Abstract
Several species of the Annonaceae plants have been used as complementary medicine for cancer-associated illnesses in some ethnic groups of northern Thailand. This study investigated the cytotoxic and cytostatic activity of methanolic extracts derived from the stems of these plants, including Uvaria longipes (Craib) L.L.Zhou, Y.C.F.Su & R.M.K.Saunders, Artabotrys burmanicus A.DC, Marsypopetalum modestum (Pierre) B.Xue & R.M.K.Saunders, and Dasymaschalon sp. Cell death induction of seven human cancer cell lines and cell cycle analyses were assessed by Annexin V and/or propidium iodide (PI) staining and analyzed by flow cytometry. Treatment of cancer cell lines with the extract of four Annonaceae plants resulted in various cytotoxic activities depending on cell type. The extract of U. longipes exhibited the highest cytotoxic activity capable of inducing cell death of several cancer cell lines, particularly against hepatocellular carcinoma cell lines (HepG2 and Hep3B). This extract was capable of inducing cell cycle arrest at the SubG1 phase. Phytochemical screening of all the extracts revealed the presence of alkaloids, sterols, tannins, anthraquinone glycoside, coumarin, and flavonoids. Determination of active compounds by high-performance liquid chromatography standards revealed bullatacin and asiminecin in all the extracts. The extract of Annonaceae stem or its compounds may provide an opportunity for the development of new therapies against cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethnomedicinal plants are sources of active compounds that have been used in many countries as food and as traditional medicine to treat a wide range of diseases (Amoo et al.2014; Tariq et al.2015, 2016; Dinda et al.2016; Maroyi 2017; Odonne et al.2017). The World Health Organization reported that interest in traditional medicine continues to grow around the world (WHO 2013). Ethnomedicinal plants possess various pharmacological and biological properties, including anti-oxidant, anti-inflammatory, anti-microbial, anti-plasmodial, and anti-helminthic activities (Amoo et al.2014; Tariq et al.2015, 2016; Dinda et al.2016; Maroyi 2017; Odonne et al.2017). Several ethnomedicinal plants contain cytotoxic compounds capable of inducing apoptotic cell death and have been used as anti-cancer agents (Jung et al.2018a, b; Mirza et al.2018; Yang et al.2018; Ye et al.2018).
Induction of apoptotic cell death is one of the promising approaches to targeting cancer cells. This process involves two main pathways that are extrinsic and intrinsic (Elmore 2007). The extrinsic pathway is stimulated when the death receptors are engaged with specific signaling molecules, while the intrinsic pathway is directly promoted by cellular sensing of extracellular and/or intracellular stresses. Both apoptotic pathways require suitable stimuli to initiate the process (Elmore 2007). Exploration of active compounds from natural products capable of activating the apoptotic process holds potential for alternative and/or complementary treatment of cancer.
The Annonaceae family of plants consist of 112 genera and approximately 2500 species which most of them are found in the tropics (Quilez et al.2018). Several species of the Annonaceae family are rich in secondary metabolites, such as alkaloids, terpenoids, flavonoids, and acetogenins (Moreira et al.2013). A number of alkaloid derivatives, such as jerantinine B (Qazzaz et al.2016), liriodenine (Li et al.2017), and vinorelbine (Capasso 2012), are apoptotic agents capable of inhibiting cell proliferation. Commonly known as custard apple, Annona squamosa Linn contains terpenoid capable of inducing apoptosis of hepatoma cells via a mitochondria-mediated pathway (Chen et al.2017). Extracts from Annona muricata containing flavonoids inhibit the growth of the human promyelocytic leukemia (HL-60) cells, probably through the disruption of MMP, reactive oxygen species (ROS) generation, and the G0/G1 cell arrest (Pieme et al.2014). In addition, bullatacin, an acetogenin isolated from the fruit of Annona atemoya, can induce apoptosis of human hepatocarcinoma cell line (2.2.15 cells) in a time- and dose-dependent manner (Chih et al.2001). These data imply the potential use of the Annonaceae plants as alternative and/or complementary medicine for cancer treatment.
