Abstract
Mosquitoes are generally considered one of the most important vectors of arboviruses, with Aedes aegypti regarded as the most important in transmission of yellow fever and dengue viruses. To investigate why there are differences in the incidence of dengue fever and Zika in different geographical areas and an absence of outbreaks in Ghana in spite of an abundance of A. aegypti mosquitoes, we established a continuous cell line from embryonic cells of A. aegypti collected in Ghana and assessed its susceptibility to dengue, yellow fever, and Zika viruses. The new cell line (designated AeAe-GH98), having an adhesive spindle-shaped web-like morphology, was serially subcultured in both VP-12 and Schneider’s medium supplemented with 10% heat-inactivated fetal bovine serum. AeAe-GH98 cells were found to have a population doubling time of 1.3 d during exponential growth. The mosquito colony used to establish the cell line was confirmed to have originated from Africa using microsatellite assay. In terms of susceptibility to Aedes-borne flaviviruses, AeAe-GH98 cells were found to have different degrees of susceptibility to yellow fever, Zika, and dengue virus infection and propagation. While susceptibility of AeAe-GH98 cells to yellow fever and Zika viruses was comparable with that of C6/36 cells, susceptibility to dengue virus was significantly lower. This cell line will serve as a useful tool for determining molecular factors influencing virus–vector susceptibility in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arboviruses of the family Flaviviridae, such as yellow fever virus (YFV), dengue virus (DENV), and Zika virus (ZIKV), are mosquito-borne and cause varying degrees of public health concern across the world. YFV, for example, caused devastating outbreaks in Africa until the introduction of vaccines that have effectively controlled infections i n many countries, including Ghana (Amoa-Bosompem et al. 2020). DENV and ZIKV, on the other hand, are largely considered emerging or re-emerging diseases across the world. Although there have been no reports of ZIKV infection or any DENV outbreaks in Ghana, there have been reports of possible local transmission of DENV there. In addition, DENV outbreaks were reported in Cote d’Ivoire and Burkina Faso in 2015 and 2016, respectively, both of which share borders with Ghana (Sudeep et al. 2009; Suzuki et al. 2017; Amoako et al. 2018; Bonney et al. 2018; Tarnagda et al. 2018; Amoa-Bosompem et al. 2020). This apparent difference in incidence of arboviral infections and the significantly greater intensity of outbreaks in South America and Asia raises questions about the possible role of the Aedes aegypti vector in transmission (Messina et al. 2014; Fredericks et al. 2019).
Continuous insect cell lines have proven to be invaluable tools for entomological research over the years (Barletta et al. 2012; Kuwata et al. 2012; Hoshino et al. 2015; Weger-Lucarelli et al. 2018). The application of cell lines has ranged from superinfection experiments testing the effect of one virus on another to the study of immune responses to pathogens and from the study of virus–vector interactions to that of virus evolution (Barletta et al. 2012; Weger-Lucarelli et al. 2018). Furthermore, the increase in the number of insect cell lines originating from different geographical areas allows for the detection and measurement of differences in response and susceptibility to pathogens between regions, and in responses to different kinds of pathogens (Lynn 1999; Sudeep et al. 2009; Barletta et al. 2012; Walker et al. 2014; Roberts et al. 2017).
The A. albopictus-derived cell line C6/36, owing to its lack of RNA interference to resist viral infections, remains one of the most used mosquito cell lines for virus isolation and characterization studies. It has also been employed in determining the effect of polypeptides or cytokine-like factors produced by insects on virus infection (Kanthong et al. 2010; Weger-Lucarelli et al. 2018). A. aegypti-derived cell lines, however, remain the most efficient in vitro tool for studying the viruses transmitted by this mosquito.
This study outlines the establishment and characterization of a cell line (designated AeAe-GH98) from A. aegypti mosquitoes collected from the northern part of Ghana and compares the susceptibility of C6/36 and AeAe-GH98 cells to two serotypes of DENV (DENV-1 isolated in Japan and DENV-2 isolated in Ghana), YFV, and ZIKV.
Materials and Methods
Culture media
VP-12 medium (Varma and Pudney 1969) supplemented with varying concentrations (20%, 15%, and 10%) of heat-inactivated fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO) was used in establishing and subculturing the AeAe-GH98 cell line. Once established, the medium for subculturing and maintenance was systematically changed to Schneider’s medium (Sigma-Aldrich) supplemented with 10% FBS by reducing the proportion of VP-12 in 25% increments.
