Abstract
Spermatogonial stem cell transplantation (SSCT) is a strategy that has demonstrated to be feasible to restore spermatogenesis in animal models when it is performed shortly after the gonadotoxic onset to destroy their endogenous germ cells. However, in the case of boys subjected to fertility preservation, future transplantations will be performed with a delay of many years. In order to study how timing of SSCT affects donor-derived spermatogenic recovery in mice, we compared the percentage of spermatogenic tubule cross-sections within testes of 59 C57BL/6NCrl mice distributed in 6 groups: group 1, untreated mice controls (n = 9); group 2, mice that received a single dose of busulfan 40 mg/kg (n = 10); group 3, mice that received two additional doses of busulfan 10 mg/kg every 5 weeks (n = 10); group 4 (SSCT-A), mice subjected to a standard SSCT performed 5 weeks after a single injection of busulfan 40 mg/kg (n = 10); group 5 (SSCT-B), mice subjected to a delayed SSCT performed 15 weeks after a single injection of busulfan 40 mg/kg (n = 10); and group 6 (SSCT-C), mice subjected to a delayed SSCT with two additional doses of busulfan 10 mg/kg every 5 weeks (n = 10). Spermatogenic recovery in standard SSCT-A and SSCT-C groups ranged between 22.29 and 22.65%, compared with a lower recovery rate of 11.54% showed in the SSCT-B group. However, donor contribution resulted higher in standard SSCT-A, representing a 69.71% of cross-sections, compared with the rest of conditions ranging from 34.69 to 35.42%. Overall, we concluded that a delay in the SSCT from the gonadotoxic onset decreases the efficiency of donor-derived spermatogenic recovery in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The improvement in long-term childhood cancer survival rates (Smith et al. 2010) has triggered that part of the scientific community focuses in strategies that warrant the best life quality to survivors, including the possibility of genetic parenthood. Because of this, fertility preservation in cancer patients and other patients that are subjected to potentially gonadotoxic treatments is gaining social and scientific interest (Picton et al. 2015; Medrano et al. 2017). However, since prepubertal boys are unable to produce sperm for freezing (Daudin et al. 2015), cryopreservation of immature testicular tissue is an experimental approach that may restore their fertility in the future if needed (Keros et al. 2007; Wyns et al. 2008; Ginsberg et al. 2010; Wyns et al. 2011; Baert et al. 2013; Poels et al. 2013; Ginsberg et al. 2014; Picton et al. 2015; Wyns et al. 2015; Medrano et al. 2017).
Cryopreservation of immature testicular tissue is based in the presence of spermatogonial stem cells (SSCs) within the seminiferous tubules with the potential to restore spermatogenesis (de Rooij 2009). Therefore, different strategies based in the potential of SSCs within immature testicular tissue to regenerate spermatogenesis are currently under research with the aim to restore fertility of patients unable to produce sperm in the next years (Picton et al. 2015; Medrano et al. 2017). Among these strategies, grafting of immature testicular tissue has shown interesting results maturating SSCs within prepubertal tissue into functional sperm able to produce healthy offspring not only in mice (Honaramooz et al. 2002) but also in rhesus macaques (Jahnukainen et al. 2012; Fayomi et al. 2019), whereas in vitro spermatogenesis, despite its success inducing the differentiation of rodent SSCs into functional sperm in vitro (Yokonishi et al. 2014; Alves-Lopes et al. 2017), has been difficult to replicate with human tissue (de Michele et al. 2017; de Michele et al. 2018; Medrano et al. 2018). Finally, spermatogonial stem cell transplantation (SSCT) into germ cell-depleted testes is a strategy that has demonstrated to be a feasible technique to restore spermatogenesis in several animal models (Brinster and Avarbock 1994; Ogawa et al. 1997; Dobrinski et al. 1999; Dobrinski 2006; Medrano et al. 2014; Kanatsu-Shinohara et al. 2016), including non-human primates (Hermann et al. 2012). The risk of malignant cell contamination within the cryopreserved donor tissue is a limitation of SSCT that poses important challenges for clinical practice, and therefore strategies for the elimination of such possible contamination are under research (Dovey et al. 2013; Valli et al. 2014). However, in contrast to the strategies mentioned above, SSCT has a special interest since it opens the possibility to future patients to have a natural conception after the spermatogenic restoration by their own auto-transplanted SSCs (Medrano et al. 2017). Although there exist some reports that aimed to perform SSCT in human patients, there is a lack of information regarding the follow-up of transplanted patients (Radford 2003).
