with FPP as a substrate produced main substance of germacrene A (49.3%). There is only 49% homology between the GAS in Artemisia annua and ST02C in ginger. Though the whole sequences of GAS gene and ST02C gene were reported, we failed to clone them in R. zedoariae and cannot detect them in the two transcriptome database of R. zedoariae leaf and tuber. Hence, we cloned the GAS gene in Artemisia annua by RT-PCR and artificially synthesized the ST02C gene according to the amino acid sequences reported in the literature above. The primer sequences were showed in Table 2.
For each reaction, the total volume was 50 μL and the reaction was as follows: The cDNA was denatured at 94°C for 5 min followed by 35 cycles of amplification (1 min at 94°C; l min at 52–58°C; 1–1.5 min at 72°C) and the final incubation at 72°C was extended to 10 min.
Introducing ST02C, HMGR, FDS, and GAS genes into cells of R. zedoariae
Four plasmids containing the cDNAs encoding HMGR, FDS, GAS as well as ST02C driven by CaMV35S promoter, were separately introduced into the Agrobacterium GV3101 strain. After infection with the Agrobacterium that contained target genes, the tuber explants were transferred to selective medium to obtain positive transformants of callus which was confirmed by PCR of target genes. The cells from positive callus were established as engineered cell strains named with different number.
Statistical analysis
All the experiments were repeated three times. Mead SE are shown for biological triplicates. The statistical significance difference was analyzed by one sample t test and the errors were analyzed using the one-way analysis of variance (ANOVA) via SPSS11.5 software.
Results
Cell culture for increased biomass – Cell distribution in two reaction vessels at different cell concentration
In order to accumulate sufficient cells to prepare for two-stage culture, the cells were inoculated into the reactor with valve 1 closed for biomass increase. The appropriate cultivation parameters for biomass increase were agitation rate 120 g and aeration rate 0.3 vvm in batch mode. When the cell concentration was allowed to rise to more than 20 g L−1, the cell cultures in both culture vessels became very thick. A large portion of cells were deposited at bottom of the reactors, which were not conducive to mass transfer and cell growth. Therefore, we lowered the cell concentration to about 5 g L−1, meanwhile, opened valve 1 to conducted perfusion run at various velocities of perfusion medium feeding and ventilating.
The gravity settling pipe in the stirred tank was used to adjust the velocity of cell transferring from stirred tank to airlift column. At the initial stage, only a minority of cells transferred into the airlift reactor. But with cell density increasing, the gravity settling became less efficient and an increasing number of cells flowed into airlift reactor. Our early researches show that the cell sedimentation efficiency was positively related to diameter of gravity settling pipe and cell density (Wang and Qi 2009). In this study, restricted by the reactor geometric structure, the diameter of the gravity settling pipe was set as 2 cm. The distribution of cells in two reactors at different cell concentration was investigated. The results in Fig. 3, indicated that most of cells were retained in the stirred tank when the cell concentration was lower than about 15 g L−1. But as cell density increased, obvious differences in cell density were detected between the two reaction vessels due to the cells transferring. Interestingly, the cell density in the stirred tank can be maintained at a relatively stable value of 18 g L−1 or so at the perfusion rate of 20%, and no obvious cell layer was observed at the bottom of the reactor. The cell concentration in the airlift column was also maintained at about 18 g L−1 by regular harvesting. Thus, the cell density in both reaction vessels was approximately similar and the two-stage cultivation mode was initiated.