We have previously shown that the extracts from leaves of four species of the Annonaceae plant, namely Uvaria longipes (Craib) L.L.Zhou, Y.C.F.Su, & R.M.K.Saunders; Dasymaschalon sp.; Artabotrys burmanicus A.D.C.; and Marsypopetalum modestum (Pierre) B.Xue & R.M.K.Saunders induced cell cycle arrest and apoptosis on human cancer cell lines (Pumiputavon et al.2017). In general, different parts of plants contain different chemical constituents. The present study was conducted to evaluate the cytotoxic activity of stem-derived methanolic extracts from these plants. The extracts were tested against human cervical carcinoma, human hepatocellular carcinoma, and human hematopoietic cell lines in vitro. High-performance liquid chromatography (HPLC) was also performed to identify the bioactive components in all the extracts.
Materials and Methods
Cell lines and culture
Human cervical carcinoma (HeLa and SiHa) (a kind gift from Assoc. Prof. Tipaya Ekalaksananan, Khon Kaen University, Thailand), human hepatocellular carcinoma (HepG2 and Hep3B) (a kind gift from Prof. Duncan R. Smith, Mahidol University, Thailand), and human myeloid leukemia (K562, U937, and RAJI) (a kind gift from Prof. Sumalee Tungpradabkul, Mahidol University, Thailand) cells were cultured in the Roswell Park Memorial Institute (RPMI) (Gibco-BRL, Grand Island, NY) medium containing 10 mM of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Merck Millipore, Darmstadt, Germany), 1 mM of sodium bicarbonate (RCI LABSCAN, Bangkok Thailand), 10% fetal bovine serum (FBS), penicillin (100 IU/ml), and streptomycin (100 μg/ml) (Gibco-BRL) (RPMI complete media) at 37°C in a 5% CO2 incubator. All cell lines have been authenticated by DNA Forensics Laboratory Pvt. Ltd., India.
Plant materials and extraction
Uvaria longipes (collection no.: Chaowasku 132), Dasymaschalon sp. (collection no.: Chaowasku 120), and Marsypopetalum modestum (collection no.: Chaowasku 164) were collected from private residences at coordinates 13.790384, 100.372378; and Artabotrys burmanicus (collection no.: Chaowasku 163) was collected from a private garden at coordinates 13.919300, 99.952555 as described previously (Pumiputavon et al.2017). The stems of the plant materials were washed with distilled water to remove dust, air dried, and ground into powder. Dry powders were soaked in methanol in large containers at room temperature for 24 h. The solvent extract was collected and filtered through filter paper. The filtrate was concentrated under vacuum in a rotary evaporator at 40°C. All the methanolic extracts were resuspended in dimethyl sulfoxide (DMSO) (Gibco-BRL) at a concentration of 100 mg/ml.
Annexin V staining assay
Cell death of human cancer cell lines (HeLa, SiHa, HepG2, Hep3B, K562, U937, and RAJI) was analyzed based on the Annexin V/propidium iodide (PI) dual staining and analyzed by flow cytometry, as described previously (Pumiputavon et al.2017).
Cell cycle analysis
The cell cycle was assessed by staining human cancer cell lines with 20 μg/ml PI and analyzed by flow cytometry, as described previously (Pumiputavon et al.2017).
Phytochemical screening
All the extracts were phytochemical-tested for alkaloids, sterols, cardiac glycosides, anthraquinone glycosides, saponins, flavonoids, and tannins by using standard methods, as described previously (Mujeeb et al.2014). The results are expressed qualitatively as (−) for the absence; and (+), (++), and (+++) for the presence of low, medium, and high content of phytochemicals, respectively.
Evaluation of acetogenins
The extracts were analyzed for five acetogenins, including annoglaxin, squamostatin-A, bullatacin, squamocin, and murisolin by a reversed-phase HPLC (RP-HPLC) system (Agilent 1200 Series, Santa Clara, CA ) with slight modifications from Yang et al. (2010). Separation was accomplished on a reversed-phase C18 column (150 × 4.6 mm inner diameter) (KINETEX® C18; Phenomenex Co., Ltd., Torrance, CA). The mobile phases consisted of A (methanol) and B (deionized water) using linear gradients of 0–40 min (85%A) and 40–60 min (85–95%A). The mobile phase pumped at a flow rate of 0.3 mL/min and the detection wavelength was 220 nm. Series of corresponding standards were measured concomitantly to construct a calibration curve used for determination of the concentrations of annoglaxin, bullatacin, squamocin, asiminecin, and murisolin in samples. The assays were performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS program. One-way ANOVA with Tukey’s HSD post hoc test was used to determine differences between groups. Significance was set at P < 0.05.