Another limitation of SSCT is the need of a receptive seminiferous epithelium for the proper donor SSC colonization. For the success of SSCT, it is necessary that the niche of SSCs, the seminiferous epithelium, remains available for the proper colonization of transplanted SSCs (Ogawa et al. 1997; Kubota and Brinster 2018). Because of this, in the mouse model, hosts are usually treated with alkylating drugs such as busulfan to deplete their endogenous germ cells before the transplant (Wang et al. 2010), recapitulating the clinical situation of human patients exposed to chemotherapy and/or radiotherapy (Meistrich 2013; Medrano et al. 2017). However, whereas in animal models SSCT is commonly performed shortly after the gonadotoxic onset to destroy their endogenous germ cells (Brinster and Avarbock 1994; Medrano et al. 2014; Kubota and Brinster 2018), in humans such future transplantation will be performed with a delay of many years since patients received their last gonadotoxic exposure and only in the case that they present azoospermia (Medrano et al. 2017). In this regard, timing for SSCT in humans will notably differ from the mouse model, and the impact of such delay in the receptivity of seminiferous epithelium for SSC colonization and therefore spermatogenic recovery is unknown.
Based in this background, here we employed a mouse model to test how a delay in time of the SSCT since the gonadotoxic onset of hosts may affect the success of the technique. Since spontaneous recovery of endogenous spermatogenesis of hosts after chemical germ cell depletion is common in mice, we also optimized a sterilizing protocol based in the repeated administration of busulfan before the transplant to keep the seminiferous epithelium depleted of endogenous germ cells for the moment of the delayed SSCT. Altogether, we finally compared SSC colonization efficiency and spermatogenic recovery in host mice subjected to standard SSCT and those subjected to a delayed SSCT with and without repeated busulfan administration.
Materials and methods
Setup of optimal repeated busulfan dose for the delayed SSCT model
After receiving an initial intraperitoneal injection of 40 mg/kg of busulfan (Merck, Darmstadt, Germany) at week 5, 12 C57BL/6NCrl (Charles River Laboratories, Barcelona, Spain) males were split in 4 groups of 3 and subjected to 2 further injections at weeks 10 and 15 with doses of 40 mg/kg, 20 mg/kg, 10 mg/kg, and 5 mg/kg, respectively. All animals were sacrificed at week 20, and the presence of spermatogenic tubule cross-sections within their testes was analyzed in order to find the optimal repeated busulfan dose to keep the seminiferous epithelium depleted of endogenous germ cells for a delayed SSCT.
Experimental design
59 C57BL/6NCrl (Charles River Laboratories) 5-week-old males were distributed in 6 groups as follows: group 1, untreated mice controls (n = 9); group 2, mice that received a single dose of busulfan 40 mg/kg at week 5 (n = 10); group 3, mice that received two additional doses of busulfan 10 mg/kg every 5 wk (n = 10); group 4 (SSCT-A), mice subjected to a standard SSCT performed 5 wk after a single injection of busulfan 40 mg/kg (n = 10); group 5 (SSCT-B), mice subjected to a delayed SSCT performed 15 weeks after a single injection of busulfan 40 mg/kg (n = 10); and group 6 (SSCT-C), mice subjected to a delayed SSCT with two additional doses of busulfan 10 mg/kg every 5 wk (n = 10). Animals from groups 1 to 3 were sacrificed at different time periods according to Fig. 1. Groups 4 to 6 received a SSCT of 200,000 cells/testis 5 wk after their last exposure to busulfan and were sacrificed 10 wk later for testis analysis (Fig. 1).
Experimental design. 59 C57BL/6NCrl 5-week-old males were distributed in 6 groups as follows: group 1, untreated mice controls (n = 9); group 2, mice that received a single dose of busulfan 40 mg/kg (n = 10); group 3, mice that received two additional doses of busulfan 10 mg/kg every 5 wk (n = 10); group 4 (SSCT-A), mice subjected to a standard SSCT performed 5 wk after a single injection of busulfan 40 mg/kg (n = 10); group 5 (SSCT-B), mice subjected to a delayed SSCT performed 15 wk after a single injection of busulfan 40 mg/kg (n = 10); and group 6 (SSCT-C), mice subjected to a delayed SSCT with two additional doses of busulfan 10 mg/kg every 5 wk (n = 10). With the exception of untreated controls, all animals received one intraperitoneal dose of busulfan 40 mg/kg at week 5, whereas those from groups 3 and 6 received two additional injections of 10 mg/kg at weeks 10 and 15. Untreated controls and animals from groups 2 and 3 were sacrificed at weeks 5, 10, and 20, whereas animals from groups 4 to 6 received a SSCT of 200,000 cells/testis 5 wk after their last exposure to busulfan and were sacrificed 10 wk later for testis analysis.
