Abstract
In this paper, four established cell lines derived from newly hatched larvae of Papilio demoleus Linnaeus and 57 single-cell clones derived from the 3 lines were used as test materials. Recombinant β-galactosidase baculovirus AcMNPV-Gal was used to infect the P. demoleus L. cell lines and the single-cell clones, and recombinant protein expression in each cell line was detected and compared. Three clonal cell lines, RIRI-PaDe-1-C1, RIRI-PaDe-2-C6 and RIRI-PaDe-3-C52, which showed significantly higher β-galactosidase expression levels than those of the parental cell lines, were selected. Five types of commercial serum-free media for insect cells, Express Five SFM, Ex-Cell 405, Sf-900III SFM, Sf-900II SFM and HyClone Serum-Free Media, were used to adapt RIRI-PaDe-2-C6 cells and RIRI-PaDe-3-C52 cells to serum-free culture conditions, and the growth characteristics of the cells and the exogenous protein expression characteristics before and after adaptation were compared. The results showed that RIRI-PaDe-2-C6 cells could stably proliferate in Ex-Cell 405, RIRI-PaDe-3-C52 cells could stably proliferate in Express Five SFM and Ex-Cell 405, and the rate of proliferation of and the level of expression of β-galactosidase in RIRI-PaDe-3-C52 cells were significantly increased in Express Five SFM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect cell lines and their culture techniques have been widely used in research fields such as insect physiology, pathology and molecular biology. Baculovirus expression systems using insect cell lines as bioreactors have been widely used to express recombinant proteins in vitro and play an important role in the fields of biology, agronomy and medicine (Granados et al. 2007; Swiech et al. 2012). Nearly half of the previously reported insect cell lines were derived from lepidopteran insect tissues. These cell lines are distributed in a few families, and the common cell lines that can be used for exogenous gene expression are Sf9, Sf21 and BTI-TN-5B1-4 cells, which cannot meet the needs of experimental studies and production (Granados et al. 1994; Drugmand et al. 2012). The establishment of new cell lines and the screening of new host insect cell lines with high exogenous gene expression levels can provide new materials for experimental research and production. However, insect cells cultured in vitro are usually derived from different insect parts. Especially for those derived from newly hatched larvae, cells are obtained by cutting and dissociating the whole larva body, followed by cell proliferation. The newly constructed cell lines contain different cell morphologies and vary widely in size, sensitivity to viruses and protein expression levels, resulting in reduced efficiency of cell utilization during culture (Pan et al. 2010). As the number of cell passages increases, cells will differentiate in production. These factors will affect the expression level and expression stability of exogenous proteins in cells (Siissalo et al. 2007). Studies have shown that clonal screening can obtain clones of homogenous cell types that are sensitive to viruses and have significantly increased recombinant exogenous gene expression levels (Matsumura et al. 2014; Liu et al. 2018). Single-cell cloning technology is an important means to purify cell lines to obtain homogenous lines.
Insect cell culture medium usually contains serum to support cell growth and proliferation. However, the use of serum also has disadvantages. The composition of serum is very complex; some components remain unidentified, and its high protein content may interfere with the purification and detection of produced proteins (Ikonomou et al. 2001). Besides, the high cost and batch-to-batch variation in serum further limit its use. The use of serum-free medium facilitates the isolation of cell culture products and reduces contamination from mycoplasma and other viruses in serum. Many studies have shown that serum-free media can be used to mass produce viruses and express recombinant proteins (Mena and Kamen 2014). Therefore, the serum-free culture of insect cells has received increasingly more attention and has become a research hotspot.
There are very few insect cell lines derived from butterfly insect tissue as a cell line–establishing material; less than 10 cell lines have been reported (Mitsuhashi 1972, 1973; Chen et al. 2009; Zhang et al. 2012; Ding et al. 2013). We used newly hatched larvae of Papilio demoleus Linnaeus as experimental materials to establish cell lines and reported 3 cell lines that could be stably passaged, RIRI-PaDe-1, RIRI-PaDe-2 and RIRI-PaDe-3 (Ding et al. 2018). Single-cell cloning of these 3 P. demoleus L. cell lines was carried out using 4 single-cell selection and culture techniques: limiting dilution method, micromanipulation method, trophoblast culture method and semi-solid micromanipulation method (Ding et al. 2018). The efficiency of establishing clonal lines using different methods was compared. We determined that the semi-solid micromanipulation method was a single-cell cloning method suitable for the P. demoleus L. cell lines, and we ultimately obtained 57 P. demoleus L. single-cell clonal lines using this method.
