Abstract
This study aimed to examine the proliferative behavior and molecular mechanisms of rat bone marrow-derived MSCs (rBMSCs) cultured under three different oxygen concentrations. Passaged rBMSCs exhibited significantly greater proliferation rates at 1% O2 and 5% O2 than those at 18% O2 and the cells exposed to 1% O2 showed the highest proliferative potential, which was evidenced by the growth curves, colony-forming efficiencies, and CCK-8 absorbance values. The rBMSCs grown under hypoxic culture conditions (1% O2 and 5% O2) had the increased percentage of cells in S + G2/M-phase and the decreased apoptotic index, compared with normoxia (18% O2). It was revealed for the first time that there were more phosphohistone H3 (PHH3)-positive cells and higher expressions of proliferating cell nuclear antigen (PCNA) in the hypoxic cultures of rBMSCs than in the normoxic culture. Hypoxia upregulated the anti-apoptotic protein Bcl-2 and downregulated the pro-apoptotic proteins Bax and the cleaved caspase-3 in cultured rBMSCs. The levels of hypoxia-inducible factor-1α (HIF-1α) and phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2) were increased in the hypoxic-cultured rBMSCs. Nevertheless, no significant difference was observed in p53 level of rBMSCs between different oxygen concentrations. In conclusion, the hypoxia exerts a promoting effect on the in vitro expansion of rBMSCs via several signaling and molecular pathways involved in the control of cell cycle and apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stem cells (MSCs) have aroused considerable interest and expectations of the employ for cell-base therapies and tissue engineering on account of their properties for self-renewal, multipotent differentiation, immunomodulation, and trophic actions (Salem and Thiemermann 2010). These stem cells can easily be isolated from a variety of somatic tissues as well. Their frequency, however, is extremely low within any tissues, and only a very limited amount of MSCs can be collected from a single donor (Sisakhtnezhad et al. 2016). Only if the efficient ex vivo expansion of isolated MSCs is carried out, the sufficient expanded cells will be harvested to meet the needs of clinical trials and many related basic researches. The investigation of optimal culture conditions for MSC expansion has been underway. A critical consideration is the optimal oxygen tension in the culture medium.
At the tissue level, MSCs reside in the hypoxic microenvironment, and for instance, O2 concentration in MSC niche of bone marrow is about 1~7%, or perhaps even lower (Mohyeldin et al. 2010). Hypoxic condition is thought to be essential for the maintenance of stem cell properties (Drela et al. 2014). However, MSCs are routinely cultured in the traditional incubators supplied by room air containing 5% CO2 and saturated water vapor, resulting oxygen concentration being approximately 18% (Sodhi et al. 2000). The oxygen concentration of gaseous atmosphere within such incubator is referred to as “normoxia” in cell culture technology. In recent years, a lot of studies have offered some evidences for the negative impacts of normoxic culture on MSCs, such as early senescence, poor growth kinetics, promotion of apoptosis, and DNA damage (Fehrer et al. 2007; Estrada et al. 2012). On the other hand, low levels of oxygen had positive effects on the in vitro survival and proliferation of MSCs principally in long-term culture; nevertheless, several studies showed converse results (Zhang et al. 2009; Holzwarth et al. 2010; Deschepper et al. 2011; Pattappa et al. 2013; Rajaraman et al. 2013; Kumar and Vaidya 2016). As far as the proliferation-promoting effect of low oxygen tension is concerned, the optimal oxygen level is still inconsistent in the scientific literature. Oxygen concentration, duration of exposure to hypoxia, procedure of hypoxic treatment, and especially intrinsic differences between various cell sources could give the explanation of these discrepancies (Buravkova et al. 2014). Certainly, the molecular mechanisms underlying the actions of hypoxia on cellular behaviors of MSCs remain to be elucidated.