SSCT
In order to transplant cell suspensions enriched for GFP + SSCs, the testes from a total of twelve 3-wk-old males from the strain Tg(CAG-EGFP)B5Nagy/J were disaggregated into single cells following a two-step digestion with collagenase IV 1 mg/mL and trypsin 0.05% (Thermo Scientific, Waltham, MA) as previously described (Ogawa et al. 1997). Resulting cells were resuspended in DMEM/F12 (Thermo Scientific) to a concentration of 20*106 cells/mL with a cell viability higher that 95% (data not shown). Since the germ cell composition of 3-week-old donors consists only in spermatogonia, no further steps for SSC enrichment were performed prior to transplantations. SSCT was performed with fresh cell donor preparations. Prior to SSCT, DNAse I (final concentration 200 μg/mL) and trypan blue (10% v/v) (all from Merck) were added to prevent cell clumping and for tracking purposes, respectively, and a volume of 10 μL (equivalent to 200,000 cells) was injected into each testis via efferent ducts as previously described (Medrano et al. 2014). Overall, 20 testes (10 animals per group) were injected in each experimental group.
Immunohistologic analysis of testes
After weighting them, testes were decapsulated, and a small fraction, 20% of the volume of each one, was designated to whole-mount staining. Remaining tissue (80% of the volume) was fixed in 10% formaldehyde (Merck) o/n at 4°C, embedded in paraffin (Merck) and sliced in 5-μm thin sections. Staining of sections with hematoxylin and eosin (Merck) was performed to quantify the number of tubule cross-sections with spermatogenic activity. For immunohistochemical analysis, de-paraffined slides were subjected to antigen retrieval by treating them with 10 mM citrate buffer pH6 for 20′ at 97°C before a blocking step with 1xPBS + 10% normal donkey serum + 1% bovine serum albumin + 0.1% Triton X-100 (all from Merck) for 1 h at room temperature. Incubation of primary antibodies (Supplemental Table I) was carried out o/n at 4°C. Secondary Alexa Fluor antibodies (Thermo Scientific) were incubated for 1 h in darkness at room temperature prior to mounting the slides with ProLong Gold antifade reagent with DAPI (Thermo Scientific). Negative controls were performed by the omission of the first antibody and with unspecific IgGs (data not shown). Slides were visualized using a fluorescence microscope DM2500 (Leica, Wetzlar, Germany). Every tubule cross-section showing at least one Vasa+ cell was considered positive for spermatogenic activity, whereas only those tubules with at least one Vasa+/GFP+ double-positive cell were counted as GFP donor-derived spermatogenesis cross-sections. All cell counts were assessed in at least two representative sections per sample with a minimal depth distance of 100 μm between them. Incomplete tubule cross-sections in which only a part of the cross-section was visible were discarded from counts to avoid bias. In order to avoid subjectivity, all counts were blind and performed by two researchers independently. Therefore, all counts were compared and repeated when discrepancy between researchers was higher than 25%. Colonization efficiency was calculated as the number of GFP+ spermatogenic tubule cross-sections per 200,000 cells transplanted in each SSCT. For whole-mount staining analysis of tubule spreads, we followed the protocol described in Gassei et al. (2014).
Statistics
Data regarding the ratio testis weight (mg)/body weight (g), the percentage of tubule cross-sections with spermatogenesis, and the percentage of tubule cross-sections with donor-derived GFP+ spermatogenesis was compared among all groups and statistically analyzed by one-way analysis of variance (Kruskal-Wallis test). For all comparisons, significance was accepted when p < 0.05.
Results
Repeated exposure to busulfan along time is necessary to prepare hosts with a germ cell-depleted niche for delayed SSCT
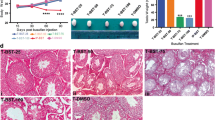
Our initial experiments were focused in the study of the spermatogenesis status of mice upon their exposure to a single dose of 40 mg/kg busulfan in order to check if this standardized sterilizing protocol (Dobrinski et al. 1999; Dobrinski 2006; Medrano et al. 2014) may be useful to prepare hosts for a delayed SSCT. Intraperitoneal administration of busulfan 40 mg/kg to 5-week-old males resulted in a decrease of the ratio testis weight (mg)/body weight (g) and an almost complete germ cell depletion when testes were analyzed at week 10 (Fig. 2a–c). However, 21.75% of tubule cross-sections spontaneously recovered spermatogenesis after 15 weeks from the gonadotoxic onset (Fig. 2b–c).