Based on a previous study, recombinant β-galactosidase baculovirus (AcMNPV-Gal) was used as a test virus to infect P. demoleus L. single-cell clones, and the expression of recombinant protein was determined. The clonal strains with expression levels significantly higher than that of the parental cell line were selected and subjected to serum-free adaptation culture. The characteristics of the P. demoleus L. cell lines as expression hosts were evaluated, providing a reference for their application.
Materials and Methods
Test cell lines and virus
Six single-cell clones derived from the Papilio demoleus Linnaeus cell line RIRI-PaDe-1, 11 single-cell clones derived from RIRI-PaDe-2 and 40 clones derived from RIRI-PaDe-3 were established and preserved by the Institute of Resource Insects of the Chinese Academy of Forestry. The medium was modified based on the classical Grace insect cell culture medium (Life Technologies, Carlsbad, CA, No. 11300027) supplemented with 20% foetal bovine serum to promote cell growth. The culture conditions were in the dark at a constant temperature of 27°C (Zhang et al. 2011).
The β-galactosidase gene was obtained from the pSV-β-Galactosidase Control Vector (Promega Corporation, Madison, WI, No. E1081, GenBank: X65335). The target fragment was obtained by PCR amplification, and restriction enzyme cleavage sites were also added. The recombinant baculovirus AcMNPV-Gal was constructed using the Bac-to-Bac baculovirus expression system (Life Technologies, Carlsbad, CA, No. 10359016), and P2 generation virus stock solution was used for the test.
Detection of recombinant β-galactosidase expression
Test cells in the exponential growth phase were diluted to 1 × 105 cells/mL and seeded in a 24-well cell culture plate with 1 mL of cell suspension per well. Each cell line was inoculated in 4 wells, 3 of which served as parallel samples and 1 of which served as the negative control. The viral titre was determined using a BacPAK baculovirus rapid titre kit (Clontech Laboratories, Mountain View, CA, No. 631406), and the virus solution was diluted so that each cell was infected with 5 virus particles (MOI = 5). The culture plate was gently shaken for 2 h in a shaker in the dark to allow the cells to fully absorb the virus; then, the medium in each well was then carefully aspirated while avoiding taking up the cells. Subsequently, 2 mL of fresh medium containing no virus was added to each well, and the cells were incubated in the dark at a constant temperature of 27°C.
After 24 h of culture, the first test was performed, followed by 1 test every 24 h; a total of 7 tests were performed. The 24-well cell culture plate inoculated with AcMNPV-Gal was shaken on a shaker for 15 min to uniformly disperse the recombinant protein secreted by the cells in the supernatant. A total of 30 μL of cell supernatant was taken from each well as the sample to be tested, and 30 μL of fresh medium was added to the original well to keep the total volume of the medium in each well constant. The enzyme activity of β-galactosidase in the sample was determined using the β-Galactosidase Enzyme Assay System with Reporter Lysis Buffer (Promega, No. E2000).
The enzymatic activities of 3 parallel samples and 1 blank control per cell line were labelled as S1, S2, S3 and C, and the average values of S1-C, S2-C and S3-C (mean ± std.) were calculated as the expression level of recombinant protein in each cell line. The enzyme activities of the test cell lines were subjected to the t test using SPSS (Ver. 19) to determine whether there was a significant difference in recombinant protein expression levels (P < 0.05).
Serum-free culture and adaptation
In this study, 5 commercially available serum-free media for insect cells, Express Five SFM (Life Technologies, Carlsbad, CA, No. 10486025), Ex-Cell 405 (SAFC Biosciences, Lenexa, KS, No. 14405C), Sf-900III SFM (Life Technologies, Carlsbad, CA, No. 12658019), Sf-900II SFM (Life Technologies, Carlsbad, CA, No. 10902088) and HyClone Serum-Free Media (Cytiva, Marlborough, MA, No. SH30350.03), were selected. The serum-free adaptation was performed with the Papilio demoleus Linnaeus single-cell clones RIRI-PaDe-2-C6 and RIRI-PaDe-3-C52 by progressively replacing the original medium.