Results
Induction of cancer cell death by stem-derived extracts from four species of the Annonaceae family
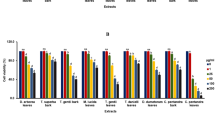
The percentages of cell death determined by flow cytometry following Annexin V and PI staining were used to evaluate the cytotoxic activity of the extracts. The populations of cells in the Annexin V+/PI− and the Annexin V+/PI+ quadrants were identified as induced cell death. The half-maximal inhibitory concentration (IC50) was calculated from the percentages of cell death (Annexin V+/PI−, Annexin V−/PI+, and the Annexin V+/PI+ quadrants) (Table 1). The degree of apoptosis varied by type of extract (stem from Uvaria longipes: SUL, Dasymaschalon sp.: SD, Artabotrys burmanicus: SAB, and Marsypopetalum modestum: SMM). SUL presented the best cytotoxic activity among the extracts, especially on hepatocellular carcinoma lines (HepG2 and Hep3B). Morphological changes were observed using an inverted microscope together with the decreases of the mean forward light scatter from flow cytometry (data not shown). These changes were observed in the extracts that had cytotoxic activity and in only the cell lines that were affected. The affected cell lines had shrunken cytoplasm, condensed chromatin, and loss of normal shape. SAB and SMM showed least cytotoxic activity on almost all cell lines even at 500 and 1000 μg/ml concentrations (Table 2). The effect of SD was prominent only on HepG2 cell line. Human myeloid leukemia lines (K562, U937, and RAJI) were less affected by all the extracts compared with cervical carcinoma and human hepatocellular carcinoma groups. These indicate that the extracts induced cytotoxicity in a cell-type specific manner. In addition, a dose-dependent manner was also observed in some extracts (Table 2 and Fig. 1).
Effect of extracts obtained from the stems of U. longipes (A), Dasymaschalon sp. (B), A. burmanicus (C), and M. modestum (D) on the induction of apoptosis in the sample SiHa cell. SiHa cells were cultured in the presence of various concentrations of the crude extracts for 24 h. The cells were then stained with Annexin V and PI, and analyzed by flow cytometry.
Effect of SUL on cell cycle arrest
From apoptosis assessment, it was evident that SUL showed the highest cytotoxic activity on most of the cancer cell lines. Therefore, SUL was further evaluated for its effect on cell cycle. After treating the cancer cell lines with various concentrations of SUL for 24 h, the cell cycle was assessed by propidium iodide staining followed by flow cytometry. SUL induced cell cycle arrest of all the cancer cell lines, except Hep3B, in a dose-dependent manner as evidenced by the increase of the sub G1-phase population compared with the controls (untreated or DMSO-treated cells) (Table 3 and Fig. 2).
Phytochemical analysis
Preliminary phytochemical screening demonstrated that all the extracts contained alkaloids, coumarins, sterols, tannins, anthraquinone, glycosides, and flavonoids. The contents of the phytochemicals are shown in Table 4. Saponins were not found in any extracts. All the extracts were analyzed for acetogenins by reversed-phase HPLC, and compared with five standard acetogenins, including annoglaxin, squamostatin-A, bullatacin, squamocin, and murisolin. The HPLC chromatogram (Fig. 3) revealed that all the extracts contained bullatacin and asiminecin (Table 5).
The HPLC chromatograms of the reference compounds and four crude methanolic stem-derived extracts. A mixture of five acetogenins including annoglaxin (1), bullatacin (2), squamocin (3), asiminecin (4), and murisolin (5); and crude methanolic stem-derived extract of U. longipes; Dasymaschalon sp.; A. burmanicus; and M. modestum.