Establishment of a continuous cell line
Embryos from fertilized A. aegypti eggs were used to initiate the primary culture following the protocol described by Kuwata et al. (2012), with modifications. Briefly, an A. aegypti colony established from mosquito larvae collected in Ghana (GH98 strain) (Amoa-Bosompem et al. 2020) were blood-fed and the eggs harvested. The harvested eggs were treated with 10% hypochlorite solution for 2 min to sterilize their surface. With the aid of a head-mounted magnifier (Mega View Pro LED, Terasaki Industries, Tokyo, Japan), 100–150 surface-sterilized eggs were cut with a stainless-steel blade (Feather® Disposable Scalpel No. 15, FEATHER Safety Razor, Osaka, Japan) in a 35-mm surface-modified tissue culture dish (Primeria Easy Grip; Corning Inc., Corning, NY) containing VP-12 medium supplemented with 20% FBS, sealed with parafilm and incubated at 28°C. The medium was replaced regularly, and migration and division of cells were observed under an inverted microscope.
Confluent cells were detached from the surface of the culture dish by scraping and subcultured in a 12.5 cm2 culture flask. The length of time needed for cells to reach confluency and the concentration of FBS in VP-12 medium reduced over time leading to the establishment of an AeAe-GH98 cell line adapted to VP-12 medium supplemented with 10% FBS. Over time, the AeAe-GH98 cells could be detached by pipetting alone. Once AeAe-GH98 cells had reached stable growth, VP-12 medium was gradually replaced with Schneider’s medium supplemented with 10% FBS for all subsequent tests.
Karyotyping analysis
Karyotyping was performed using a previously described method (Freshney 2005; Kuwata et al. 2012; Hoshino et al. 2015) with modifications. Briefly, cells (that had gone through 40 rounds of passages) were seeded in a 75 cm2 flask at a concentration of 1 × 105 cells/mL and incubated at 28°C for 3 d before treatment with 10−6 M colchicine overnight. Cells were collected by centrifugation at 100×g for 5 min and resuspended in hypotonic solution. Ice-cold Carnoy’s fixative reagent was added at a ratio of 1:1. Cells were again collected by low speed centrifugation and resuspended in Carnoy’s fixative reagent. The fixation step was repeated once. Finally, cells were resuspended in 200 μL of Carnoy’s fixative reagent, mounted on a slide, and stained with Giemsa solution.
Cell growth rate
AeAe-GH98 cells were seeded at a concentration of 1 × 104 cells/mL in a 6-well culture plate and incubated at 28°C in a polyethylene bag. A number of cells were counted in triplicate on days 2, 4, 5, 6, 7, and 8 using a Countess Cell Counting Chamber Slide and a Countess II FL Automated Cell Counter (Thermo Fisher Scientific, Waltham, MA). The cell concentrations were used to construct a growth curve by plotting a graph of cell concentration against day and an additional inset graph of rate of cell divisions per hour [(CB-CA)/time (hr.)] against day.
Population assignment of the AeAe-GH98 cell line and screening for persistently infecting viruses
Microsatellite loci were genotyped to determine the origin of the A. aegypti colony used in establishing the AeAe-GH98 cell line. Briefly, genomic DNA was extracted from individual mosquitoes using the MagExtractor-Genome (TOYOBO, Osaka, Japan) following the manufacturer’s protocol. The microsatellite markers and primers used were trinucleotide repeats A1, B2, B3, and A9 and dinucleotide repeats AC2, CT2, AG2, AC4, AC1, AC5, AG1, and AG5, developed by Slotman et al. (2007) and Brown et al. (2011). Multiplex pairings and primer/fluorophore combinations were as previously described by Itokawa et al. (2020). Polymerase chain reactions (PCRs) were performed using a Type-it Microsatellite PCR Kit (Qiagen, Hilden, Germany). Each 10-μL reaction was composed of 5 μL of 2 × Type-it Multiplex PCR master mix, 0.8 μL (2.5 μm) of primer, 0.8 μL (2.5 μm) of fluorescent-labeled M13 primer, 1 μL of genomic DNA, and 2.4 μL of Milli-Q water. The thermocycler conditions were: 95°C for 2 min; 35 cycles of 98°C for 5 s, 55°C for 90 s, and 72°C for 20 s; and 72°C for 10 min. The PCR product was purified with Agencourt AMPure XP (Beckman Coulter, Brea, CA) and electrophoresed using an ABI 3130 sequencer in the presence of the 500 LIZ size standard (GeneScan, Thermo Fisher Scientific). The sequence reaction mixture was made up of 10 μL Hi-Di™ Formamide, 0.1 μL 500 LIZ size standard, and 0.5 μL purified PCR product. Analysis of the genotype data was done by discriminant analysis of principal components (DAPC) using the adegenet package (Jombart 2008; Jombart et al. 2010) in R 3.6 (R Core Team 2019).
