Abstract
To promote the differentiation of human-induced pluripotent stem cells (hiPSCs) into myocardium through a standard chemically defined and small-molecule-based induction protocol (CDM3), and preliminarily prepare myocardial patches that provide experimental data and theoretical support for further maturation through other in vitro experiments and safety studies in vivo. After resuscitation, culture, and identification of hiPSCs, they were inoculated onto Matrigel-coated polycaprolactone (PCL). After 24 h, cell growth was observed by DAPI under a fluorescence microscope and the stemness of hiPSCs was identified by OCT4 fluorescence. After fixation, scanning electron microscopy was performed to observe the morphology of cells on the patch surface. On days 1, 3, 5, and 7 of culture, cell viability was determined by Cell Counting Kit-8 (CCK-8) assay and a curve was drawn to observe cell growth and proliferation. After co-culture with Matrigel-covered PCL for 24 h, hiPSCs were divided into control and CDM3 groups, and cultured for an additional 6 d. On the eighth day, cell growth was observed by DAPI under a fluorescence microscope, hiPSC stemness was identified by OCT4 fluorescence, and cardiomyocytes were identified by cardiac troponin T (cTnT) and α-actin expression. hiPSCs co-cultured with Matrigel-covered PCL for 24 h emitted green fluorescence indicating OCT4, showing that hiPSCs maintained their stemness on Matrigel-covered PCL scaffolds. DAPI emitted blue fluorescence, indicating that cells grew clonally with uniform cell morphology. Scanning electron microscopy showed that hiPSCs adhered and grew on PCL covered with Matrigel, with clearly visible cell outlines indicating normal morphology. Assessment of cell viability by the CCK-8 method showed that hiPSCs proliferated and grew on PCL scaffolds covered with Matrigel. After 6 d of culture, immunofluorescence showed that control group hiPSCs highly expressed the stem cell marker OCT4 but not myocardial markers cTnT or α-actin. In contrast, notable expression of myocardial markers cTnT and α-actin but not OCT4 occurred in the CDM3 group. hiPSCs can proliferate and grow on PCL scaffolds covered with Matrigel. Under the influence of CDM3, hiPSCs differentiated into cardiomyocyte-like cells, allowing the preliminary preparation of myocardial patches that can provide a better method for clinical treatment of myocardial infarction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases have become an important problem endangering human health all over the world (Virani et al. 2020). Following the occurrence of myocardial infarction, numbers of surviving cardiomyocytes decrease, eventually leading to the onset of arrhythmia and end-stage heart failure (Sutton and Sharpe 2000). At present, with the exception of heart transplantation, there is no treatment that can reverse myocardial damage caused by myocardial infarction, especially for patients with massive myocardial infarction (Anderson and Morrow 2017). Stem cells have attracted wide attention owing to their self-renewal ability and potential for cell differentiation. Stem cell transplantation is considered one of the most promising therapies for myocardial infarction to repair damaged myocardial structures and restore cardiac function (Laflamme and Murry 2011; Cahill et al. 2017; Yu et al. 2017).
Among various stem cells, induced pluripotent stem cells (iPSCs) have low immune rejection, unlimited self-proliferation, and full-lineage differentiation potential. Accordingly, hiPSCs are currently considered an important cell source for organ regeneration/repair (Karagiannis et al. 2019). Although various induction schemes have been used to obtain cardiomyocytes, CDM3-induced cardiomyocyte differentiation allows efficient and stable production of human-induced pluripotent stem cell–derived cardiomyocytes (hiPSC-CM) with similar myocardial marker protein expression, electrophysiology, and microstructure to human mature cardiomyocytes, showing great advantages. According to cTnT flow cytometry evaluation, the positive rate of cTnT was 80 ~ 95%, which showed great advantage. The CDM3 protocol refers to a chemically defined medium consisting of 3 components: the basal medium RPMI 1640, l-ascorbic acid 2-phosphate, and rice-derived recombinant human albumin, combined with small-molecule-based induction differentiation, which work together, and is an efficient and stable induction method for obtaining human-induced pluripotent stem cell–derived cardiocytes (Burridge et al. 2014; Tan et al. 2018; Hou et al. 2022; Minter-Dykhouse et al. 2022).