Discussion
The Annonaceae family has been recognized as a source of very rich secondary metabolites, including terpenoids, alkaloids, steroids, polyphenols, and flavonoids (Moreira et al.2013). In the present study, the cytotoxic and cytostatic activities of methanolic extracts of stems of these four species against human cervical carcinoma (HeLa and Caski), human hepatocellular carcinoma (HepG2 and Hep3B), and human myeloid leukemia (K562, U937, and RAJI) cancer cell lines were evaluated. The results showed that the extracts induced cell death in a dose-dependent and cell-type specific manner. The extract obtained from the stem of U. longipes induced the accumulation of the SubG1 phase of some cancer cell lines, also in a dose-dependent and cell-type specific manner.
The phytochemical analysis showed the presence of alkaloids, coumarins, sterols, tannins, anthraquinone glycosides, and flavonoids in all the extracts. Alkaloids have several therapeutic properties such as anti-inflammatory, hepatoprotective, and anti-cancer activities (Takshak 2018). Coumarins regulate a number of cellular pathways and have been reported as showing anti-cancer activity on different types of cancers (Thakur et al.2015). Tannins exhibit potential anti-cancer effect in vitro, including triggering apoptosis and cell cycle arrest, inhibition of invasion and metastases, and inhibiting angiogenesis (Cai et al.2017), though further study in vivo is needed. Sterol has been proposed as a possible therapeutic agent against cancer by inhibition of various regulatory molecules in the cholesterol homeostasis of cancer cells (Gabitova et al.2014). The activities of flavonoids on cancer cells include anti-proliferative effect, inhibiting tumor formation, promoting apoptosis, and inhibiting the activation of nuclear factor kappa B (NF-κB) signaling pathway (Tungmunnithum et al.2018). All the extracts in our study contained alkaloids, coumarins, sterols, tannins, anthraquinone glycosides, and flavonoids. An exception was in the case of coumarins, which were not detected in the stem-derived methanolic extracts of M. modestum. The lack of flavonoids in M. modestum is consistent with the lowest cytotoxic activity of this extract as compared with the others.
Bullatacin and asiminecin were detected in all the extracts by HPLC. These two active compounds have been reported for various biological activities. Bullatacin isolated from an Annonaceae plant is capable of inducing apoptosis of human hepatoma cell line through a mechanism that reduces intracellular cyclic AMP (cAMP) and cyclic GMP (cGMP) levels (Chiu et al.2003). An in vivo study showed that treatment of mice with bullatacin results in reduction in tumor growth with improved hematologic parameters (Chen et al.2013). Moreover, bullatacin can induce apoptosis of human cervical cancer HeLa and human leukemia HL-60 cells by inhibition of NADH oxidase activity (Morre et al.1995). Asiminecin has shown promising cytotoxicity and exhibiting potential for development as a potential anti-cancer agent (Mangal et al.2015). The cytotoxic activity has been demonstrated on the HT-29 human colon cancer cell line (Zhao et al.1994). However promising these results may seem, they are just preliminary findings. All the extracts should be tested for cytotoxicity against normal cells, such as human mononuclear cells. It is also worth noting that the fruits, seeds, and leaves of the Annonaceae family contain a neurotoxic compound called annonacin which is possibly responsible for atypical Parkinsonism/dementia clusters (Smith et al.2016). It has been reported that the stem of the Annonaceae family contains annonacin which is cytotoxic and insecticidal and inhibited the formation of crown gall tumors on potato discs (Alkofahi et al.1988). Whether the Annonaceae plants tested in our study contain annonacin needs further investigation. Phytochemical analysis and the cytotoxicity tests would guarantee the suitability and safety of these extracts in therapeutic application against different cancer cell lines in the future.
Conclusions
Stem-derived methanolic extracts of U. longipes, Dasymaschalon sp., A. burmanicus, and M. modestum exhibited evidence of cell death induction in a cell-type specific manner. The extract of U. longipes showed the highest cytotoxic activity. Cancer cell lines treated with this extract showed increased percentage of cells in SubG1 phase of the cell cycle. All the extracts in this study offer a potential source of bullatacin and asiminecin, which might be an alternative or complementary remedy for cancer management.