Cell retention efficiency of the bioreactor system
When perfusion mode was conducted, cell cultures were continuously separated from medium by settling device. The settling cells then mixed with the culture solution again that circulated clockwise in airlift column. Cell retention efficiency was determined by detecting the cell loss in discarded medium. Table 3 depicted the influence of perfusion rate, aeration rate and helix pitch of spiral tube on cell retention. The results suggested that the helix pitch of spiral pipe was a key factor for cell settling. The smaller was the helix pitch, the more efficient were cells separated from the medium. Ventilation in the airlift column was another significant contributor to cell settling. Cell retention efficiency improved obviously with the increase of aeration rate, and only minority of the cells transferred to exhausted medium at aeration rate of 0.5 vvm. Table 3 also indicated that lower cell loss was detected when ventilation rate was 0.5 vvm and the helix pitch of spiral tube was 1 cm. The combination of high aeration rate and small helix pitch can improve the rotation speed of cell cultures in the spiral tube, which can be attributed to the increasing of centrifugal force. Nevertheless, excessive ventilating caused negative effect on cell growth and division in the airlift column (Table 3). Under the conditions of aeration rate 0.3 vvm, helix pitch 1 cm, and perfusion rate 10%, both cell retention and cell proliferation were kept at an appropriate levels of 0.16 ± 0.04 d−1 (stirred-tank), 0.14 ± 0.03 d−1 (air-lift), and 0.06 ± 0.03 mg L−1 (Table 3). The cells escaping from centrifugal force were further retained by a gravity settling pipe connected to the base of helical pipe. In agreement with the previous experiments, high perfusion medium feed rate was a negative factor for cell settling (Wang and Qi 2009).
Cell growth in the reactor system
During the process of two-stage culture, the cells need to be proliferated continuously in the first stage to present enough cells for β-elemene synthesis in the second stage. The cells tended to grow stably when the cell concentration was maintained to about 18 g L−1 in both vessels, and then the two-stage cultivation mode was performed with various factors of precursors, chemical additives and ultraviolet irradiation in airlift column. Before two-stage culture, the cell growth rate in the stirred tank was slightly higher than that in the airlift column (Table 3) due to different mass transfer efficiency between them (Fig. 4). However, when a two-stage run was started, the biomass of cells in airlift column did not increase because the additives and ultraviolet radiation made a relatively tough environment for cell growth and proliferation. In order to optimize the culture conditions for two-stage culture, the influence of various operating parameters on cell growth was investigated. Table 3 indicated that all of the factors of perfusion rate, aeration rate, and helix pitch were relevant to cell growth. In the stirred tank, the perfusion medium feeding and aeration rates were important influences on cell growth though not prominent (data not shown). The optimum culture conditions was A2B1C2, under which the cell growth rate reached 0.17 d−1 and less cell loss was detected in discarded medium (Table 3). As mentioned above, the combination of high aeration rate and small helix pitch was not conductive to cell growth in airlift column due to the shear force generated by medium rotation. In addition, during the process of two-stage culture, more and more cells accumulated to the bottom of the airlift column for the reasons of cell sedimentation; hence, the redundant cells were continuously harvested and maintained the cell density at a relative stable value of approximately 18 g L−1.
Cell culture for β-elemene synthesis – Abiotic elicitation and precursor feeding
The anabolism of secondary metabolites in plants is influenced by various abiotic and biological stresses. It was reported that salicylic acid, ethylene, and methyl jasmonic acid could activate transcription factors in response to such stresses, which were involved in the synthesis of terpenes (Kai et al. 2004; Ana et al. 2014; Niels et al. 2015). Based on the previous studies, the following experimental strategy was designed to enhance the flux towards β-elemene synthesis in R. zedoariae cells. Salicylic acid, ethylene, and methyl jasmonic acid were added to the cell cultures at 20 μg L−1. FPP molecules as a precursor of sesquiterpene pathway also tested with the concentration of 100 mg L−1. Before addition, all the elicitors and precursors were filter-sterilized with membrane filter (0.22 μ). Cells were harvested 7 d after addition.
The results (Table 4) revealed that the addition of methyl jasmonic acid increased β-elemene production. Both salicylic acid and ethylene were also conductive to β-elemene accumulation in R. zedoariae cells, but salicylic acid and ethylene caused cell browning with the extending of incubation time. Ultraviolet irradiation did not promote β-elemene accumulation in R. zedoariae cells because most of ultraviolet radiation was blocked out by glass wall of the reactor.