The serum-free medium and the original medium (Grace + 20% FBS) were mixed at ratios of 3:7, 1:1, 7:3 and 4:1, respectively, to prepare the adaptation media, which were used to replace the original medium to adapt the test cells. In this process, when the cells were fully adapted to the current serum concentration, the medium was replaced with the next gradient of adaptation medium, until the culture medium was replaced fully with serum-free medium. In this process, the morphology and growth of the test cells were closely observed.
Cell growth curve and doubling time calculation
Test cells in the exponential growth phase were diluted to 2 × 105 cells/mL and seeded in a 96-well cell culture plate with 100 μL of cell suspension per well. Each cell line was inoculated in 4 wells as parallel samples; a total of 7 plates were inoculated and incubated at a constant temperature of 27°C in the dark. One plate was taken every 24 h for a cell viability assay. Cell viability assays were performed using Promega’s CellTiter 96 Aqueous One Solution Cell Proliferation Assay (No. G3582) and were conducted for 7 consecutive days. In Excel, the assay day was used as the abscissa, and the absorbance value was used as the ordinate; therefore, a cell density curve as a function of culture time was plotted. According to the curve trend, we determined the time when cells entered the exponential growth phase, and the cell population doubling time was then calculated.
Results
Determination of β-galactosidase expression levels in RIRI-PaDe-1 cells and single-cell clones
The RIRI-PaDe-1 cell line and its 6 clonal cell lines were infected with recombinant AcMNPV-Gal. All the tested cells expressed β-galactosidase, and expression peaked 72–120 h after infection (Table 1). The results showed that the expression of β-galactosidase in the single-cell clone RIRI-PaDe-1-C1 (0.249 ± 0.064 mU/μL) was significantly higher than that of the parental cell line (0.147 ± 0.053 mU/μL) (P < 0.05).
Determination of β-galactosidase expression levels in RIRI-PaDe-2 cells and single-cell clones
The RIRI-PaDe-2 cell line and its 10 clonal cell lines were infected with recombinant AcMNPV-Gal. All the tested cells expressed β-galactosidase, and protein expression peaked 72–120 h after infection. Cell lines were sorted according to β-galactosidase expression levels in descending order. The top 6 cell lines with the highest expression are listed in Table 2. β-Galactosidase expression in the clonal strain RIRI-PaDe-2-C6 (0.216 ± 0.038 mU/μL) was significantly higher than that in the parental cell line RIRI-PaDe-2 (0.119 ± 0.023 mU/μL) (P < 0.05).
Determination of β-galactosidase expression levels in RIRI-PaDe-3 cells and single-cell clones
The RIRI-PaDe-3 cell line and its 40 clonal cell lines were infected with recombinant AcMNPV-Gal. All the tested cells expressed recombinant protein. Cell lines were sorted according to β-galactosidase expression in descending order. The top 6 cell lines with the highest expression levels are listed in Table 3. The results showed that under the same experimental conditions, 4 single-cell clones expressed higher levels of β-galactosidase than did the parental cell line RIRI-PaDe-3, but further statistical tests found that β-galactosidase expression in these cell lines was not significantly different from that in the parental cell line.
In summary, AcMNPV-Gal infected all test cells, but the expression of β-galactosidase was different in all cell lines. Among them, the single-cell clonal lines RIRI-PaDe-1-C1 and RIRI-PaDe-2-C6 expressed significantly higher levels of β-galactosidase than did their parental cell lines. We failed to identify single-cell clones of RIRI-PaDe-3 with significantly higher β-galactosidase expression than the parental cell line. However, the clone with the highest expression level, RIRI-PaDe-3-C52, has a relatively simple genetic background. Horizontal comparison of β-galactosidase expression by these 3 clones revealed that the expression levels in RIRI-PaDe-2-C6 cells and RIRI-PaDe-3-C52 cells were significantly higher than those in RIRI-PaDe-1-C1 cells.