Owing to blood scour and mechanical contraction of the heart, implantation and survival rates of transplanted cells are low. To overcome this, carrier scaffolds are used to provide a good environment for transplanted cells, thereby improving cell survival, retention, and repair abilities (Gerecht-Nir et al. 2006). Polycaprolactone has been widely used in cardiac tissue engineering because of its plastic mechanical properties, good biocompatibility, and easy processing. The purpose of this study was to promote myocardial differentiation of hiPSCs using a CDM3 protocol after co-culture of PCL coated with Matrigel, and construction of a preliminary myocardial mesh that provides experimental data and theoretical support for further studies of maturity through in vitro experiments and safety through in vivo animal experiments.
Materials and methods
Materials
hiPSCs, PGM1 Stem Cell Medium, PSCeasy Stem Cell Recovery Medium, PSCeasy Stem Cell Digestion Solution, PSCeasy Stem Cell Substrate Working Solution, and a Human Cardiomyocyte Differentiation Kit were purchased from Cellapy (Beijing, China). The Substrate Working Solution is mainly composed of Corning 354277 Matrigel products. The Human Cardiomyocyte Differentiation Kit consists of cardiomyocyte differentiation medium and three cardiomyocyte differentiation additives. Cardiomyocyte differentiation medium mainly refers to the chemically defined CDM3 medium, which consists of the basal medium RPMI 1640, l-ascorbic acid 2-phosphate, and rice-derived recombinant human albumin. Cardiomyocyte Differentiation Additive I is mainly composed of 6 µM CHIR99021. Cardiomyocyte Differentiation Additive II is mainly composed of 2 µM WNT-C59. There is no special component in myocardial differentiation medium III; only the CDM3 medium is sufficient. A Cell Counting Kit-8 (CCK-8) was purchased from Yisheng (Shanghai, China). Immunofluorescently labeled antibodies were purchased from Abcam (Cambridge, UK). PCL patches were from the Department of Chemical Engineering at Tsinghua University (Beijing, China) and Suzhou Institute of Environmental Innovation at Tsinghua University. Patches were prepared as follows. At 25–30 °C and < 50% humidity, a PCL solution was subjected to an electrospinning device to form fibers deposited on a collection device. The fiber diameter was 1200 nm, the average fiber elastic modulus was 0.5 GPa, and the material thickness was 0.5 mm.
Resuscitation and culture of hiPSCs
hiPSCs frozen in liquid nitrogen were immersed in a 37 °C water bath and quickly thawed. Next, cells were diluted in 3 mL of recovery medium, centrifuged at 200 × g for 5 min, and resuspended in 2 mL of recovery medium. Cells were seeded on culture plates coated with the Substrate Working Solution 1 d in advance and cultured overnight. From the next day onward, the PGM1 Stem Cell Medium was used to maintain the culture and changed every day. At 80–90% confluence, the cells were digested and passaged onto a fresh culture plate coated with the substrate working solution.
Co-culture of hiPSCs with PCL scaffolds coated with Matrigel
Each PCL support was cut into 5-mm-diameter circular pieces with a hole punch, sterilized by ultraviolet irradiation for 1 h on both sides, and then placed into 96-well plates. Next, 100 μL of bottom working liquid was added to each well and the plates were kept at 37℃ overnight. After abandonment, hiPSCs in the cell culture plate were dissociated into a single-cell suspension, inoculated drop by drop onto the PCL scaffold covered with Matrigel, and incubated overnight to ensure cell attachment. Subsequently, the medium was changed daily.
Experimental grouping and induced differentiation of human-induced pluripotent stem cells
hiPSCs and PCL coated with Matrigel were co-cultured for 24 h and divided into control and CDM3 groups. The control group was cultured with PGM1 Stem Cell Medium for 6 d, while the CDM3 group was cultured with Human Cardiomyocyte Differentiation Complete Medium for 6 d.