Much progress has been made on the upstream pathway of terpenes synthesis in plants (Liu et al. 2005). It has been confirmed that germacrene A can be converted to β-elemene at high temperature without any biochemical catalysts(Leila et al. 2015). Harro et al. (2002) reported that FPP was converted into germacrene A with one-step synthesis catalyzed by GAS. In this study, the content of β-elemene was obviously increased in R. zedoariae cells compared to the control when FPP was fed to the cell suspensions as precursor. However, the GAS gene cannot be detected in two transcriptome database of R. zedoariae, and we also failed to clone this gene with experimental means. Hence, whether there are some differences for β-elemene synthesis in plants need to be further proved.
Expression patterns of HMGR, DXS, and FDS genes in suspension cells
HMGR, DXS, and FDS are important rate-limiting enzymes for terpene synthesis in plants. In this study, the expression patterns of HMGR, DXS and FDS genes in cell lines with higher β-elemene content were determined by PCR.
Figure 5 indicated that in cells of R. zedoariae, the IPPs used for β-elemene synthesis are mainly from MVA pathway because the expression level of HMGR gene was higher than that of DXS gene. As a key enzyme in the branch points of sesquiterpene synthetic pathway, FDS gene showed an active expression pattern. Accordingly, HMGR and FDS should be two key points in controlling β-elemene metabolism.
Expression patterns of HMGR, DXS, and FDS genes in suspension cells. Control (H), Control (D), and Control (F) represented the experiment control of HMGR, DXS and FDS gene respectively. Means ± SE of triplicates are shown. For each compound, columns with different letters are significantly different at p < 0.05 by ANOVA.
Raising β-elemene content by overexpression of HMGR, FDS, and ST02C genes in cells of R. zedoariae
The complete sequences of HMGR and FDS genes were obtained in a transcriptome database of R. zedoariae, and then confirmed by sequence alignment online at the URL of https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastx&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome . After cloning in R. zedoariae, the HMGR (Fig. 9) and FDS (Fig. 10) genes were transferred into plant expression vectors respectively used for transformation of R. zedoariae. ST02C gene and GAS gene and could catalyze FPP converted to germacrene A in different plants (Figs. 6 and 7). We failed to clone either of these genes in R. zedoariae. Therefore, we cloned GAS gene from Artemisia annua (Fig. 8) and synthesized the ST02C gene (Fig. 7). The plant expression vectors containing ST02C gene or GAS gene were constructed as well for transformation of R. zedoariae. As shown in Fig. 6, a total of 26 positive lines were obtained (Figs. 9 and 10).
Vector construct and PCR analysis for the presence of four genes in cell lines of R. zedoariae. Lane M represents the DNA Marker DL400. Lane + represents positive control. Lane– represents negative control. LaneF1~F8 represent cell lines from transformation with pCAMBIA2300-FDS. LaneS1~S8 represent cell lines from transformation with pCAMBIA2300-ST02C. LaneH1~H8 represent cell lines from transformation with pCAMBIA2300-HMGR. LaneG1~G9 represent cell lines from transformation with pCAMBIA2300-GAS.
The β-elemene content in transgenic cell lines of R. zedoariae is shown in Fig. 11. Cells overexpressing HMGR can efficiently promote accumulation of β-elemene in cell lines from H2 to H5 with the range of 0.15–0.17% (w/v), which was higher than the control (0.11% (w/v). This implied that the upstream approach of β-elemene biosynthesis was based more on the MVA pathway in R. zedoariae. The levels of β-elemene in FDS-trans formed lines also showed higher content of β-elemene ranging from 0.14% (w/v) to 0.18% (w/v) compared to the control level of 0.12% (w/v). The highest β-elemene content of 0.22% (w/v) was detected in ST02C-trans-formed lines (S5) when compared to the control lines (0.11 (w/v). Whether the ST02C gene was present in R. zedoariae or another gene was involved in the β-elemene biosynthesis needs to be confirmed through further analyses. Most of transgenic lines showed higher levels of β-elemene, reflecting that transgenic technology is a promising way to improve β-elemene accumulation in R. zedoariae cells.