Setup of a chemical sterilization protocol to avoid spermatogenic recovery for delayed SSCT. (a) Representation of the ratio of testis weight (mg)/body weight (g) from week 5 to 20 for all tested conditions. (b) Representation of the percentage of tubule cross-sections with active spermatogenesis from week 5 to 20 for all tested conditions. (c) Illustrative pictures of the testicular histology at weeks 10 and 20 of untreated controls, mice treated with one single dose of busulfan 40 mg/kg at week 5, and mice treated with one initial dose of busulfan 40 mg/kg at week 5 and two further doses of 10 mg/kg every 5 wk. Black asterisks indicate tubule cross-sections with recovered spermatogenesis. Scale bar corresponds to 100 μm. (d) Results from the quantification of the percentage of tubule cross-sections with active spermatogenesis at week 20 for all tested conditions. Data is presented as mean ± standard error. Asterisks indicate statistical differences with p < 0.05.
Since such 21.75% of spontaneous spermatogenic recovery may interfere in the proper colonization of SSCs upon a delayed SSCT (Ogawa et al. 1997; Kubota and Brinster 2018), in a second experimental phase, we decided to test four sterilizing protocols based in an initial germ cell depletion with busulfan 40 mg/kg at week 5, followed by two additional injections of busulfan every 5 weeks in order to limit spontaneous germ cell recovery (Fig. 1).
All four protocols tested resulted in a successful germ cell-depleted seminiferous epithelium of mice at week 20, ranging from 0 to 6.53% of spermatogenic tubule cross-sections (Fig. 2d). However, triple dose of busulfan 40 mg/kg and initial dose of busulfan 40 mg/kg followed by two additional doses of busulfan 20 mg/kg every 5 wk resulted in the premature death of animals before they reach week 30. On the other hand, the administration of an initial dose of busulfan 40 mg/kg followed by two additional doses of either busulfan 10 mg/kg or 5 mg/kg every 5 wk did not disturb survival rate up to week 30 (data not shown) but were effective to maintain spontaneous spermatogenic recovery with values under 7% (Fig. 2d). Based on this, we decided to use an initial injection of busulfan 40 mg/kg at week 5, followed by two additional injections of 10 mg/kg every 5 weeks as the protocol to continue with subsequent experiments of delayed SSCT.
A delay of SSCT since the first exposure of hosts to busulfan decreases the colonization outcome of donor SSCs
We observed a slight but significant decreased ratio of testis weight (mg)/body weight (g) in those testes from animals subjected to a delayed SSCT when compared with standard SSCT (Fig. 3a). Colonization of the seminiferous epithelium of hosts by GFP+ donor SSCs and spermatogenic recovery after SSCT is shown in Fig. 3b–d and Supplemental Fig. 1, respectively. Overall, a total of 13,813 tubule cross-sections were evaluated (117.06 ± 36.66 tubule cross-sections per testis, with a range between 60 and 392).
Results from the analysis of standard and delayed SSCT models. (a) Comparative among the three SSCT models of the ratio of testis weight (mg)/body weight (g). (b) Illustrative pictures of the GFP signal found in fresh tubule spreads from mice subjected to SSCT. (c) Illustrative pictures of the results of tubule spreads whole-mount immunostaining of Plzf (green)/GFP (red) and c-kit (green)/GFP (red). White arrows indicate Plzf (green)/GFP (red) and c-kit (green)/GFP (red) colonies from donor SSCs. Dashed lines indicate the edge of seminiferous tubules. (d) Illustrative pictures of the results of immunostaining of Vasa (green)/GFP (red) in testicular paraffin sections from mice subjected to SSCT. White asterisks indicate GFP+ tubule cross-sections with active spermatogenesis. Dashed lines indicate the edge of seminiferous tubules. (e) Results of the percentage of tubule cross-sections with host/donor spermatogenesis and Sertoli cell only (SCO) in all three SSCT models. (f) Ratio of GFP+ tubules with donor germ cells among spermatogenic tubule cross-sections. (g) Comparative among the three SSCT models of the colonization efficiency, calculated as the number of GFP+ donor spermatogenic tubule cross-sections per 200,000 cells transplanted in each SSCT. Scale bars correspond to 250 μm. Data is presented as mean ± standard error. Asterisks indicate statistical differences with p < 0.05.