In this study, we examined the effects of three oxygen concentrations (1, 5, and 18% O2) on the long-term expansion of passaged rat bone marrow-derived MSCs (rBMSCs). This is because the elaborate efforts to expand human MSCs under hypoxic conditions have largely been made so far, and few of the similar studies for the ex vivo expansion of rat MSCs have been documented. Not only were the frequently used indicators regarding in vitro proliferative kinetics employed in our experiment but phosphohistone H3 (PHH3) and proliferating cell nuclear antigen (PCNA), which were not at all or hardly used utilized in previous studies of MSC expansion, were detected simultaneously. Furthermore, we explored the cellular mechanisms underlying actions of oxygen tension on ex vivo expansion of MSCs in two aspects of cell cycling and apoptosis, since the result of cellular expansion is dependent on both mitotic activity and cell death. To our knowledge, very little experimental data about cell division and apoptosis has been reported for ex vivo expansion of rBMSCs under hypoxia in the same study up to date. At the molecular level, we quantified the expressions of some proteins which are related closely to the cell proliferation, cell apoptosis, and hypoxic adaption, including Bax, Bcl-2, caspase-3, p53, HIF-1α, and pERK1/2. The results obtained in our study showed that the low oxygen tensions were observed to promote the ex vivo expansion of rBMSCs through several signaling and molecular pathways involved in the control of cell cycle and apoptosis. These observations could contribute to the improvement of MSC expansion in vitro and the elucidation of cellular and molecular mechanisms underlying proliferation-promoting action of hypoxia on the MSCs.
Materials and methods
Cell isolation and culture
MSCs were harvested by flushing the cavity of femurs and tibias of male Sprague-Dawley rats weighing 50–70 g with low-glucose, phenol red-free Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Gaithersburg, MD). The nucleated bone marrow cells (NBMCs) were counted and plated at 1 × 104 cells/cm2. The cells were incubated for 48 h at 37°C and 7.5% CO2 with the growth medium, which is the DMEM containing 15% inactivated fetal bovine serum (FBS; Gibco, Gaithersburg, MD), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA). Cultures were then washed with phosphate-buffered saline (PBS) and replaced with the fresh medium. The attached cells were cultured until they reached 80–90% confluence, then passaged using 0.25% trypsin (Hyclone, GE Healthcare Life Sciences, Pittsburgh, PA). At every passage, the cells were replated at a density of 1 × 103 cells/cm2; the culture medium was discarded and replaced with fresh medium every 3~4 d. All experiments were performed with cells from passage 2nd to 5th.
rBMSCs characterization
Flow cytometry analysis of MSC surface markers was performed as described previously (Park et al. 2013). Briefly, MSCs were detached, washed, pelleted, and resuspended in DMEM medium without phenol red, and then incubated with either phycoerythrin or FITC-conjugated antibodies specific for CD29, CD90, CD11b/c, and CD45 (BD Biosciences, San Jose, CA) for 20 min at room temperature with antibodies in a final volume of 100 μL and eventually resuspended in 4% paraformaldehyde until analysis on flow cytometer (FC500 Beckman Coulter).
The third passage cells (at 80–90% confluence) were subjected to adipogenic, osteogenic, and chondrogenic differentiation in vitro, according to established protocols (Park et al. 2013). For adipogenic differentiation, MSCs were cultured in DMEM with 10% FBS, 1 mM dexamethasone, 0.5 mM isobutyl-1-methylxanthine, 100 mM indomethacin, and 10 μg mL−1 insulin. After 14 d, cells were fixed with stained with Oil Red O (Sigma-Aldrich, St. Louis, MO) to visualize lipid droplets. Osteogenic differentiation was induced by DMEM contained with 10% FBS, 10 nM dexamethasone, 10 mM β-glycerophosphate, and 0.2 mM ascorbic acid. After 28 d, the mineralization of the extracellular matrix was determined by 1% Alizarin Red (pH 4.2) (Sigma-Aldrich) staining. Chondrogenic differentiation was induced in pellet culture for 14–21 d and performed using SD rat MSC chondrogenic differentiation medium (Cyagen, Santa Clara, CA) according to the instructions of manufacturer.
Hypoxic conditioning
The passaged rBMSCs divided into three groups in which oxygen concentrations were 1, 5, and 18%, respectively. These cells were cultured in a specialized chamber (C-Chamber and Oxygen controller ProOx 110, BioSpherix, Parish, NY) housed within a standard cell culture incubator (Thermo Forma, model 3110; Forma Scientific, Marietta, OH). The chambers were, respectively, flushed with sterile, humidified gas mixture containing different concentrations of oxygen (1, 5, or 18%) and 7.5% CO2 with a balance of N2. The cells were cultured under the three indicated oxygen concentrations in all of the following experiments.
Proliferation assay
The passaged cells were cultured in 24-well plates for 7 d. The growth curves were drawn based on the number of cells per day during a period of 7 d. As for the assay of viable cells, rBMSCs were cultured in 96-well plates for 5 d, and then, cells were analyzed using the CCK-8 assay kit according to the instructions of manufacturer (Promega Madison, WI).