According to the kit instructions, Human Cardiomyocyte Differentiation Additives I, II, and III were mixed with CDM3 medium to form Human Cardiomyocyte Differentiation Complete Medium I, II, and III, respectively. When hiPSCs and PCL were co-cultured for 24 h, myocardial differentiation was initiated. Briefly, at day 0, 80–90% confluent hiPSCs were cultured in CDM3 medium with 6 µM CHIR99021 for 48 h. At day 2, the medium was changed to CDM3 medium supplemented with 2 µM WNT-C59 and continued to incubate for 48 h. The medium was refreshed on day 4 and every other day for the CDM3 medium.
Immunofluorescence staining
hiPSCs cultured on a cell culture plate or PCL scaffold and hiPSC-CM cultured on a PCL scaffold were observed by immunofluorescence imaging. Briefly, cells were fixed with 4% paraformaldehyde at room temperature for 20 min, permeabilized by 0.5% Triton-X100 at room temperature for 10 min, and blocked with 4% bovine serum albumin at room temperature for 1 h. Rabbit anti-rat Oct4 (1:200), rabbit anti-rat cardiac troponin T (cTnT, 1:200), and rabbit anti-rat α-actin (1:250) primary antibodies were added for incubation at 4℃ overnight. After washing three times with PBS, goat anti-rabbit IgG Alexa Fluor 488 was applied at room temperature for 2 h in the dark. To stain cell nuclei, DAPI was added at room temperature for 10 min. Cells were washed with PBS three times for 5 min each time between each step. Fluorescence images were captured with a Leica PDMI 4000 B inverted fluorescence microscope (Wetzlar, Germany).
Scanning electron microscopy
Specimens were washed with PBS, fixed with 2.5% glutaraldehyde, stored at 4 °C, and prepared as electron microscopy specimens to observe the surface morphology of the PCL material coated with Matrigel and cell morphology of hiPSCs cultured on Matrigel-coated PCL.
Determination of cell proliferation by the CCK-8 method
hiPSCs were co-cultured with PCL coated with Matrigel in basal medium for 7 d, and cell proliferation was measured by CCK-8 on days 1, 3, 5, and 7 of culture. Each group was set up with four replicate wells, and the absorbance of each well at 450 nm was measured with a Synergy 4 plate reader (BioTek, Winooski, VT, USA).
Data analysis and statistics
Three experiments were conducted. Data were statistically processed by SPSS 20.0 (IBM, Armonk, NY) and expressed as mean + standard deviation (x + s). Statistical analysis was performed using the independent sample mean test. Differences were considered significant at P < 0.05.
Results
Morphology and identification of hiPSCs
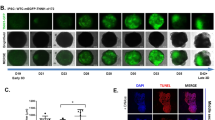
After 24 h of culture, hiPSCs were cloned and grown in traditional culture dishes coated with Matrigel. The resulting colonies were flat, dense, and regular in shape (Fig. 1).
OCT4 is a specific marker protein of iPSCs. Expression of OCT4 in stem cells was observed as the emission of green immunofluorescence (Fig. 2), whereas nuclei appear blue (Fig. 3). Following culture of hiPSCs on patches for 24 h, immunofluorescence indicates that the morphology of hiPSCs was consistent across the three groups. Specifically, hiPSCs displayed characteristics of stem cells including large nucleoli, small cytoplasm, and strong expression of OCT4, indicating that the cells remained undifferentiated (Fig. 2A–C). Nuclear DAPI staining was strong, indicating that hiPSCs had adhered to PCL (Fig. 3A–C). Green fluorescence on Matrigel-coated plastic indicated that hiPSCs mostly grew as monolayers (Fig. 2A). In contrast, the green fluorescence of the two cell-patch groups mostly overlapped and displayed bright and dark phases, indicating that hiPSCs not only grew on the PCL surface but also migrated to the interior of the three-dimensional network (Fig. 2B, C). The Matrigel-coated PCL group had brighter green fluorescence than the PCL group, indicating that hiPSCs grew in large numbers and Matrigel promoted both stem cell adhesion and migration on PCL.