Statistical analysis of the number of spermatogenic tubule cross-sections in all SSCT groups led us to find that standard SSCT (SSCT-A) and delayed SSCT of hosts treated with two further repeated doses of busulfan 10 mg/kg (SSCT-C) gave the best results in terms of spermatogenic recovery with an average of 22.65% and 22.29% of tubule cross-sections with spermatogenic activity, respectively, compared with a spermatogenic recovery rate of 11.54% in the case of delayed SSCT without repetition of busulfan exposure (SSCT-B) (Fig. 3e). Interestingly, whereas a 69.71% of GFP+ cross-sections were found within spermatogenic tubules in standard SSCT (SSCT-A), a lower contribution of donor SSC colonization to spermatogenic recovery was shown in the rest of conditions ranging from 34.69 to 35.32% of GFP+ cross-sections within spermatogenic tubules (Fig. 3f). A similar behavior was found for the colonization efficiency, where standard SSCT (SSCT-A) showed a significantly higher efficiency with 28.83 GFP+ spermatogenic tubule cross-sections per 200,000 transplanted cells, compared with the rest of conditions ranging from 4.71 to 7.62 (Fig. 3g).
Discussion
Since the first demonstration of spermatogenic recovery in sterilized mice subjected to a transplant of SSCs in the early 1990s (Brinster and Avarbock 1994), SSCT has been successfully replicated in several animal models, including non-human primates (Brinster and Avarbock 1994; Dobrinski et al. 1999; Dobrinski 2006; Hermann et al. 2012; Medrano et al. 2014; Picton et al. 2015). Based in these observations, testicular tissue cryopreservation became an experimental strategy to preserve the fertility of prepubertal boys that will be subjected to a potentially gonadotoxic onset, as is the case of cancer patients and some hematological diseases (Picton et al. 2015; Medrano et al. 2017; Daudin et al. 2015; Poels et al. 2013; Baert et al. 2013; Keros et al. 2007; Wyns et al. 2008; Wyns et al. 2011; Wyns et al. 2015; Ginsberg et al. 2010; Ginsberg et al. 2014; Meistrich 2013; Onofre et al. 2018). However, since future SSCTs to restore spermatogenesis of human patients will be performed with a delay of several years after their gonadotoxic exposure, here we aimed to study if such delay in the transplantation timing may have any influence on the spermatogenic recovery of hosts using a mouse model.
Despite the existence of Kit mutant mouse strains that lack endogenous spermatogenesis and have been used elsewhere for SSCT (Kanatsu-Shinohara et al. 2016), we considered to use chemical germ cell ablation using alkylating agents such as busulfan in order to replicate better the clinical situation in human patients. Also, the gonadotoxic exposure to mouse hosts at week 5 coincides with the initiation of puberty, resembling better the clinical situation of prepubertal/pubertal patients that are selected for fertility preservation and are the ones that would be subjected to SSCT with their own cryopreserved SSCs in the future once they become adults. However, we found that unlike humans, mice showed an incredible ability to spontaneously regenerate their spermatogenesis upon busulfan exposure (Fig. 2b–c), highlighting a higher plasticity and recovery potential of murine SSCs upon a gonadotoxic onset due to the ability of aligned differentiating spermatogonia to behave as SSCs when spermatogonial chains are broken into single spermatogonia (de Rooij 2009; Kubota and Brinster 2018). Therefore, surviving differentiating spermatogonia in mouse can transform back into SSCs that trigger a rapid regeneration of germ cells and resume spermatogenesis (de Rooij 2009; Wang et al. 2010; Kubota and Brinster 2018). In contrast, the SSC niche in humans is composed by a greater number of A dark/A pale spermatogonia, but these putative SSCs lack of the plasticity shown in murine SSCs, and therefore the few surviving undifferentiated SSCs after a gonadotoxic chemotherapeutic treatment in humans require a very long time to regenerate spermatogenesis, resulting in prolonged severe oligozoospermia/azoospermia that may last forever (de Rooij 2009; Wang et al. 2010; Meistrich 2013; Medrano et al. 2016; Kubota and Brinster 2018). Because of this, in order to replicate a seminiferous epithelium depleted of endogenous germ cells in hosts of our delayed mouse SSCT model, here we determined an optimal chemical sterilizing protocol that included two further doses of 10 mg/kg of busulfan every 5 wk after an initial dose of busulfan 40 mg/kg at week 5 (Fig. 2b–d).