For colony-forming unit-fibroblast (CFU-F) assays, rBMSCs were seeded at a density of 1 × 103 cells/cm2 in 6-well plates. After a 9-d culture period, the cultures in plates were rinsed three times with PBS and stained with Wright-Giemsa dye (Sigma). Individual colonies made up of at least 20 cells were counted microscopically. The yields of CFU-F were expressed as the number of CFU-F colonies per well.
Apoptosis assay
After cultivation of 5 d, cells were then collected and analyzed by FITC Annexin V Apoptosis Detection Kit I according to the instructions of manufacturer (BD, Pharmingen). The apoptotic index was calculated by dividing the number of cells in (Q2 + Q4) phases by the total number of cells.
Cell cycle assay
Following a culture period of 5 d, cell cycle analysis was performed using propidium iodide (PI)-staining. Cells were washed with PBS three times and resuspended in ice-cold 70% ethanol, incubated 4°C for 24 h, and treated with 200 μg/mL RNase A for 30 min at 37°C. The samples were incubated in 40 μg/mL PI for 5 min at room temperature and placed on ice until analysis by flow cytometry. The proliferation index was calculated by the cell numbers in S-G2/M phase divided by the total analyzed cells.
PHH3 staining
Cells were incubated for 5 d and were assessed by immunostaining for phosphorylated form of histone H3 on serine 10 (PHH3, H3S10p). Cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, blocked with 1% BSA, stained with polyclonal antibody against phospho-histone H3 (S10) (Immunoway) overnight at 4°C, and then incubated with a 1:1000 dilution of fluorescein isothiocyanate-conjugated rabbit anti-mouse secondary antibody (Immunoway) in dark. The nuclei were stained for 15 min with DAPI (4, 6-diamidino-2-phenylindole, Sigma-Aldrich) at room temperature in dark. Fluorescence images were visualized with a fluorescence microscope (TH4-200, Olympus, Tokyo, Japan). For each sample, a minimum of 500 cells from more than five random fields of the slide were counted and mitosis cells were scored. The mitotic index is calculated by dividing the number of cells in mitosis by the total number of cells.
Western blotting
Western blotting analyses were performed as described in a previous study (Liang et al. 2016). The following primary antibodies were used: anti-PCNA, anti-Bax, anti-Bcl-2, anti-cleaved caspase 3, anti-p53, anti-HIF-1a, anti-pERK1/2, and anti-β-actin (diluted at 1:1000; Abcam, Cambridge, MA). The blots were incubated with a goat anti-rabbit or rabbit anti-mouse HRP-conjugated secondary antibody (dilut- 213 ed at 1:1000; Abcam). The immunoreactive bands were visualized with an enhanced chemiluminescent detection system (Tanon-5500, Shanghai, China). Densitometric analysis of the blots was performed using image J software.
Data analysis
Each of the results was obtained from a minimum of three independent experiments and data were expressed as means ± standard deviation (SD). PASW Statistics 18.0 (SPSS Inc., Chicago, IL) was used in all statistical analyses. Comparison of the growth curves of MSCs under different oxygen conditions was analyzed by two-way repeated measure ANOVAs followed by Sidak test. One-way ANOVA with the Fisher’s least significant difference post hoc test was performed as comparison for the results of Western blotting, CCK-8 assay, and CFU-F assay. Comparison of the results of PHH3 assay and cell cycle analysis was assessed by Chi-square test with the R × C contingency tables. Significance was set at a P value less than 0.05 (p < 0.05) for all experiments.
Results
Identification of rBMSCs
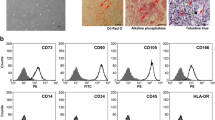
As shown in Fig. 1A-a, passaged rBMSCs exhibited spindle or fibroblastic morphology. These culture-expanded cells maintained their potential to differentiate along osteoblastic, adipogenic, and chondrogenic lineages (Fig. 1A-b/c/d). Flow cytometric immunophenotyping of rBMSCs showed the cells expressed mesenchymal lineage-associated cell surface markers CD90 (99.3%) and CD29 (99.6%), whereas the surface antigens of CD11b/c (1.3%) and CD45 (2.8%) were negative (Fig. 1B). In brief, the characteristics of passaged rBMSCs met the minimum criteria of MSCs, namely adherence to plastic, immunophenotype, and multilineage differentiation potential.