Discussion
Application of the two-stage culture system
Two-stage cultivation is a common strategy used for plant cell culture. For traditional two-stage run, both cell proliferation and target substance synthesis proceed in different bioreactor systems. Traditional two-stage culture suffered from the drawbacks of slow adaptation of cells to the new growth environment and an increased risk of contamination during cell transfer between bioreactors. In this study, a combined system consisting of stir-tank and airlift bioreactors was used to conduct a two-stage perfusion cultivation of R. zedoariae suspension cells. The application of this apparatus can accomplish two-stage culture in one bioreactor system. The continuous cell culture was also achieved via a cell settling device composed by a spiral-type centrifugal settling pipe and a gravity settling pie that built into the air-lift column.
In the two-stage culture system, the stress of additives and ultraviolet radiation made the airlift column a tough environment for cell growth. The medium in the airlift column did not flow back to the stirred tank due to the medium inlet in airlift column was above the liquid surface. Therefore, the two reaction vessels were relatively independent each other though they were connected by a glass pipe. Cells in the stirred tank grew well and were continuously presented to the airlift column for β-elemene synthesis. In the airlift column, β-elemene content in cells was increased by additives and genetic transformation; meanwhile, normal growth of cells was maintained. Both biomass and secondary metabolites production were achieved simultaneously in one bioreactor system. This bioreactor culture system is stable, convenient, and inexpensive, with potential industrial applications. We plan further studies on scale-up and volume increase of this bioreactor system in future.
Preliminary study on the metabolic pathway of β-elemene in R. zedoariae
Progress has been made on the upstream pathway of terpene synthesis in plants. Advances in understanding the downstream pathway are urgently needed, but have not yet been achieved in most plants. Two independent pathways of IPP biosynthesis, the MVA and MEP pathways, have been found in higher plants. In some plants, the precursors of sesquiterpenes and triterpenes are largely from the contribution of MVP pathway, whereas the MEP pathway is involved to the synthesis of diterpenes as well as tetraterpenes (Liu et al. 2005). HMGR and DXS are the key rate-limiting enzymes of the MVA and MEP biosynthetic pathway respectively (Aquil et al. 2009). These enzymes often serve as important regulatory sites in the upstream pathway for terpene synthesis. In the present study, the HMGR gene was actively expressed in cells with high β-elemene content. In addition, cells overexpressing HMGR can more efficiently promote accumulation of β-elemene compared to the control line, implying that the upstream approach of β-elemene biosynthesis are based more on the MVA pathway in R. zedoariae. FDS was considered as a key enzyme in the branch points of sesquiterpene synthetic pathway in plants (Ping et al. 2012). The present study also indicated that it is essential to increase the metabolic flux towards sesquiterpenes via overexpression of FDS gene in R. zedoariae. ST02C is a key enzyme for β-elemene biosynthesis in ginger. In this study, the highest β-elemene content of 0.22% (w/v) was detected in ST02C-transformed lines, but we failed to obtain the nucleotide sequence of this gene from R. zedoariae. Whether these genes are absent in genome of R. zedoariae or a new pathway is present in R. zedoariae for β-elemene synthesis needs to be confirmed. There is only 49% homology between the GAS in Artemisia annua and ST02C in ginger, so it can also be speculated that another gene, similar in function to the ST02C or GAS, may be present in R. zedoariae. Further studies will be conducted to characterize more candidate genes in rhizoma zedoaria to obtain fine transgenic cell lines with higher β-elemene content.