For determination of the presence or absence of persistently infecting viruses, both the mosquito colony and the established cell line were subjected to next-generation sequencing assays following a previously described method (Kobayashi et al. 2017; Amoa-Bosompem et al. 2020). Reads were assembled and analyzed on the NCBI Blast database, Blastn and Blastx.
Flavivirus susceptibility
In determining the susceptibility of mosquitoes from northern Ghana to DENV, YFV, and ZIKV, AeAe-GH98 cells were inoculated with all three flaviviruses following a previously reported protocol (Kuwata et al. 2012) with modifications. Briefly, AeAe-GH98 cells were seeded in a 6-well culture flask at a concentration of 1 × 105 cells/mL, placed in a polyethylene bag, and incubated at 28°C overnight. The cells were inoculated with DENV-1 (D1/Hu/Saitama/NIID100/2014), DENV-2 (DENV-2/GH/NMIMR-BC-UG-F299/2017), YFV (live vaccine strain 17D-204), or ZIKV (MR766/UGANDA/1985) with a multiplicity of infection (MOI) value of 0.l. Cell supernatants were harvested on days 0, 2, 3, 4, and 5. The virus titer was determined by the focus-forming assay on Vero cells (Japanese Collection of Research Bioresources Cell Bank) as previously described by Amoa-Bosompem et al. (2020). A graph of virus titer against time was plotted. All samples were run in triplicate with C6/36 cells (European Collection of Authenticated Cell Culture) as controls.
Results
Primary cell culture and morphology
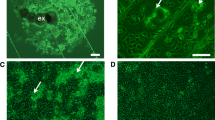
The initial cell cultures were composed of cells with different shapes and sizes and with some web-like properties (Fig. 1a,b). Continuous subculturing resulted in a mesh-like cylindrical cell population arising by the 12th passage (Fig. 1c). By the 42nd passage, however, the population constituted a single layer of web-like spindle-shaped adhesive cells (Fig. 1e,f) with an average cell length of 80 μm (Fig. 1f). At present, this AeAe-GH98 continuous cell line has undergone > 50 passages and has been maintained for over 3 years by subculturing, with a split ratio of 1:8–1:9 at 10–14-d intervals.
Karyotyping analysis
The chromosomes of AeAe-GH98 cells were examined under the microscope after staining with Giemsa (Fig. 2). The diploid AeAe-GH98 cells had a chromosome number of 6 consisting of 3 pairs of unevenly sized chromosomes (2n = 6). The observed difference in size is consistent with A. aegypti chromosomes, with the smallest, largest, and intermediate reported to be chromosomes 1, 2, and 3, respectively (Clements 1992; Timoshevskiy et al. 2014).
Cell growth rate
To determine the AeAe-GH98 cell growth rate, cell concentration was measured on days 2–7 post seeding. The cells reached the log phase of growth at least 3 days post seeding. The population doubling time at the start of the log phase (days 4–5) was calculated to be 1.93 d, reducing to ~ 1.3 d during the peak period of cell growth (Fig. 3).
Growth rate of AeAe-GH98 cultured in Schneider’s medium supplemented with 10% FBS at 28°C. The main graph is a graph of cell concentration against day showing the rate of increase in cell concentration over time. The inset graph is a graph of rate of cell division per hour against day showing the number of dividing cells and rate of increase in cell division over time. Plotted values are mean ± standard deviation.