References
Alkofahi A, Rupprecht JK, Smith DL, Chang CJ, McLaughlin JL (1988) Goniothalamicin and annonacin: bioactive acetogenins from Goniothalamus giganteus (Annonaceae). Experientia 44:83–85
Amoo SO, Aremu AO, Van Staden J (2014) Unraveling the medicinal potential of South African Aloe species. J Ethnopharmacol 153:19–41
Cai Y, Zhang J, Chen NG, Shi Z, Qiu J, He C, Chen M (2017) Recent advances in anticancer activities and drug delivery systems of tannins. Med Res Rev 37:665–701
Capasso A (2012) Vinorelbine in cancer therapy. Curr Drug Targets 13:1065–1071
Chen Y, Chen JW, Zhai JH, Wang Y, Wang SL, Li X (2013) Antitumor activity and toxicity relationship of annonaceous acetogenins. Food Chem Toxicol 58:394–400
Chen YY, Cao YZ, Li FQ, Zhu XL, Peng CX, Lu JH, Chen JW, Li X, Chen Y (2017) Studies on anti-hepatoma activity of Annona squamosa L. pericarp extract. Bioorg Med Chem Lett 27:1907–1910
Chiu HF, Chih TT, Hsian YM, Tseng CH, Wu MJ, Wu YC (2003) Bullatacin, a potent antitumor Annonaceous acetogenin, induces apoptosis through a reduction of intracellular cAMP and cGMP levels in human hepatoma 2.2.15 cells. Biochem Pharmacol 65:319–327
Chih HW, Chiu HF, Tang KS, Chang FR, Wu YC (2001) Bullatacin, a potent antitumor annonaceous acetogenin, inhibits proliferation of human hepatocarcinoma cell line 2.2.15 by apoptosis induction. Life Sci 69:1321–1331
Dinda B, Kyriakopoulos AM, Dinda S, Zoumpourlis V, Thomaidis NS, Velegraki A, Markopoulos C, Dinda M (2016) Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J Ethnopharmacol 193:670–690
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Gabitova L, Gorin A, Astsaturov I (2014) Molecular pathways: sterols and receptor signaling in cancer. Clin Cancer Res 20:28–34
Jung JI, Kim SY, Park KY, Sydara K, Lee SW, Kim SA, Kim J (2018a) In vitro combinatorial anti-proliferative and immunosuppressive effects of Brucea javanica extract with CX-4945 and imatinib in human T-cell acute lymphoblastic leukemia cells. Biomed Pharmacother 106:403–410
Jung KH, Rumman M, Yan H, Cheon MJ, Choi JG, Jin X, Park S, Oh MS, Hong SS (2018b) An ethyl acetate fraction of Artemisia capillaris (ACE-63) induced apoptosis and anti-angiogenesis via inhibition of PI3K/AKT signaling in hepatocellular carcinoma. Phytother Res 32:2034–2046. https://doi.org/10.1002/ptr.6135
Li ZH, Gao J, Hu PH, Xiong JP (2017) Anticancer effects of liriodenine on the cell growth and apoptosis of human breast cancer MCF-7 cells through the upregulation of p53 expression. Oncol Lett 14:1979–1984
Mangal M, Khan MI, Agarwal SM (2015) Acetogenins as potential anticancer agents. Anti Cancer Agents Med Chem 16:138–159
Maroyi A (2017) Ethnopharmacology and therapeutic value of Bridelia micrantha (Hochst.) Baill. in tropical Africa: a comprehensive review. Molecules (Basel, Switzerland) 22
Mirza MB, Elkady AI, Al-Attar AM, Syed FQ, Mohammed FA, Hakeem KR (2018) Induction of apoptosis and cell cycle arrest by ethyl acetate fraction of Phoenix dactylifera L. (Ajwa dates) in prostate cancer cells. J Ethnopharmacol 218:35–44
Moreira IC, Roque NF, Vilegas W, Zalewski CA, Lago JH, Funasaki M (2013) Genus xylopia (Annonaceae): chemical and biological aspects. Chem Biodivers 10:1921–1943
Morre DJ, de Cabo R, Farley C, Oberlies NH, McLaughlin JL (1995) Mode of action of bullatacin, a potent antitumor acetogenin: inhibition of NADH oxidase activity of HeLa and HL-60, but not liver, plasma membranes. Life Sci 56:343–348
Mujeeb F, Bajpai P, Pathak N (2014) Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed Res Int 2014:497606
Odonne G, Houel E, Bourdy G, Stien D (2017) Treating leishmaniasis in Amazonia: a review of ethnomedicinal concepts and pharmaco-chemical analysis of traditional treatments to inspire modern phytotherapies. J Ethnopharmacol 199:211–230