Identification of rBMSCs. (A) The morphology and tri-lineage differentiation potential of rBMSCs. (a) A representative microphotograph of rBMSCs stained with Wright-Giemsa (scale bar, 100 μm). (b) A representative microphotograph of rBMSCs differentiated into osteocytes stained with alizarin red (scale bar, 50 μm). (c) A representative microphotograph of rBMSCs differentiated into adipocytes stained with oil red O (scale bar, 50 μm). (d) A representative microphotograph of rBMSCs differentiated into chondrocytes stained with alcian blue (scale bar, 20 μm). (B) CD profile of passaged rBMSCs by flow cytometric analysis: CD 29 (99.6%); CD 45 (2.8%); CD 90 (99.3%); CD 11 b/c (1.3%).
Hypoxia enhances proliferative potential of rBMSCs in vitro
Firstly, the proliferation rate of rBMSCs was examined by direct cell counting. As shown by the growth curves (Fig. 2A), rBMSCs in 1% O2 group had higher growth capacity than those of 5% O2 (P < 0.01) or normoxia group (P < 0.01) throughout the 7-d culture. Moreover, the increase in proliferation rate was even more obvious from the 5th to the 7th day. The proliferation-promoting effect of hypoxia on rBMSCs was concentration-dependent and 1% O2 was the most efficient oxygen concentration.
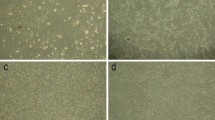
Effects of indicated oxygen conditions on proliferation characteristic of rBMSCs. (A) Growth curves: cell numbers (cells/cm2) were determined throughout the culture for the indicated oxygen concentrations and the results were analyzed by repeated measures ANOVAs with Sidak. (B) Cell viability of rBMSCs cultured under different oxygen concentrations by cck-8 assay. (C) Representative views of colonies at day 9. (D) The CFU-F numbers of rBMSCs cultured under different oxygen concentrations. Each point or column with a bar represents mean ± SD (*P < 0.05 and **P < 0.01; n = 3).
Then, we evaluated growth behavior of rBMSCs by a CCK-8 assay for quantization of viable cell numbers after 5 d of culture. As shown in Fig. 2B, the reduced oxygen concentrations resulted in an increasing trend in the absorbance of the CCK-8 assay. A significant difference in CCK-8 assay was witnessed between 1% O2 group and normoxia group (P < 0.05), but there was no significant difference between 1% O2 group and 5% O2 group or between 5% O2 group and normoxia group.
We confirmed this trend by CFU-F efficiency assays performed on passage 3 MSCs. As shown in Fig. 2C/D, the yields of CFU-F colony increased markedly under 1% O2 condition compared with normoxia (P < 0.01) and 5% O2 (P < 0.05). The yield of CFU-F colony of 5% O2 group was higher than that of normoxia group, there was no statistically significant difference between these two groups. The results indicated that MSCs cultured at 1% O2 exhibited a higher self-renewal capacity.
Hypoxia reduces the apoptosis of rBMSCs
Annexin V-FITC/PI double staining was used to determine the effect of the three oxygen concentrations on apoptosis. The apoptotic indexes of 1% O2, 5% O2, and normoxia groups were 7.5 ± 0.74, 13.6 ± 0.95, and 16.8 ± 1.60%, respectively (Fig. 3A/B). The apoptotic index of 1% O2 group was much lower than that of the other two groups (P < 0.01), and 5% O2 group also had a lower apoptotic index than normoxia group (P < 0.05).
Effects of indicated oxygen conditions on apoptosis in rBMSCs. (A) Flow cytometric analysis of apoptotic cells after Annexin V/PI staining. (B) Apototic indexes based on flow cytometry analysis with Annexin V/PI staining. (C) The levels of apoptosis-related proteins in rBMSCs exposed to the indicated conditions for 5 d measured by Western blotting. (D) The ratio of Bax to β-actin band density values. (E) The ratio of Bcl-2 to β-actin band density values. (F) The ratio of cleaved caspase-3 to β-actin band density values. The band density values were analyzed using Image J software. Bar graphs represent mean ± SD (*P < 0.05 and **P < 0.01; n = 3).
We extended our studies by examining the expression of several apoptosis-related proteins by Western blotting. Bax, Bcl-2, and cleaved caspase-3 are the key mediators of apoptosis. Compared with normoxic culture, hypoxia down-regulated the expression of Bax and cleaved caspase 3; at the same time, the expression of anti apoptotic proteins Bcl-2 was significantly up-regulated (see Fig. 3D/E/F). These results confirm that low oxygen concentrations reduce the frequency of commitment to apoptosis of rBMSCs in vitro.