In the subsequent comparisons among the different SSCT experiments performed in this study, we observed that a germ cell-depleted seminiferous epithelium in hosts increases the spermatogenic recovery (SSCT-A and SSCT-C conditions), compared with those hosts that already showed spontaneous germ cell recovery at the moment of SSCT (SSCT-B condition) (Fig. 3e). However, despite the similar spermatogenic recovery rates observed in both SSCT-C hosts treated with two additional doses of busulfan 10 mg/kg and standard SSCT (SSCT-A), the percentage of GFP+ spermatogenic cross-sections resulting from the colonization of donor SSCs in SSCT-C represented a very low contribution, similar to the one shown in SSCT-B (Fig. 3e–g). Of note, the lower colonization rate of donor SSCs in both delayed SSCT models highlights the possibility of a deteriorated somatic niche resulting from busulfan effects on the testicular somatic cell compartment. Indeed, in addition to the direct damage of busulfan to Sertoli cells and Leydig cells implied in the survival and regulation of spermatogonia, recent reports describe the important role of endothelial cells in the testicular SSC niche (Bhang et al. 2018). Therefore, busulfan may affect the proper stromal vascularization in testes, resulting in a biased localization of donor SSCs to vascular network that may limit the survival of colonizing SSCs in both delayed SSCT experiments (SSCT-B and SSCT-C conditions). In this regard, the use of mesenchymal cells or their secretome in combination with SSCT has demonstrated to be a possible method to improve the formation of a proper vascular network in the testis and helps donor spermatogonial colonization (Kadam et al. 2018; Sagaradze et al. 2019).
On the other hand, the high spermatogenic recovery due mainly to endogenous surviving spermatogonia shown in SSCT-C can also be explained by a selection process of the more resistant SSCs of hosts. According to this hypothesis, host surviving SSCs selected by repeated exposure to low-dose busulfan may show an enhanced fitness in terms of mitotic activity to repopulate the seminiferous epithelium and trigger the quick spermatogenic recovery observed in SSCT-C, in a similar manner that the already reported enhanced fitness that murine SSC acquire under the selection process of aging (Martin et al. 2014). Alternatively, the periodic exposition to low-dose busulfan in hosts from SSCT-C may also induce repeated breaks of the chains of aligned spermatogonia derived from surviving SSCs after the initial gonadotoxic onset, resulting in an increased number of endogenous SSCs ready to spread over seminiferous epithelium and regenerate spermatogenesis.
Overall, the main conclusion that we obtained from our data employing a mouse model is that a delay in SSCT significantly decreases the donor SSC colonization of seminiferous tubules, indicating that the outcome of future clinical setup for adult patients which seminiferous epithelium has been germ cell depleted for long time may be affected. Therefore, although the use of testicular tissue for fertility preservation is a feasible strategy to restore fertility in many animal models, our results indicate that increasing the number of SSCs for transplant may be needed to counter the handicap of timing delay in humans, in order to improve SSC colonization and clinical recovery of spermatogenesis of patients. However, although our study has focused in the comparison of SSC colonization efficiency upon a delayed SSCT among 3 different SSCT conditions, other important parameters such as the status of the somatic cell compartment of hosts and the possible genetic incompatibilities between donor and host mouse strains were not evaluated. Because of this, our study must be considered a pilot that highlights the important role of timing of SSCT for its success. Therefore, our interpretations of the low colonization rates of donor cells upon a delayed SSCT observed in this study must be confirmed in future studies. Moreover, since the SSC niche of mice differs in terms of plasticity from humans, further studies considering delayed SSCT with non-human primates showing an SSC niche physiology closer to humans (Hermann et al. 2012) are mandatory to clarify this question.
Conclusions
In this study, we observed how a delay in the SSCT timing decreases the receptivity of testes to donor SSC’s colonization. Therefore, this is an important question that must be considered in the future clinical setup for human patients in which the delay between the gonadotoxic onset that depleted their endogenous germ cells before puberty and the transplant will comprise several years.
Data availability
All authors declare that all data and materials included in this manuscript comply with field standards.