Green fluorescence staining of OCT4 stem cells for dry identification protein. (A ~ C) Stem cells co-cultured with hiPSC and cell culture plates coated with Matrigel were evaluated. (A) hiPSC nuclei were stained blue by DAPI (× 200); (B) hiPSC expresses green fluorescence Photodry marker OCT4 (× 200); (C) A and B chimeric (× 200); (D ~ F) Stem cell co-cultured with hiPSC and PCL coated with Matrigel were identified. (D) hiPSC nucleus dyed blue by DAPI (× 200); (E) hiPSC expressed green fluorescence dry marker OCT4 (× 200); (F) mosaic in D and E (× 200).
Green fluorescence staining of OCT4 stem cells for dry identification protein. A ~ C was the control group; (A) hiPSC nuclei were stained blue by DAPI (× 200); (B) hiPSC expressed green fluorescence dry marker OCT4 (× 200); (C) A and B chimeric (× 200); D ~ F are CDM3 group; (D) hiPSC nucleus was stained blue by DAPI (× 200); (E) there was no obvious green fluorescence dry marker of hiPSC expression OCT4 (× 200); (F) mosaic in D and E (× 200).
Immunofluorescence detection of myocardial markers
After 24 h of co-culture of hiPSCs and PCL coated with Matrigel, control and CDM3 groups were cultured with the corresponding medium for 6 d. Immunofluorescence shows that the hiPSC stem cell marker OCT4 was highly expressed in the control group (Fig. 3A–C), whereas no significant green fluorescence indicating OCT4 expression was observed in the CDM3 group (Fig. 3D–F). Correspondingly, cTnT (Fig. 4A–C) and α-actin (Fig. 5A–C) were not significantly expressed in the cells of the control group, whereas there was notable expression of cTnT (Fig. 4D–F) and α-actin (Fig. 5D–F) in the CDM3 group, indicating that the preliminary preparation of myocardial mesh was successful.
cTnT myocardial marker protein was stained with green fluorescence. A ~ C was the control group; (A) hiPSC nuclei were stained blue by DAPI (× 200); (B) there was no obvious expression of cTnT myocardial marker protein in hiPSC (× 200). (C) A and B chimeric (× 200); D ~ F are CDM3 group; (D) hiPSC nucleus was stained blue by DAPI (× 200); (E) hiPSC expressed cTnT myocardial marker protein (× 200); (F) mosaic in D and E (× 200).
α-Actin myocardial marker protein was stained with green fluorescence. A ~ C was the control group; (A) hiPSC nuclei were stained blue by DAPI (× 200); (B) there was no obvious expression of α-actin myocardial marker protein in hiPSC (× 200). (C) A and B chimeric (× 200); D ~ F are CDM3 group; (D) hiPSC nucleus was stained blue by DAPI (× 200); (E) hiPSC expressed α-actin myocardial marker protein (× 200); (F) mosaic in D and E (× 200).
Scanning electron microscopy observation
After 24 h of co-culture of hiPSC and PCL, electron microscopy showed that the surface of PCL was covered by white Matrigel. In PCL coated with Matrigel, large cells were embedded into the Matrigel, and cell profiles were clearly visible, mostly oval, forming cell colonies (Fig. 6A, B).
Determination of cell proliferation by CCK-8
hiPSCs were cultured in basal medium on PCL covered with Matrigel for 7 d. The optical density value was 1.422 ± 0.015 on day 1, 1.751 ± 0.037 on day 3, and 1.898 ± 0.024 on day 5 and on day 7. The resulting proliferation curve demonstrates that hiPSCs grew and proliferated on PCL coated with Matrigel (Fig. 7).
Discussion
Myocardial infarction is the main cause of chronic heart failure, which leads to the formation of fibrous scar tissue following necrosis of cardiomyocytes. Current therapies focus on controlling the symptoms of heart failure and cannot reverse its pathophysiological process. However, its poor prognosis and high mortality rate compel the search for new treatments.