Adaptation of RIRI-PaDe-2-C6 cells in serum-free and low-serum medium and changes in proliferation kinetics and exogenous protein expression after adaptation
The P. demoleus L. single-cell clone RIRI-PaDe-2-C6 was adapted by gradually replacing the original Grace + 20% FBS medium with 5 types of serum-free media. When the medium was replaced with 70% Ex-Cell 405 serum-free medium, some cells became round and were prone to grow in clumps. With 100% serum-free medium, cell clumping was significant during the first month of culture, and then, the cells gradually returned to uniform adherent growth. After 4 mo of serum-free culture and adaptation, the cells stably proliferated and were passage in Ex-Cell 405; thus, serum-free adaptation was successful. When the other 4 types of serum-free media were used for adaptation, the cells exhibited growth arrest and apoptosis, and serum-free medium adaptation was not achieved (Fig. 1).
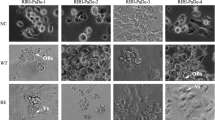
Morphology of the P. demoleus L. clonal line RIRI-PaDe-2-C6 during serum-free medium adaptation. (a) Cell morphology before adaptation to serum-free medium. (b) Cell morphology after 3 mo of culture in 100% Express Five SFM. (c) Cell morphology after 1 mo of culture in 100% Sf-900II SFM. (d) Cell morphology after 1 mo of culture in 100% Sf-900III SFM. (e) Cell morphology after 3 mo of culture in 100% HyClone Serum-Free Media. (f) Cell morphology after 3 mo of culture in 100% Ex-Cell 405.
The cell growth curves before and after serum-free adaptation are shown in Fig. 2. After 24 h of inoculation, the viable cell density of the cells gradually increased, reached a maximum at 144 h, and then began to decline. The doubling times of RIRI-PaDe-2-C6 cells before and after serum-free adaptation in Ex-Cell 405 were 66.92 h and 87.59 h, respectively, indicating that the proliferation rate of RIRI-PaDe-2-C6 cells significantly decreased after serum-free adaptation.
RIRI-PaDe-2-C6 cells before and after adaptation were infected with the recombinant virus AcMNPV-Gal, and the expression of recombinant β-galactosidase protein was detected at 24, 48, 72, 96, 120, 144 and 168 h after infection. Recombinant β-galactosidase protein expression in RIRI-PaDe-2-C6 cells before and after adaptation in Ex-Cell 405 serum-free medium is shown in Fig. 3. The results showed that the expression of recombinant β-galactosidase protein in RIRI-PaDe-2-C6 cells post adaptation was not significantly higher than that before adaptation (t = − 0.177, df = 4, P = 0.868 > 0.05) and that the infection time (144 h) taken to reach the highest β-galactosidase expression level after adaptation was significantly longer than that before adaptation (96 h).
Adaptation of RIRI-PaDe-3-C52 cells in serum-free and low-serum medium and changes in proliferation kinetics and exogenous protein expression after adaptation
The clonal cell line RIRI-PaDe-3-C52 was adapted by gradually replacing the original Grace+20% FBS medium with 5 types of serum-free media. The cells were able to grow and gradually adapt to the serum-free medium in Express Five SFM and Ex-Cell 405 medium. In the other 3 types of media, the cells showed reduced cell viability; a small number of adherent cells became round and had increased cell inclusions; some cells were broken or misshaped; and finally, the cells could not be passaged normally (Fig. 4).
Morphology of the P. demoleus L. clonal line RIRI-PaDe-3-C52 during serum-free medium adaptation. (a) Cell morphology before adaptation to serum-free medium. (b) Cell morphology after 3 mo of culture in 100% Express Five SFM. (c) Cell morphology after 1 mo of culture in 100% Sf-900II SFM. (d) Cell morphology after 1 mo of culture in 100% Sf-900III SFM. (e) Cell morphology after 3 mo of culture in 100% HyClone Serum-Free Media. (f) Cell morphology after 3 mo of culture in 100% Ex-Cell 405.