References
Alves-Lopes JP, Soder O, Stukenborg JB (2017) Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 130:76–89
Baert Y, Van Saen D, Haentjens P, In't Veld P, Tournaye H, Goossens E (2013) What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod 28:1816–1826
Bhang DH, Kim BJ, Kim BG, Schadler K, Baek KH, Kim YH, Hsiao W, Ding BS, Rafii S, Weiss MJ, Chou ST, Kolon TF, Ginsberg JP, Ryu BY, Ryeom S (2018) Testicular endothelial cells are a critical population in the germline stem cell niche. Nat Commun 9:4379
Brinster RL, Avarbock MR (1994) Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A 91:11303–11307
Daudin M, Rives N, Walschaerts M, Drouineaud V, Szerman E, Koscinski I, Eustache F, Saias-Magnan J, Papaxanthos-Roche A, Cabry-Goubet R, Brugnon F, Le Lannou D, Barthelemy C, Rigot JM, Freour T, Berthaut I, Giscard d'Estaing S, Touati F, Melin-Blocquaux MC, Blagosklonov O, Thomas C, Benhamed M, Schmitt F, Kunstmann JM, Thonneau P, Bujan L (2015) Sperm cryopreservation in adolescents and young adults with cancer: results of the French national sperm banking network (CECOS). Fertil Steril 103:478–486 e471
de Michele F, Poels J, Vermeulen M, Ambroise J, Gruson D, Guiot Y, Wyns C (2018) Haploid germ cells generated in organotypic culture of testicular tissue from prepubertal boys. Front Physiol 9:1413
de Michele F, Poels J, Weerens L, Petit C, Evrard Z, Ambroise J, Gruson D, Wyns C (2017) Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum Reprod 32:32–45
de Rooij DG (2009) The spermatogonial stem cell niche. Microsc Res Tech 72:580–585
Dobrinski I (2006) Germ cell transplantation in pigs--advances and applications. Soc Reprod Fertil Suppl 62:331–339
Dobrinski I, Avarbock MR, Brinster RL (1999) Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod 61:1331–1339
Dovey SL, Valli H, Hermann BP, Sukhwani M, Donohue J, Castro CA, Chu T, Sanfilippo JS, Orwig KE (2013) Eliminating malignant contamination from therapeutic human spermatogonial stem cells. J Clin Invest 123:1833–1843
Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H, Shetty G, Meistrich ML, Houser L, Robertson N, Roberts V, Ramsey C, Hanna C, Hennebold JD, Dobrinski I, Orwig KE (2019) Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 363:1314–1319
Gassei K, Valli H, Orwig KE (2014) Whole-mount immunohistochemistry to study spermatogonial stem cells and spermatogenic lineage development in mice, monkeys, and humans. Methods Mol Biol 1210:193–202
Ginsberg JP, Carlson CA, Lin K, Hobbie WL, Wigo E, Wu X, Brinster RL, Kolon TF (2010) An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Hum Reprod 25:37–41
Ginsberg JP, Li Y, Carlson CA, Gracia CR, Hobbie WL, Miller VA, Mulhall J, Shnorhavorian M, Brinster RL, Kolon TF (2014) Testicular tissue cryopreservation in prepubertal male children: an analysis of parental decision-making. Pediatr Blood Cancer 61:1673–1678
Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, Peterson K, Masterson K, Ramsey C, Ward T, Lienesch M, Volk A, Cooper DK, Thomson AW, Kiss JE, Penedo MC, Schatten GP, Mitalipov S, Orwig KE (2012) Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 11:715–726
Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S (2002) Sperm from neonatal mammalian testes grafted in mice. Nature 418:778–781
Jahnukainen K, Ehmcke J, Nurmio M, Schlatt S (2012) Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res 72:5174–5178
Kadam P, Ntemou E, Baert Y, Van Laere S, Van Saen D, Goossens E (2018) Co-transplantation of mesenchymal stem cells improves spermatogonial stem cell transplantation efficiency in mice. Stem Cell Res Ther 9:317
Kanatsu-Shinohara M, Morimoto H, Shinohara T (2016) Fertility of male germline stem cells following spermatogonial transplantation in infertile mouse models. Biol Reprod 94:112
Keros V, Hultenby K, Borgstrom B, Fridstrom M, Jahnukainen K, Hovatta O (2007) Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod 22:1384–1395
Kubota H, Brinster RL (2018) Spermatogonial stem cells. Biol Reprod 99:52–74
Martin LA, Assif N, Gilbert M, Wijewarnasuriya D, Seandel M (2014) Enhanced fitness of adult spermatogonial stem cells bearing a paternal age-associated FGFR2 mutation. Stem Cell Reports 3:219–226
Medrano JV, Andres MDM, Garcia S, Herraiz S, Vilanova-Perez T, Goossens E, Pellicer A (2017) Basic and clinical approaches for fertility preservation and restoration in cancer patients. Trends Biotechnol 36:199–215. https://doi.org/10.1016/j.tibtech.2017.10.010
Medrano JV, Martinez-Arroyo AM, Sukhwani M, Noguera I, Quinonero A, Martinez-Jabaloyas JM, Pellicer A, Remohi J, Orwig KE, Simon C (2014) Germ cell transplantation into mouse testes procedure. Fertil Steril 102:e11–e12