Hypoxia accelerates the cell cycle progression of rBMSCs
The results of flow cytometry (Fig. 4A/B) showed a significant increase in the percentage of S + G2/M phase cells (also known as proliferation index) in the 1% O2 group, as compared with the normoxia group (P < 0.01) and 5% O2 group(P < 0.05). However, there was no obvious difference between 5% O2 group and normoxia group.
Effects of indicated oxygen conditions on cell cycle in rBMSCs. (A) Flow cytometric of cells after PI staining. (B) The proliferation index of three experimental groups based on flow cytometric analysis with PI staining. The data were analyzed by the partitioned Chi-square method for multiple comparisons. (C) The expression of PCNA in rBMSCs measured by Western blotting and the ratio of PCNA to β-actin band density values. The band density values were analyzed using Image J software. (D) The representative immunofluorescence microphotographs with PHH3 labeling. A scale bar of 100 μm is shared among all microphotographs in this panel. (E) Mitotic indexes of rBMSCs under different oxygen concentrations based on the result of PHH3 immunostaining; bar graphs represent mean ± SD (*P < 0.05 and **P < 0.01; n = 3).
Proliferating cell nuclear antigen (PCNA) has been identified as a proliferation marker and expresses in the nuclei of cells during the S phase of the cell cycle (Hu et al. 2016). As shown in the results of Western blotting (Fig. 4C), the expression of PCNA in the 1% O2 group was higher than those in 5% O2 group (P < 0.05) and the normoxia group (P < 0.05).
Immunohistochemical staining of phosphohistone H3 (PHH3) is specific for cells in late G2 and M phases of the cell (Villani et al. 2016). Therefore, the mitotic index (MI) was examined by the PHH3 immunostaining after 5-d culturing under those three oxygen conditions (Fig. 4D/E). The mitotic index in 1% O2 groups, 5% O2 group and the nomoxia group were 35.5 ± 8.0, 19.7 ± 8.4 and 10.3 ± 4.2‰, respectively. MI of rBMSCs cultured at 1% O2 was significantly higher than that of normoxic condition (P < 0.01) or 5% O2 (P < 0.05) (Fig. 4E). However, there was no statistical difference between 5% O2 group and normoxia group.
All these results provided evidences that the positive effect of sufficiently low oxygen concentration on the proliferation of MSCs could be mediated through accelerating of cell cycle progression.
Hypoxia-induced proliferation in rBMSCs is involved in HIF and ERK pathways
As shown in Fig. 5, the expression of HIF-1α was upregulated in rBMSCs with oxygen concentrations decreasing. Compared with the normoxia group, 5% O2 group had the significantly higher expression of HIF-1a (P < 0.05) and the 1% O2 group showed an even more obvious increase (P < 0.01), which confirms HIF-1α is associated closely with hypoxic adaption of BMSCs and may be a key regulator in facilitating BMSCs proliferation under hypoxic conditions.
Western blot analysis of HIF-1α, Phospho-ERK1/2, and p53 in rBMSCs under indicated oxygen conditions. (A) The expression of HIF-1α and the ratio of HIF-1a to β-actin band density values. (B) The expression of Phospho-ERK1/2 and ERK1/2 expression and the ratio of P-ERK1/2 to total ERK1/2 band density values. (C) The expression of p53 and the ratio of p53 to β-actin band density values. The band density values were also analyzed using Image J software. Bar graphs represent mean ± SD (*P < 0.05 and **P < 0.01; n = 3).
Western blotting analyses revealed that the expression of phospho-ERK1/2 was markedly higher in 1% O2 group than in normoxia group (P < 0.05). Although no significant differences in phospho-ERK1/2 were witnessed between 5% O2 group and normoxia group, neither between 1% O2 group and 5% O2 group, a decreasing trend appeared in the expression of phospho-ERK1/2 in rBMSCs with the oxygen concentrations increasing. These results indicated that sufficiently low oxygen concentration could affect the level of phospho-ERK1/2.
Western blotting data showed that culturing rBMSCs at different oxygen concentrations had no obvious effect on the expression of p53.