It can be seen from the growth curve (Fig. 5) that there was a significant difference in the proliferation kinetics of RIRI-PaDe-3-C52 cells after adaptation in different serum-free media. The calculated doubling time for cells adapted in Ex-Cell 405 was 108.34 h, which was significantly increased compared with that before adaptation (75.79 h), indicating that the proliferation rate of RIRI-PaDe-3-C52 cells in Ex-Cell 405 was significantly slower than that before adaptation. However, the cell population doubling time of the cells adapted in Express Five SFM was 38.22 h, which was significantly shorter than that before adaptation, indicating that the proliferation rate of RIRI-PaDe-3-C52 cells in Express Five SFM was significantly higher than that before adaptation.
Figure 6 shows a histogram of β-galactosidase expression in RIRI-PaDe-3-C52 cells as a function of AcMNPV-Gal infection time before and after serum-free adaptation. The results showed that β-galactosidase expression levels reached the highest level in RIRI-PaDe-3-C52 cells 96 and 120 h after infection. The independent sample t test was used to compare expression levels before and after adaptation. The results showed that β-galactosidase expression in RIRI-PaDe-3-C52 cells adapted in Express Five SFM medium was significantly higher than that before adaptation (t = − 11.683, df = 4, P = 0.000 < 0.05). There was no significant difference in β-galactosidase expression in RIRI-PaDe-3-C52 cells before and after adaptation in Ex-Cell 405 medium (P > 0.05).
Discussion
In summary, we believe that single-cell cloning technology can indeed be used to screen clonal cells with exogenous protein expression levels higher than that of the parental cell line. Insect cell culture in serum-free medium with different formulations is also very different. Two types of media, Express Five SFM and Ex-Cell 405, are suitable for serum-free adaptation of P. demoleus L. cell lines. In particular, after adaptation in Express Five SFM, the expression of recombinant β-galactosidase significantly increased in the RIRI-PaDe-3-C52 clonal line, which is worthy of further investigation.
Both parental P. demoleus L. cell lines and single-cell clones expressed recombinant β-galactosidase. However, compared with the commonly used host cell line Sf9 (the highest enzymatic activity of Sf9-expressed β-galactosidase was 4.132 ± 0.942 U/mL), the expression level was still low. We speculate that the main reason for this result is that P. demoleus L. is not a natural host of AcMNPV and that the number of cells infected by the recombinant virus was lower than that of Sf9 cells. Baculovirus in nature has a high degree of host specificity, and 1 type of virus can only infect a few closely related insects. However, this host specificity is relative. Each virus has an original host, but it can often infect several alternative hosts. The host range of AcMNPV is wide, and it can infect a variety of lepidopteran insects. It is for this reason that AcMNPV is the most commonly used vector for insect cell baculovirus expression systems and is used to construct recombinant viruses capable of expressing high levels of exogenous protein in sensitive host cells (Yuan et al. 2012). Thus, the selection of recombinant viral expression vectors is an important factor affecting the expression of exogenous proteins. At present, one possible improvement is to increase the host range by modifying the AcMNPV host range determination genes, thereby increasing the number of infected cells and, ultimately, obtaining higher exogenous protein expression levels.
Conclusions
By implementing the study, we confirmed that the single-cell cloning technique can indeed obtain the cell strains with significantly higher heterologous protein expression characteristics than the paternal cell lines developed from P. demoleus. Further serum-free adaption study confirmed that the cell strains can adapt to the growth environment of Express Five SFM and Ex-Cell 405, which provides a reference for future large-scale culture research.