In recent years, stem cell therapy has attracted much attention because of its self-renewal ability and targeted differentiation potential. Among many candidate stem cells, iPSCs have been widely studied owing to their low immune rejection, unlimited self-proliferation, and full-spectrum differentiation potential. Studies have shown that iPSCs can differentiate into skeletal muscle, chondrocytes, hepatocytes, nerve cells, cardiomyocytes, and other cell types (Csobonyeiova et al. 2021; Kerr et al. 2021; Lent et al. 2021; Nguyen et al. 2021; Iberite et al. 2022). However, direct transplantation of cells into areas of myocardial infarction does not produce a good therapeutic effect, and most cells are lost to flushing and beating of the heart muscle. To improve the rate of cell implantation, cardiac tissue engineering has emerged. The co-culture of cells and carriers to prepare cardiac mesh for transplantation into the infarct area can greatly improve the efficiency of implantation.
At present, sources of carrier scaffolds are mainly divided into natural and synthetic materials. Natural biomaterials are extracted from natural biological sources, which has the advantage of simulating the cellular microenvironment. However, because natural materials do not have good mechanical properties, they often face great challenges when applied to heart tissues with high mechanical shrinkage strength. Synthetic materials can be used to construct scaffolds with definite physical and mechanical properties according to requirements, and can even be combined with different scaffolds in various ways to improve their performance with high repeatability, including materials like polycaprolactone, polylactic acid, and polyurethane (Streeter and Davis 2018).
Among these scaffolds, polycaprolactone has been approved by the United States Food and Drug Administration as a biomedical material for its unique characteristics. Polycaprolactone made by electrospinning technology provides a favorable environment for cell adhesion and growth, and has good biocompatibility. In addition, it has good mechanical properties, can maintain a certain mechanical strength to resist physiological stress generated by the implant site, and is consistent with the mechanical characteristics of the target region. Moreover, polycaprolactone has low biodegradability and the transplanted scaffolds should provide enough degradation time to permit tissue attachment and growth until the new tissues can stably function. Currently, scaffolds based on polycaprolactone are completely degraded in 3–4 yr. Polycaprolactone is also characterized by low immunogenicity and non-toxicity, making it suitable for cardiac tissue engineering (Siddiqui et al. 2018; Sowmya et al. 2021). Related studies (Wanjare et al. 2017; Hendrickson et al. 2021; Sridharan et al. 2021) have shown that both polycaprolactone scaffolds and polycaprolactone-gelatin scaffolds can be co-cultured with hiPSC-CM to form myocardial patches.
At present, various differentiation induction methods are available to promote the myocardial differentiation of stem cells, such as 5-azacytidine (Rachel et al. 2020), basic fibroblast growth factor (Rosenblatt-Velin et al. 2005), and exosomes (Ramesh et al. 2021). However, the cardiomyoid cells obtained by these methods have the risk of low maturity and arrhythmia following transplantation to areas of myocardial infarction (Anderson et al. 2014; Chong et al. 2014). In our research group, myocardial mesh has been prepared by inducing differentiation methods such as vitamin C (Shijun et al. 2016), contact co-culture (Zhang et al. 2022), and 5-azacytidine glycoside (Niu et al. 2013). However, the obtained myocardial mesh has similar low maturity and potential risk of arrhythmia after transplantation.
Studies at home and abroad have shown that CDM3-induced myocardial differentiation can more efficiently and stably obtain hiPSC-CM. Moreover, the obtained cardiomyoid cells have more similar myocardial marker protein expression, electrophysiology, and microstructure to mature human cardiomyocytes. Therefore, in this study, the standard chemical definition and small-molecule induction scheme (CDM3) were adopted. After 24 h of co-culture of hiPSC and PCL coated with Matrigel, myocardial differentiation of hiPSCs was promoted under the action of CDM3 induction, allowing preliminary preparation of a myocardial mesh.