Population assignment and screening for persistently infecting viruses
Genetic characterization of the A. aegypti colony was performed to establish the AeAe-GH98 cell line. DAPC was conducted to genotype the 12 microsatellite loci followed by comparison with previously reported genotypes of global A. aegypti populations (Gloria-Soria et al. 2016). DAPC split the individual genotypes into two distinct populations, wild A. aegypti mosquitoes of African origin (Fig. 4, right-hand side, dark gray) and those originating outside Africa (Fig. 4, left-hand side, pale gray) as previously reported (Gloria-Soria et al. 2016). The genotypes of individuals in the colony from which the cell line was established (population 40) was found to cluster with the wild A. aegypti mosquitoes of African origin (Fig. 4). The AeAe-GH98 cell line was found to be virus-free. RNA-seq analysis did not find any nucleotide acid sequence similar to known RNA virus genomes.
Results of discriminant analysis of principal components using 12 microsatellite genotypes. Population 40 indicates genotypes of individuals sampled from the colony from which the AeAe-GH98 cell line was established. Other genotypes (dark gray, right-hand side (from African countries); pale gray, left-hand side (from non-African countries)) are those reported by Gloria-Soria et al. (2016). 1, Argentina; 2, Australia; 3, Brazil; 4, Cameroon; 5, Colombia; 6, Costa Rica; 7, Dominican Republic; 8, French Polynesia; 9, Gabon; 10, Grenada; 11, Guinea Bissau; 13, Indonesia; 14, Kenya; 15, Madeira; 16, Mauritius; 17, Mexico; 25, Hawaii; 26, Pakistan; 27, Puerto Rico; 28, Saudi Arabia; 29, Senegal; 30, South Africa; 31, Sri Lanka; 32, Thailand; 33, Philippines; 34, Trinidad; 35, Uganda; 36, USA; 37, Venezuela; 38, Vietnam (Ho Chi Minh); 39, Vietnam (Hanoi); 40, Laboratory strain GH98.
Flavivirus susceptibility
To determine susceptibility to flaviviruses, AeAe-GH98 cells were inoculated with DENV, YFV, or ZIKV exhibiting a MOI value of 0.1, and viral concentration was monitored over time. YFV and ZIKV were observed to replicate efficiently in AeAe-GH98 cells with titers in the region of 105 focus-forming units (FFU)/mL at 5 days post inoculation (dpi) (Fig. 5). There was no observable cytopathic effect (CPE) on the new cell line as a result of YFV or ZIKV infection, nor any effect on replication (data not shown). In contrast, both serotypes of DENV replicated poorly in the new cell line. Only DENV-1 was observed to have established infection at 3 dpi with a concentration of 673 FFU/mL, but it did not replicate effectively to increase viral titer (Fig. 5 and Fig. S1A). All viruses were observed to replicate significantly better but with different degrees of efficiency in C6/36 cells (Fig. S1B). Neither DENV serotype caused an observable CPE in AeAe-GH98 cells.
Concentration of DENV, YFV, and ZIKV in C6/36 and AeAe-GH98 cells at 5 d post inoculation (dpi). Virus concentrations were determined using the focus-forming assay on Vero cells. AeAe-GH98 was observed to be susceptible to YFV and ZIKV infection and replication. Each point denotes the mean ± standard deviation of assays run in triplicate.
Discussion
Mosquitoes remain one of the most important vectors of arbovirus transmission. While there are hundreds of mosquitoes capable of transmitting at least one of the mosquito-borne arboviruses, mosquitoes of the genus Aedes are some of the most efficient and thus medically important vectors of arboviruses (Liang et al. 2015). Differences in the susceptibility of mosquitoes to arbovirus infection and their efficiency as vectors have led to research into the virus–vector relationship. Some of this research has focused on the molecular basis of susceptibility as well as immunological responses to viral infection, with the aim of developing effective strategies for controlling infections (Conway et al. 2014). While the use of wild or laboratory bred mosquitoes may be ideal, insect cell lines provide a very useful alternative for in vitro elucidation of all these factors, including susceptibility of a mosquito population to arboviruses (Inoue 1989; Lynn 1999; Kuno 2007; Kanthong et al. 2010; Barletta et al. 2012; Walker et al. 2014; Weger-Lucarelli et al. 2018). This study therefore established an A. aegypti cell line from Aedes mosquitoes from Ghana and determined its susceptibility to DENV, YFV, and ZIKV.