Discussion
The present study shows clearly that 1% O2 has a greater promoting effects on the ex vivo expansion of rBMSCs than 5% O2 and normoxia. This finding differs from some of the previous studies in which the researchers compared the proliferation characteristics of MSCs under more than one hypoxic condition. Taken together, two of the studies believed 5% O2 showed a significant increase compared to the other O2 tensions, while D’Ippolito and coauthors found human MSCs can grow best at 3% O2 (D’Ippolito et al. 2006; Bertolo et al. 2015; Ahmed et al. 2016). Some of the studies even found that low O2 had negative or no obvious effects on growth behavior of MSCs (Holzwarth et al. 2010; Pattappa et al. 2013). It is noteworthy that those studies all used human MSCs. So, the inconsistent results may be caused by difference in cell sources.
The cell cycle and apoptotic effects were then assessed intensively under hypoxic conditions in vitro. Unlike conclusions of some previous studies which state that low O2 concentrations enhance proliferative potential of MSCs, meanwhile it has no detectable effect on cell cycle (D’Ippolito et al. 2006) or apoptosis (D’Ippolito et al. 2006; Fehrer et al. 2007); we believe hypoxia, especially 1% O2, not only promotes the cell cycle progression but also inhibits apoptosis. These changes in cellular level are accompanied by a significant elevation in the ratio of Bcl-2/bax and decrease of cleaved caspase 3, as well as increased expression of PHH3 and PCNA.
At a molecular level, apoptosis is mediated by two main protein families: the Bcl-2 family and the caspase family (Antico Arciuch et al. 2012). Bcl-2 and Bax are the most widely studied regulators of apoptosis, and caspase-3 is regarded as the key executor of apoptosis (Hua et al. 2015). Previous studies have showed that over-expression of Bcl-2 can protect MSCs against apoptosis (Li et al. 2007) and silencing of caspase-3 can increase cell proliferation, the cell cycle, and the anti-apoptotic capacity of MSCs under hypoxia in vitro (Hua et al. 2015).
As far as we know, there was no report on the PHH3 and PCNA for hypoxia-related research of MSCs until now. Phosphorylation of the amino terminus of the histone H3 (Ser 10) closely with mitotic chromatin condensation is specific for late G2 and M phases of the cell cycle (Ginter et al. 2016). PHH3 has been considered as a superior mitosis-specific marker and a proliferation marker. Evidences have been proven the utility of PHH3 staining in assessing mitotic activity in various kinds of cancer cells (Villani et al. 2016; Chow et al. 2017). Proliferating cell nuclear antigen (PCNA) is a cofactor of DNA polymerase δ in eukaryotic cells and is essential for replication (Strzalka and Ziemienowicz 2011). PCNA has been identified as another proliferation marker and its expression is specific for the S phase of the cell cycle (Strzalka and Ziemienowicz 2011). Quite a few experiments revealed that the low oxygen concentrations increased cell proliferation and were correlated with the high-level expression of PCNA in various kinds of cells including mice cochlear spiral ganglion stem/progenitor cells, MC3T3-E1 cells, bovine satellite cells, human pulmonary artery smooth muscle cells, and so on (Wang et al. 2007; Kook et al. 2008; Chen et al. 2015; Wang et al. 2015). Therefore, the high-level expression of PHH3 and PCNA further confirms that 1% O2 can enhance the proliferative potential and also indicates that the pro-proliferative effect should be partially dependent on the up-regulation of two proteins to promote cell cycle procession.
A well-known mechanism of hypoxia-mediated regulation is the activation of hypoxia-inducible factor-1α (HIF-1α). Some researchers have proposed that low oxygen concentrations leading to up-regulation of HIF-1α are termed truly hypoxia (Karlsen et al. 2011). Most of the views show that the hypoxic microenvironment of MSCs implies more stabilization of HIF-1α (Nekanti et al. 2010; Ni et al. 2014; Lv et al. 2017). However, Berniakovich and Giorgio drew a conclusion that HIF-1α was not involved in affecting mouse bone marrow MSCs under hypoxia, because they found that 3% O2 was insufficient of inducing the detectable level of HIF-1α, and knocking down HIF-1α did not change O2 effects on MSCs (Berniakovich and Giorgio 2013). Palomaki and his team claimed that HIF-1α protein was stabilized under normoxic condition in MSCs derived from pediatric bone marrow and umbilical cord blood (Palomaki et al. 2013). Consistent with the previous general views, this study observed that an increase in the stabilization of HIF-1α was increased in rBMSCs with the oxygen concentration decreasing, which indicates that the HIF-1α is a key regulator for the hypoxia-mediated proliferation.