After 24 h of hiPSC co-culture on PCL coated with Matrigel, immunofluorescence showed that hiPSCs maintained stem cell dryness and proliferated on the scaffold. Scanning electron microscopy clearly revealed the white Matrigel coating on the PCL, and the outline of a cell on the material surface can be seen. After 6 d of continuous culture, hiPSCs still highly expressed the stem cell marker OCT4 but not myocardial markers cTnT or α-actin. After 6 d of culture in differentiation medium, the CDM3 group showed myocardial differentiation of hiPSCs. hiPSC-CM expressed cTnT and α-Actin, but no longer expressed the dry marker OCT4, preliminarily indicating hiPSC myocardial differentiation. hiPSCs were co-cultured on PCL coated with Matrigel in PGM1 Stem Cell Medium for 7 d. OD values were determined by the CCK-8 method at 1, 3, 5, and 7 d. The resulting growth curve revealed that hiPSCs prolificated stably on PCL coated with Matrigel. Thus, the experimental results of this study prove that hiPSCs can grow and proliferate on PCL coated with Matrigel, whereas CDM3 promotes the differentiation of hiPSC into cardiomyoid cells; in addition, a myocardial mesh was preliminarily prepared.
The results of this experiment demonstrate that CDM3 can promote myocardial differentiation of hiPSCs on PCL and preliminary preparations of myocardial mesh. In subsequent studies, the differentiation efficiency and maturity of cardiomyoid cells in myocardial mesh prepared by our CDM3 scheme will be demonstrated through quantitative experiments in vitro. The safety of myocardial mesh obtained by our CDM3 scheme was verified by animal experiments in vivo. In the future, further improvements of myocardial mesh maturity and clinical applications still need research and demonstration.
Data availability
Not applicable.
References
Anderson JL, Morrow DA (2017) Acute myocardial infarction. N Engl J Med 376(21):2053–2064
Anderson ME, Goldhaber J, Houser SR et al (2014) Embryonic stem cell–derived cardiac myocytes are not ready for human trials. Circ Res 115(3):335–338
Burridge PW, Matsa E, Shukla P et al (2014) Chemically defined generation of human cardiomyocytes. Nat Methods 11(8):855–860
Cahill TJ, Choudhury RP, Riley PR (2017) Heart regeneration and repair after myocardial infarction: translational opportunities for novel therapeutics. Nat Rev Drug Discovery 16(10):699–717
Chong JJH, Yang X, Don CW et al (2014) Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510(7504):273–277
Csobonyeiova M, Polak S, Nicodemou A et al (2021) iPSCs in modeling and therapy of osteoarthritis. Biomedicines 9(2):186
Gerecht-Nir S, Radisic M, Park H et al (2006) Biophysical regulation during cardiac development and application to tissue engineering. Int J Dev Biol 50(2–3):233–243
Hendrickson T, Mancino C, Whitney L et al (2021) Mimicking cardiac tissue complexity through physical cues: a review on cardiac tissue engineering approaches. Nanomed: Nanotechnol Biol Med 33:102367
Hou X, Ma S, Fan W et al (2022) Chemically defined and small molecules-based generation of sinoatrial node-like cells. Stem Cell Res Ther 13(1):158
Iberite F, Gruppioni E, Ricotti L (2022) Skeletal muscle differentiation of human iPSCs meets bioengineering strategies: perspectives and challenges. NPJ Regen Med 7(1):23
Karagiannis P, Takahashi K, Saito M et al (2019) Induced pluripotent stem cells and their use in human models of disease and development. Physiol Rev 99(1):79–114
Kerr CM, Richards D, Menick DR et al (2021) Multicellular human cardiac organoids transcriptomically model distinct tissue-level features of adult myocardium. Int J Mol Sci 22(16):8482
Laflamme MA, Murry CE (2011) Heart regeneration. Nature 473(7347):326–335
Minter-Dykhouse K, Nelson TJ, Folmes C (2022) Uncoupling of proliferative capacity from developmental stage during directed cardiac differentiation of pluripotent stem cells. Stem Cells Dev 31(17–18):521–528
Nguyen R, Da Won BS, Qiao L et al (2021) Developing liver organoids from induced pluripotent stem cells (iPSCs): an alternative source of organoid generation for liver cancer research. Cancer Lett 508:13–17