References
Ana R, Takehiko S, Magdalena C, Berta A, Jose G, Aurelio GC, Carlos JO, Maria JR, Lorenzo Z, Leandro P (2014) Terpene down-regulation triggers defense responses in transgenic orange leading to resistance against fungal pathogens. Plant Physiol 164(1):321–339
Aquil S, Husaini AM, Abdin MZ, Rather GM (2009) Overexpression of the HMG-CoA reductase gene leads to enhanced artemisinin biosynthesis in transgenic Artemisia annua plants. Planta Med 75:1453–1458
Christopher JD, Derek JM, William CD, Bernard JV (2009) Kinetic simulation of a centrifugal bioreactor for high population density hybridoma culture. Biotechnol Prog 25(6):1650–1659
Christopher JD, Bernard JV, Cornelius FL (2011) Fluid flow through a high cell density fluidized-bed during centrifugal bioreactor culture. Biotechnol Prog 26(4):1014–1023
De la Torre T, Mottschall M, Lesjean B, Drews A, Iheanaetu A, Kraume M (2010) Filterability assessment in membrane bioreactors using an in situ filtration test cell. Water Sci Technol 61(11):2809–2812
Duan GY, Nils C, Jens S, Dirk W, Oliver E (2013) The metabolic interplay between plants and phytopathogens. Metabolites 3(1):1–23
Harro JB, Jan K, Francel WV, Iris GA, Jan WK, Eelco W (2002) Isolation and characterization of two Germacrene a synthase cDNA clones from Chicory1. Plant Physiol 129(1):134–144
Hyun JK, David RG (2012) Suites of terpene synthases explain differential terpenoid production in ginger and turmeric tissues. PLoS One 7(12):1–23
Kai A, RK M, Maurice WS, Michel AH, Robert CS (2004) Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol 135(4):2025–2037
Leila P, Hamid RM, Astrid K, Rudolf B, Ulo N (2015) Germacrene A synthase in yarrow (Achillea millefolium) is an enzyme with mixed substrate specificity: gene cloning, functional characterization and expression analysis. Front Plant Sci 6:1–15
Liu Y, Wang H, Ye HC (2005) Advances in the plant isoprenoid biosynthesis pathway and its metabolic engineering. J Integr Plant Biol 47:769–782
Marisol OV, Susan H, Sun MH, Jin YW, Lee EK, Gary JL (2016) Plant cell culture strategies for the production of natural products. BMB Rep 49(3):149–158
Niels JN, Chen XY, Mindy YW, Adam JM, Ramon LP, Andrew CA, So l AG, Ross GA (2015) Natural variation in monoterpene synthesis in kiwifruit: transcriptional regulation of terpene synthases by NAC and ETHYLENE-INSENSITIVE3-like transcription factors. Plant Physiol 167(4):1243–1258
Ping LL, Jun NW, Yan PG, Song G, Guang YR (2012) Adaptive evolution of the chrysanthemyl diphosphate synthase gene involved in irregular monoterpene metabolism. BMC Evol Biol 12:214–225
Qi L, Cheng R, Shi TL (2014) Regulatory network rewiring for secondary metabolism in Arabidopsis thaliana under various conditions. BMC Plant Biol 14:180
Wang GR, Chen QZ, Tang N, Li BL, Fan DL, Tang KX (2015) A split airlift bioreactor for continuous culture of Glycyrrhiza inflata cell suspensions. Plant Cell Tissue Organ Cult 121:121–126
Wang GR, Qi NM, Wang ZM (2010) Application of a stir-tank bioreactor for perfusion culture and continuous harvest of G. inflata suspension cells. Afr J Biotechnol 9(3):347–351
Wang GR, Qi NM (2009) Perfusion culture of G. inflata suspension cells in a stir-tank bioreactor. Aust J Bot 57:240–246
Zhao YS, Wu JJ, Zheng F, Tang Q, Yang LJ, Li LN, Wu WY, Hann SS (2015) β-elemene inhibited expression of DNA methyltransferase 1 through activation of ERK1/2 and AMPKα signaling pathways in human lung cancer cells: the role of Sp1. J Cell Mol Med 19(3):630–641
Zhou Y, Xie M, Song Y, WP ZHR, Tian YX, Wang Y, Bai SJ, Zhao YC, Chen XY, She GM (2016) Two traditional Chinese medicines Curcumae Radix and Curcumae Rhizoma: an ethnopharmacology, phytochemistry, and pharmacology review. Evid Based Complement Alternat Med 4:7–30
Funding
This work was supported by the Shanghai natural science fund (No.BS150004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Ewen Mullins
Rights and permissions
About this article
Cite this article
Wang, G.R., Wang, H. Cell suspension culture of Rhizoma zedoariae in a two-stage perfusion bioreactor system for β-elemene production. In Vitro Cell.Dev.Biol.-Plant 55, 209–220 (2019). https://doi.org/10.1007/s11627-018-9943-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-018-9943-9