VP-12 medium supplemented with FBS was used in establishing the cell line, consistent with previous reports that describe it as ideal for culturing cells from a variety of mosquito species (Kuwata et al. 2012; Hoshino et al. 2015). Schneider’s medium supplemented with FBS was shown to be equally ideal in maintaining the cell culture once it had been established. However, it was important to gradually change the medium from VP-12 to Schneider’s to prevent the cultures from crushing due to shock and also to allow the monitoring of any adverse effect Schneider’s medium may have had. Schneider’s medium, which is commercially available, was used for subsequent tests because of its relative ease in preparation compared with VP-12 which is not commercial.
This is the first report of a successfully established A. aegypti cell line from mosquitoes originally collected from West Africa (Ghana). DAPC analysis (Jombart et al. 2010) clustered the 12 microsatellite genotypes with published genotypes of A. aegypti from African countries rather than with those from non-African groups (Fig. 4). Thus, genetically, the AeAe-GH98 cells could be expected to be a more suitable experimental model for African A. aegypti than other pre-existing cell lines.
The successful establishment of the A. aegypti cell line afforded the opportunity to determine the susceptibility of mosquitoes from northern Ghana to DENV, YFV, and ZIKV in vitro. The AeAe-GH98 cell line was found to be generally non-susceptible to DENV, though DENV-1 was able to successfully colonize the cell but without continuous replication. The non-susceptibility of the cell line to DENV-2 (isolated from patients in Ghana, and which also happens to be the dominant strain in the outbreak in Burkina Faso in 2016) may be an indication of the reason for the absence of an outbreak in the northern part of Ghana, in particular during the 2015/2016 outbreak, despite the relatively higher frequency of movement of people between Burkina Faso and northern Ghana and an abundance of the Aedes vector (Appawu et al. 2006; Bonney et al. 2018; Tarnagda et al. 2018; Amoa-Bosompem et al. 2020).
In contrast, the successful replication of YFV and ZIKV in the new cell line might indicate sustained competence of Aedes mosquitoes in Ghana as vectors of YFV and the possibility of ZIKV transmission on exposure. The presence of a competent vector underlines the need for continuous vaccination complemented with vector control measures (which should include ZIKV as a focus group) to prevent future YFV and/or ZIKV outbreaks, especially since the last reported cases of YFV infections, in 2011, were in northern Ghana (Online document: International Federation of Red Cross and Red Crescent 2011; Online document: World Health Organization 2012).
Last but not least, a lot of work is going into determining the immunological and genetic factors that may influence vector susceptibility to arbovirus infection in vitro (Kanthong et al. 2010; Barletta et al.. 2012; Walker et al. 2014). The successful establishment of a cell line that has different degrees of susceptibility to YFV, ZIKV (both of which were originally detected in Africa), and DENV (all strains originally detected in Asia and the Americas) will allow for in vitro comparison and possible elucidation of factors key to vector susceptibility. Steps are therefore being taken to use the AeAe-GH98 cell line in determining the immunological and genetic factors that may influence vector susceptibility and/or competence.
Conclusion
The establishment of an Aedes mosquito cell line, of African origin, with different levels of susceptibility to medically important flaviviruses will be useful in establishing the factors influencing vector competence in vitro and will allow for in vitro determination of possible ways of breaking the transmission of medically important flaviviruses.