Abbreviations
- MOI:
-
Multiplicity of infection
References
Chen YR, Solter LF, Chien TY, Jiang MH, Lin HF, Fan HS, Lo CF, Wang CH (2009) Characterization of a new insect cell line (NTU-YB) derived from the common grass yellow butterfly, Eurema hecabe (Linnaeus) (Pieridae: Lepidoptera) and its susceptibility to microsporidia. J Invertebr Pathol 102(3):256–262
Ding WF, Feng Y, Zhang X, Li X, Wang CY (2013) Establishment and characterization of a cell line developed from the neonate larvae of Papilio demoleus Linnaeus (Lepidoptera: Papilionidae). In Vitro Cell Dev Biol Anim 49(2):108–113
Ding WF, Zhang X, Li X, Liu ZG (2018) Expression of three reporter genes in four cell lines developed from Papilio demoleus Linnaeus (Lepidoptera: Papilionidae). In Vitro Cell Dev Biol Anim 54(3):194–199
Drugmand J-C, Schneider Y-J, Agathos SN (2012) Insect cells as factories for biomanufacturing. Biotechnol Adv 30(5):1140–1157
Granados RR, Li GX, Blissard GW (2007) Insect cell culture and biotechnology. Virol Sin 22(2):83–93
Granados RR, Li GX, Derksen ACG, McKenna KA (1994) A new insect cell line from Trichoplusia ni (BTI-Tn-5B1-4) susceptible to Trichoplusia ni single enveloped nuclear polyhedrosis virus. J Invertebr Pathol 64(3):260–266
Ikonomou L, Bastin G, Schneider YJ, Agathos SN (2001) Design of an efficient medium for insect cell growth and recombinant protein production. In Vitro Cell Dev Biol Anim 37(9):549–559
Liu ZG, Ding WF, Xie SC, Sun N, Zhang X, Li X, Feng Y (2018) Establishment and characterization of cell clones from the Papilio cell line RIRI-PaDe-3 by a high-efficiency clonal method. Cytotechnology 70(4):1235–1245
Matsumura T, Tatsumi K, Noda Y, Nakanishi N, Okonogi A, Hirano K, Liu L, Osumi T, Tada T, Kotera H (2014) Single-cell cloning and expansion of human induced pluripotent stem cells by a microfluidic culture device. Biochem Biophys Res Commun 453(1):131–137
Mena JA, Kamen AA (2014) Insect cell technology is a versatile and robust vaccine manufacturing platform. Expert Rev Vaccines 10(7):1063–1081
Mitsuhashi J (1972) Primary culture of the haemocytopoietic tissue of Papilio xuthus Linne. Appl Entomol Zool 7(1):39–41
Mitsuhashi J (1973) Establishment of cell lines from the pupal ovaries of the swallow tail, Papilio xuthus Linne: Lepidoptera: Papilionidae. Appl Entomol Zool 8(2):64–72
Pan MH, Cai XJ, Liu M, Lv J, Tang H, Tan J, Lu C (2010) Establishment and characterization of an ovarian cell line of the silkworm, Bombyx mori. Tissue Cell 42(1):42–46
Siissalo S, Laitinen L, Koljonen M, Vellonen K-S, Kortejärvi H, Urtti A, Hirvonen J, Kaukonen AM (2007) Effect of cell differentiation and passage number on the expression of efflux proteins in wild type and vinblastine-induced Caco-2 cell lines. Eur J Pharm Biopharm 67(2):548–554
Swiech K, Picanço-Castro V, Covas DT (2012) Human cells: new platform for recombinant therapeutic protein production. Protein Expr Purif 84(1):147–153
Yuan WT, Ju CY, Yi TC, Shuo CW, Villaflores O (2012) A bi-cistronic baculovirus expression vector for improved recombinant protein production. Bioeng Bugs 3(2):127–130
Zhang X, Feng Y, Ding WF, Chen XM, Wang CY, Ma T (2011) Establishment and characterization of an embryonic cell line from Gampsocleis gratiosa (Orthoptera: Tettigoniidae). In Vitro Cell Dev Biol Anim 47(4):327–332
Zhang X, Feng Y, Ding WF, Chen XM, Wang CY, Ma T (2012) Characterization of a new insect cell line that is derived from the neonate larvae of Papilio xuthus (Lepidoptera: Papilionidae) and its susceptibility to AcNPV. Tissue Cell 44(3):137–142
Funding
This work was supported by the Fundamental Research Funds for the Central Non-profit Research Institution of CAF (No. CAFYBB2016ZD005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Ding, WF., Liu, ZG., Sun, N. et al. Screening of single-cell clonal lines from Papilio demoleus Linnaeus cell lines for exogenous protein expression and adaptation in serum-free culture. In Vitro Cell.Dev.Biol.-Animal 56, 444–451 (2020). https://doi.org/10.1007/s11626-020-00484-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-020-00484-z