One of the HIF-1’s downstream signals is extracellular signal-regulated kinase 1/2 (ERK1/2) (Fabian et al. 2014). Previous studies have showed that hypoxia can phosphorylate ERK1/2 in many cell lineages (Ciccarelli and Giustetto 2014). The activation of ERK1/2 under hypoxia in human BMSCs has also been proved (Fabian et al. 2014). Similarly, some experiments conclude that hypoxia and serum deprivation increase the expression of phospho-ERK1/2 in rBMSCs (Gao et al. 2010; Liu et al. 2011). On the other hand, some experiments got the opposite results in rBMSCs (Song et al. 2009; Hou et al. 2014). Our results support that hypoxia can make the ratio of phospho/total-ERK1/2 increase in rBMSCs, which infers that the hypoxia-mediated proliferation in rBMSCs might be dependent on the level of phosphorylated ERK1/2. Moreover, studies demonstrate that the ERK pathway plays a powerful role in stimuli-induced phosphorylation of histone H3 (Ciccarelli and Giustetto 2014). Thus, further work should be required to investigate whether the high level of phospho-ERK1/2 can cause histone H3 to be phosphorylated during adaption to hypoxia in rBMSCs.
In the present study, there was no obvious change in the expression of p53 in rBMSCs under hypoxic culture. The p53 tumor suppressor protein is an eminent transcription regulator of cell cycle entry and a pro-apoptotic protein, which is frequently thought to be associated with hypoxia (Sermeus and Michiels 2011) and to be repressed by high level of HIF-1α in MSCs (Lv et al. 2017). Nevertheless, the relationship between p53 and hypoxia is not occurring in all cases (Sermeus and Michiels 2011). It seems to be dependent on the level and duration of hypoxia and the cell sources. In the case of adipose tissue-derived stromal cells (ASCs), p53 was downregulated when the cells were cultured under 5% O2 (Andreeva et al. 2015) and under 2% O2 (Choi et al. 2015), while p53 level did not change obviously after hBMSCs were cultured in extreme hypoxia (0.5% O2) for 3 d (Crowder et al. 2013).
Conclusion
Our study strongly suggests that hypoxia has a promoting effect on the expansion of rBMSCs in vitro, which is concentration-dependent. One percent is recommended as a more suitable oxygen concentration for ex vivo expansion of rBMSCs. The mechanisms underlying the hypoxia-induced proliferation of rBMSCs are associated with promoting the cell division and reducing the apoptosis partially through the pathways of Bcl-2, Bax, caspase 3, PCNA, PHH3, HIF-1α. However, further experiments need to be performed to fully elucidate the mechanisms of the hypoxia-mediated proliferation in rBMSCs. Our findings should help to optimize the cultured condition for the collection of rBMSCs.
References
Ahmed NE, Murakami M, Kaneko S, Nakashima M (2016) The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs). Sci Rep 6:35476. https://doi.org/10.1038/srep35476
Andreeva ER, Lobanova MV, Udartseva OO, Buravkova LB (2015) Response of adipose tissue-derived stromal cells in tissue-related O2 microenvironment to short-term hypoxic stress. Cells Tissues Organs 200:307–315. https://doi.org/10.1159/000438921
Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC (2012) Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal 16:1150–1180. https://doi.org/10.1089/ars.2011.4085
Berniakovich I, Giorgio M (2013) Low oxygen tension maintains multipotency, whereas normoxia increases differentiation of mouse bone marrow stromal cells. Int J Mol Sci 14:2119–2134. https://doi.org/10.3390/ijms14012119
Bertolo A, Hafner S, Taddei AR, Baur M, Potzel T, Steffen F, Stoyanov J (2015) Injectable microcarriers as human mesenchymal stem cell support and their application for cartilage and degenerated intervertebral disc repair. Eur Cell Mater 29:70–80 discujssion 80–71
Buravkova LB, Andreeva ER, Gogvadze V, Zhivotovsky B (2014) Mesenchymal stem cells and hypoxia: where are we? Mitochondrion 19(Pt A):105–112. https://doi.org/10.1016/j.mito.2014.07.005