References
Amoako N, Duodu S, Dennis FE, Bonney JHK, Asante KP, Ameh J, Mosi L, Hayashi T, Agbosu EE, Pratt D, Operario DJ, Fields B, Liu J, Houpt ER, Armah GE, Stoler J, Awandare GA (2018) Detection of dengue virus among children with suspected malaria, Accra, Ghana. Emerg Infect Dis 24:1544–1547
Amoa-Bosompem M, Kobayashi D, Murota K, Faizah NA, Itokawa K, Fujita R, Osei JHN, Agbosu E, Pratt D, Kimura S, Kwofie DK, Ohashi M, Bonney JHK, Dadzie S, Sasaki T, Ohta N, Isawa H, Sawabe K, Iwanaga S (2020) Entomological assessment of the status and risk of mosquito-borne arboviral transmission in Ghana. Viruses 12(2):147
Appawu M, Dadzie S, Abdul H, Asmah H, Boakye D, Wilson M, Ofori-Adjei D (2006) Surveillance of viral hemorrhagic fevers in Ghana: entomological assessment of the risk of transmission in the northern regions. Ghana Med J 40:137–141
Barletta ABF, Silva MCLN, Sorgine MHF (2012) Validation of Aedes aegypti Aag-2 cells as a model for insect immune studies. Parasit Vectors 5:148
Bonney JHK, Hayashi T, Dadzie S, Agbosu E, Pratt D, Nyarko S, Asiedu-Bekoe F, Ido E, Sarkodie B, Ohta N, Yamaoka S (2018) Molecular detection of dengue virus in patients suspected of Ebola virus disease in Ghana. PLoS One 13:e0208907
Brown JE, McBride CS, Johnson P, Ritchie S, Paupy C, Bossin H, Lutomiah J, Fernandez-Salas I, Ponlawat A, Cornel AJ, Black WC IV, Gorrochotegui-Escalante N, Urdaneta-Marquez L, Sylla M, Slotman M, Murray KO, Walker C, Powell JR (2011) Worldwide pattern of genetic differentiation imply multiple ‘domestications’ of Aedes aegypti, a major vector of human diseases. Proc R Soc B 278:2446–2454
Clements AN (1992) The biology of mosquitoes Vol 1. Development Nutrition and Reproduction. CABI Publishing, Wallingford, CT
Conway MJ, Colpitts TM, Fikrig E (2014) Role of the vector in arbovirus transmission. Annu Rev Virol 1:71–88
Fredericks AC, Russel TA, Wallace LE, Davidson AD, Fernandez-Sesma A, Maringer K (2019) Aedes aegypti (Aag2)-derived clonal mosquito cell lines reveal the effects of pre-existing persistent infection with the insect-specific bunyavirus Phasi Charoen-like virus on arbovirus replication. PLoS Negl Trop Dis 13(11):e0007346
Freshney RI (2005) Culture of animal cells: a manual of basic techniques, 5th edn. Wiley and Sons, Inc., Hoboken, NJ
Gloria-Soria A, Ayala D, Bheecarry A, Calderon-Arguedas O, Chadee DD, Chiappero M, Coetzee M, Elahee KB, Fernandez-Salas I, Kamal HA, Kamgang B, Khater EI, Kramer LD, Kramer V, Lopez-Solis A, Lutomiah J, Martins A Jr, Micieli MV, Paupy C, Ponlawat A, Rahola N, Rasheed SB, Richardson JB, Saleh AA, Sanchez-Casas RM, Seixas G, Sousa CA, Tabachnick WJ, Troyo A, Powell JR (2016) Global genetic diversity of Aedes aegypti. Mol Ecol 25(21):5377–5395
Hoshino K, Isawa H, Kuwata R, Tajima S, Takasaki T, Iwabuchi K, Sawabe K, Kobayashi M, Sasaki T (2015) Establishment and characterization of two new cell lines from the mosquito Armigeres subalbatus (Coquillett) (Diptera: Culicidae). In Vitro Cell Dev Biol-Animal 51:672–679
Inoue H (1989) Establishment of insect continuous cell lines and its utilization for virus multiplications in vitro. Japan Agricultural Research Quarterly 23(1):31–36
Itokawa K, Hu J, Sukehiro N, Tsuda Y, Komagata O, Kasai S, Tomita T, Minakawa N, Sawabe K (2020) Genetic analysis of Aedes aegypti captured at two international airports serving to the Greater Tokyo Area during 2012–2015. PLoS One 15(4):e0232192
Jombart T (2008) adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24(11):1403–1405
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet 11:94
Kanthong N, Laosutthipong C, Flegel WT (2010) Response to dengue virus infections altered by cytokine-like substances from mosquito cell cultures. BMC Microbiol 10:290
Kobayashi D, Ohashi M, Osei JHN, Agbosu E, Opoku M, Agbekudzi A, Joannides J, Fujita R, Sasaki T, Bonney JHK, Dadzie S, Isawa H, Sawabe K, Ohta N (2017) Detection of a novel putative phlebovirus and first isolation of Dugbe virus from ticks in Accra, Ghana. Ticks Tick Borne Dis 8:640–645