Pieme CA, Kumar SG, Dongmo MS, Moukette BM, Boyoum FF, Ngogang JY, Saxena AK (2014) Antiproliferative activity and induction of apoptosis by Annona muricata (Annonaceae) extract on human cancer cells. BMC Complement Altern Med 14:516
Pumiputavon K, Chaowasku T, Saenjum C, Osathanunkul M, Wungsintaweekul B, Chawansuntati K, Wipasa J, Lithanatudom P (2017) Cell cycle arrest and apoptosis induction by methanolic leaves extracts of four Annonaceae plants. BMC Complement Altern Med 17:294
Qazzaz ME, Raja VJ, Lim KH, Kam TS, Lee JB, Gershkovich P, Bradshaw TD (2016) In vitro anticancer properties and biological evaluation of novel natural alkaloid jerantinine B. Cancer Lett 370:185–197
Quilez AM, Fernandez-Arche MA, Garcia-Gimenez MD, De la Puerta R (2018) Potential therapeutic applications of the genus Annona: local and traditional uses and pharmacology. J Ethnopharmacol 225:244–270
Smith RETK, Shejwalkar P, Hara K (2016) Neurotoxicity of fruits, seeds and leaves of plants in the Annonaceae Family. Austin Neurol Neurosci 1:1005
Takshak S (2018) Bioactive Compounds in Medicinal Plants: A condensed Review. SEJ Pharmaconosy and Natural Medicine 1:1
Tariq A, Adnan M, Amber R, Pan K, Mussarat S, Shinwari ZK (2016) Ethnomedicines and anti-parasitic activities of Pakistani medicinal plants against Plasmodia and Leishmania parasites. Ann Clin Microbiol Antimicrob 15:52
Tariq A, Mussarat S, Adnan M (2015) Review on ethnomedicinal, phytochemical and pharmacological evidence of Himalayan anticancer plants. J Ethnopharmacol 164:96–119
Thakur A, Singla R, Jaitak V (2015) Coumarins as anticancer agents: a review on synthetic strategies, mechanism of action and SAR studies. Eur J Med Chem 101:476–495
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines (Basel, Switzerland):5
World Health Orgnization (2013) WHO traditional medicine strategy: 2014-2023. WHO Press. Geneva, Switzerland 1–76
Yang H, Zhang N, Zeng Q, Yu Q, Ke S, Li X (2010) HPLC method for the simultaneous determination of ten Annonaceous acetogenins after supercritical fluid CO2 extraction. Int J Biomed Sci 6:202–207
Yang J, Lee YJ, Kwon YS, Kim MJ (2018) Anticancer activity of an Oplopanax elatus stem extract and biologically active isolated compounds. Curr Pharm Biotechnol 19:258–264. https://doi.org/10.2174/1389201019666180515105447
Ye R, Dai N, He Q, Guo P, Xiang Y, Zhang Q, Hong Z, Zhang Q (2018) Comprehensive anti-tumor effect of Brusatol through inhibition of cell viability and promotion of apoptosis caused by autophagy via the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomed Pharmacother 105:962–973
Zhao GX, Miesbauer LR, Smith DL, McLaughlin JL (1994) Asimin, asiminacin, and asiminecin: novel highly cytotoxic asimicin isomers from Asimina triloba. J Med Chem 37:1971–1976
Acknowledgments
The authors express their gratitude to Kritsadee Rattanathammethee and Narumon Techawong for their technical assistance, and to Dr. Paul Kowal and to “Hi-Tech Outsourcing Services” for editing the manuscript.
Funding
This study was supported by Chiang Mai University Research Group Grant (JW) and the Office of the Higher Education Commission/Thailand Research Fund (TRF) MRG5680080 (PL). The funding sources had no role in the study design: collection, analysis, and interpretation of data; writing of the report; and decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Pumiputavon, K., Chaowasku, T., Saenjum, C. et al. Cytotoxic and cytostatic effects of four Annonaceae plants on human cancer cell lines. In Vitro Cell.Dev.Biol.-Animal 55, 723–732 (2019). https://doi.org/10.1007/s11626-019-00393-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-019-00393-w