Niu H, Mu J, Zhang J et al (2013) Comparative study of three types of polymer materials co-cultured with bone marrow mesenchymal stem cells for use as a myocardial patch in cardiomyocyte regeneration. J Mater Sci - Mater Med 24(6):1535–1542
Rachel K, Pathak S, Moorthi A et al (2020) 5-Azacytidine incorporated polycaprolactone-gelatin nanoscaffold as a potential material for cardiomyocyte differentiation. J Biomater Sci Polym Ed 31(1):123–140
Ramesh S, Govarthanan K, Ostrovidov S et al (2021) Cardiac differentiation of mesenchymal stem cells: impact of biological and chemical inducers. Stem Cell Rev Rep 17(4):1343–1361
Rosenblatt-Velin N, Lepore MG, Cartoni C et al (2005) FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Investig 115(7):1724–1733
Shijun X, Junsheng M, Jianqun Z et al (2016) In vitro three-dimensional coculturing poly3-hydroxybutyrate-co-3-hydroxyhexanoate with mouse-induced pluripotent stem cells for myocardial patch application. J Biomater Appl 30(8):1273–1282
Siddiqui N, Asawa S, Birru B et al (2018) PCL-based composite scaffold matrices for tissue engineering applications. Mol Biotechnol 60(7):506–532
Sowmya B, Hemavathi AB, Panda PK (2021) Poly (epsilon-caprolactone)-based electrospun nano-featured substrate for tissue engineering applications: a review. Prog Biomater 10(2):91–117
Sridharan D, Palaniappan A, Blackstone BN et al (2021) In situ differentiation of human-induced pluripotent stem cells into functional cardiomyocytes on a coaxial PCL-gelatin nanofibrous scaffold. Mater Sci Eng C 118:111354
Streeter BW, Davis ME 2018 Therapeutic cardiac patches for repairing the myocardium. Cham:Springer International Publishing 1144 1–24
Sutton MG, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101(25):2981–2988
Tan X, Dai Q, Guo T et al (2018) Efficient generation of transgene- and feeder-free induced pluripotent stem cells from human dental mesenchymal stem cells and their chemically defined differentiation into cardiomyocytes. Biochem Biophys Res Commun 495(4):2490–2497
Van Lent J, Verstraelen P, Asselbergh B et al (2021) Induced pluripotent stem cell-derived motor neurons of CMT type 2 patients reveal progressive mitochondrial dysfunction. Brain 144(8):2471–2485
Virani S, Alonso A, Benjamin E J, et al (2020) Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 141(9)
Wanjare M, Hou L, Nakayama KH et al (2017) Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells. Biomater Sci 5(8):1567–1578
Yu H, Lu K, Zhu J et al (2017) Stem cell therapy for ischemic heart diseases. Br Med Bull 121(1):135–154
Zhang Z, Zhou F, Zheng J, et al (2022) Preparation of myocardial patches from DiI-labeled rat bone marrow mesenchymal stem cells and neonatal rat cardiomyocytes contact co-cultured on polycaprolactone film. Biomed Mater (Bristol) 17(4)
Acknowledgements
We thank Liwen Bianji (Edanz) (www.liwenbianji.cn/) for basic language editing of a draft of this manuscript.
Funding
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: 2018 National Natural Science Foundation (81870181) and 2022 National Natural Science Foundation (82270255).
Author information
Authors and Affiliations
Contributions
DY carried out the molecular genetic studies, participated in the immunoassays, and drafted the manuscript. BP carried out flow cytometry. ZJw participated in the co-culture. YB participated in the design of the study and performed the statistical analysis. MJS and ZF conceived of the study, participated in its design and coordination, helped to draft the manuscript, etc. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was approved by the ethics committee of Beijing Anzhen Hospital, Capital Medical University. The batch number of the ethics approval document is GZR-3–072. The Beijing Anzhen Hospital and national animal care and use guidelines were followed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, Y., Zhou, F., Zheng, J. et al. Effect of CDM3 on co-culture of human-induced pluripotent stem cells with Matrigel-covered polycaprolactone to prepare cardiac patches. In Vitro Cell.Dev.Biol.-Animal 59, 256–263 (2023). https://doi.org/10.1007/s11626-023-00764-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-023-00764-4