Kuno G (2007) Host range specificity of flaviviruses: correlation with in vitro replication. J Med Entomol 44(1):93–101
Kuwata R, Hoshino K, Isawa H, Tsuda Y, Tajima S, Sasaki T, Takasaki T, Kobayashi M, Sawabe K (2012) Establishment and characterization of a cell line from the mosquito Culex tritaeniorhynchuis (Diptera: Culicidae). In Vitro Cell Dev Biol-Animal 48:369–376
Liang G, Gao X, Gould EA (2015) Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerg Microbes Infect 4:e18
Lynn DE (1999) Development of insect cell lines: virus susceptibility and applicability to prawn cell culture. Methods Cell Sci 21:173–181
Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, Duda K, Bhatt S, Katzelnick L, Howes RE, Battle KE, Simmons CP, Hay SI (2014) Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol 22(3):138–146
Online document: International Federation of Red Cross and Red Crescent (2011) Ghana: yellow fever outbreak- Nov 2011. In: Reliefweb. https://reliefweb.int/disaster/ep-2011-000178-gha
Online document: World Health Organization (2012) Yellow fever in Ghana. In: World Health Organization. https://www.who.int/csr/don/2012_02_03b/en/
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. http://www.R-project.org
Roberts GC, Zothner C, Remenyi R, Merits A, Stonehouse NJ, Harris M (2017) Evaluation of a range of mammalian and mosquito cell lines for use in Chikungunya virus research. Sci Rep 7:14641
Slotman MA, Kelly NB, Harrington LC, Kitthawee S, Jones JW, Scott TW, Caccone A, Powell JR (2007) Polymorphic microsatellite markers for studies of Aedes aegypti (Diptera: Culicidae), the vector of dengue and yellow fever. Mol Ecol Notes 7(1):168–171
Sudeep AB, Parashar D, Jadi RS, Basu A, Mokashi C, Arankalle VAA, Mishra AC (2009) Establishment and characterization of a new Aedes aegypti (L.) (Diptera: Culicidae) cell line with special emphasis on virus susceptibility. In Vitro Cell Dev Biol-Animal 45:491–495
Suzuki T, Kutsuna S, Taniguchi S, Tajima S, Maeki T, Kato F, Lim CK, Saijo M, Tsuboi M, Yamamoto K, Morioka S, Ishikane M, Hayakawa K, Kato Y, Ohmagari N (2017) Dengue Virus Exported from Côte d’Ivoire to Japan, June 2017. Emerg Infect Dis 23:1758–1760
Tarnagda Z, Cissé A, Bicaba BW, Diagbouga S, Sagna T, Ilboudo AK, Tialla D, Lingani M, Sondo KA, Yougbaré I, Yaméogo I, Sow HE, Sakandé J, Sangaré L, Greco R, Muscatello DJ (2018) Dengue fever in Burkina Faso, 2016. Emerg Infect Dis 24:170–172
Timoshevskiy VA, Kinney NA, deBruyn BS, Mao C, Tu Z, Severson DW, Sharakhov IV, Sharakova MV (2014) Genomic composition and evolution of Aedes aegypti chromosomes revealed by the analysis of physically mapped supercontigs. BMC Biol 12:27
Varma MGR, Pudney M (1969) The Growth and serial passage of cell lines from Aedes aegypti (L.) larvae in different media. J Med Entom 6(4):432–439
Walker T, Jeffries CL, Mansfield KL, Johnson N (2014) Mosquito cell lines: history, isolation, availability and application to assess the threat of arboviral transmission in the United Kingdom. Parasit Vectors 7:382
Weger-Lucarelli J, Rückert C, Grubaugh ND, Misencik MJ, Armstrong PM, Stenglein MD, Ebel GD, Brackney DE (2018) Adventitious viruses persistently infect three commonly used mosquito cell lines. Virology 521:175–180
Funding
This study was funded by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) and the Research Program on Emerging and Re-emerging Infectious Diseases from the Japan Agency for Medical Research and Development (AMED). This study was also supported in part by JSPS KAKENHI Grant Numbers JP16J09470, JP15H04614, and JP18H02856 and by the Sasakawa Scientific Research Grant from The Japan Science Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Amoa-Bosompem, M., Kobayashi, D., Itokawa, K. et al. Establishment and characterization of a cell line from Ghanaian Aedes aegypti (Diptera: Culicidae) focusing on Aedes-borne flavivirus susceptibility. In Vitro Cell.Dev.Biol.-Animal 56, 792–798 (2020). https://doi.org/10.1007/s11626-020-00504-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-020-00504-y