Medrano JV, Rombaut C, Simon C, Pellicer A, Goossens E (2016) Human spermatogonial stem cells display limited proliferation in vitro under mouse spermatogonial stem cell culture conditions. Fertil Steril 106:1539–1549 e1538
Medrano JV, Vilanova-Perez T, Fornes-Ferrer V, Navarro-Gomezlechon A, Martinez-Triguero ML, Garcia S, Gomez-Chacon J, Povo I, Pellicer A, Andres MM, Novella-Maestre E (2018) Influence of temperature, serum, and gonadotropin supplementation in short- and long-term organotypic culture of human immature testicular tissue. Fertil Steril 110:1045–1057 e1043
Meistrich ML (2013) Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril 100:1180–1186
Ogawa T, Arechaga JM, Avarbock MR, Brinster RL (1997) Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol 41:111–122
Onofre J, Faes K, Kadam P, Vicini E, van Pelt AMM, Goossens E (2018) What is the best protocol to cryopreserve immature mouse testicular cell suspensions? Reprod BioMed Online 37:6–17
Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, Mitchell RT, Pennings G, Rives N, Tournaye H, van Pelt AM, Eichenlaub-Ritter U, Schlatt S, Diseases ETFOFPIS (2015) A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod 30:2463–2475
Poels J, Van Langendonckt A, Many MC, Wese FX, Wyns C (2013) Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod 28:578–589
Radford J (2003) Restoration of fertility after treatment for cancer. Horm Res 59(Suppl 1):21–23
Sagaradze G, Basalova N, Kirpatovsky V, Ohobotov D, Nimiritsky P, Grigorieva O, Popov V, Kamalov A, Tkachuk V, Efimenko A (2019) A magic kick for regeneration: role of mesenchymal stromal cell secretome in spermatogonial stem cell niche recovery. Stem Cell Res Ther 10:342
Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, Smith FO, Reaman GH (2010) Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28:2625–2634
Valli H, Sukhwani M, Dovey SL, Peters KA, Donohue J, Castro CA, Chu T, Marshall GR, Orwig KE (2014) Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil Steril 102:566–580 e567
Wang DZ, Zhou XH, Yuan YL, Zheng XM (2010) Optimal dose of busulfan for depleting testicular germ cells of recipient mice before spermatogonial transplantation. Asian J Androl 12:263–270
Wyns C, Collienne C, Shenfield F, Robert A, Laurent P, Roegiers L, Brichard B (2015) Fertility preservation in the male pediatric population: factors influencing the decision of parents and children. Hum Reprod 30:2022–2030
Wyns C, Curaba M, Petit S, Vanabelle B, Laurent P, Wese JF, Donnez J (2011) Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum Reprod 26:737–747
Wyns C, Van Langendonckt A, Wese FX, Donnez J, Curaba M (2008) Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod 23:2402–2414
Yokonishi T, Sato T, Komeya M, Katagiri K, Kubota Y, Nakabayashi K, Hata K, Inoue K, Ogonuki N, Ogura A, Ogawa T (2014) Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun 5:4320
Acknowledgments
We would like to thank all the staff of the animal facility from the Valencia University for their help and patience. We are also grateful to Dr. Isabel Fariñas from the Valencia University for providing us GFP donors for our experiments.
Funding
This work was supported by a private donation of the Celtic Submarí club- Villareal C.F. to Hospital Universitario y Politécnico La Fe intended to promote the scientific research on fertility preservation in child with cancer and an AES project grant (PI16/00931) conceded by the Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Contributions
JVM, ENM, AP, and MMA conceived this work. JVM, IA, ANG, and IN conducted the experiments. JVM analyzed data and wrote the manuscript. All listed authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The use of 71 C57BL/6NCrl 5-wk-old male mice as hosts and 12 Tg(CAG-EGFP)B5Nagy/J 3-wk-old males as donors of testicular cells for this study was approved by the Ethical Committee for Animal Welfare of the University of Valencia (ref.: A1513161658035). Mice were housed in purified air-ventilated racks in order to prevent any infection, fed ad libitum, and with controlled dark/light cycle.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
All statistical analyses were performed using SPSS v.25 (IBM).
Additional information
Editor: Tetsuji Okamoto
Supplementary information
Rights and permissions
About this article
Cite this article
Medrano, J.V., Acimovic, I., Navarro-Gomezlechon, A. et al. Timing of spermatogonial stem cell transplantation affects the spermatogenic recovery outcome in mice. In Vitro Cell.Dev.Biol.-Animal 57, 21–29 (2021). https://doi.org/10.1007/s11626-020-00531-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-020-00531-9