Chen HC, Lee JT, Shih CP, Chao TT, Sytwu HK, Li SL, Fang MC, Chen HK, Lin YC, Kuo CY, Wang CH (2015) Hypoxia induces a metabolic shift and enhances the Stemness and expansion of Cochlear spiral ganglion stem/progenitor cells. Biomed Res Int 2015:359537. https://doi.org/10.1155/2015/359537
Choi JR, Pingguan-Murphy B, Wan Abas WA, Yong KW, Poon CT, Noor Azmi MA, Omar SZ, Chua KH, Xu F, Wan Safwani WK (2015) In situ normoxia enhances survival and proliferation rate of human adipose tissue-derived stromal cells without increasing the risk of tumourigenesis. PLoS One 10:e0115034. https://doi.org/10.1371/journal.pone.0115034
Chow KL, Tse KY, Cheung CL, Wong KW, Cheung AN, Wong RW, Chan AN, Yuen NW, Ngan HY, Ip PP (2017) The mitosis-specific marker phosphohistone-H3 (PHH3) is an independent prognosticator in uterine smooth muscle tumours: an outcome-based study. Histopathology 70:746–755. https://doi.org/10.1111/his.13124
Ciccarelli A, Giustetto M (2014) Role of ERK signaling in activity-dependent modifications of histone proteins. Neuropharmacology 80:34–44. https://doi.org/10.1016/j.neuropharm.2014.01.039
Crowder SW, Horton LW, Lee SH, McClain CM, Hawkins OE, Palmer AM, Bae H, Richmond A, Sung HJ (2013) Passage-dependent cancerous transformation of human mesenchymal stem cells under carcinogenic hypoxia. FASEB J 27:2788–2798. https://doi.org/10.1096/fj.13-228288
Deschepper M, Oudina K, David B, Myrtil V, Collet C, Bensidhoum M, Logeart-Avramoglou D, Petite H (2011) Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J Cell Mol Med 15:1505–1514. https://doi.org/10.1111/j.1582-4934.2010.01138.x
D'Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC (2006) Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 39:513–522. https://doi.org/10.1016/j.bone.2006.02.061
Drela K, Sarnowska A, Siedlecka P, Szablowska-Gadomska I, Wielgos M, Jurga M, Lukomska B, Domanska-Janik K (2014) Low oxygen atmosphere facilitates proliferation and maintains undifferentiated state of umbilical cord mesenchymal stem cells in an hypoxia inducible factor-dependent manner. Cytotherapy 16:881–892. https://doi.org/10.1016/j.jcyt.2014.02.009
Estrada JC, Albo C, Benguria A, Dopazo A, Lopez-Romero P, Carrera-Quintanar L, Roche E, Clemente EP, Enriquez JA, Bernad A, Samper E (2012) Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ 19:743–755. https://doi.org/10.1038/cdd.2011.172
Fabian Z, Ramadurai S, Shaw G, Nasheuer HP, Kolch W, Taylor C, Barry F (2014) Basic fibroblast growth factor modifies the hypoxic response of human bone marrow stromal cells by ERK-mediated enhancement of HIF-1alpha activity. Stem Cell Res 12:646–658. https://doi.org/10.1016/j.scr.2014.02.007
Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gully C, Gassner R, Lepperdinger G (2007) Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell 6:745–757. https://doi.org/10.1111/j.1474-9726.2007.00336.x
Gao F, Hu XY, Xie XJ, Xu QY, Wang YP, Liu XB, Xiang MX, Sun Y, Wang JA (2010) Heat shock protein 90 protects rat mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways. J Zhejiang Univ Sci B 11:608–617. https://doi.org/10.1631/jzus.B1001007
Ginter PS, Shin SJ, Liu Y, Chen Z, D'Alfonso TM (2016) Phosphohistone H3 expression correlates with manual mitotic counts and aids in identification of "hot spots" in fibroepithelial tumors of the breast. Hum Pathol 49:90–98. https://doi.org/10.1016/j.humpath.2015.10.012
Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, Muller I (2010) Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol 11:11. https://doi.org/10.1186/1471-2121-11-11
Hou M, Cui J, Liu J, Liu F, Jiang R, Liu K, Wang Y, Yin L, Liu W, Yu B (2014) Angiopoietin-like 4 confers resistance to hypoxia/serum deprivation-induced apoptosis through PI3K/Akt and ERK1/2 signaling pathways in mesenchymal stem cells. PLoS One 9:e85808. https://doi.org/10.1371/journal.pone.0085808
Hu H, Chen M, Dai G, Du G, Wang X, He J, Zhao Y, Han D, Cao Y, Zheng Y, Ding D (2016) An inhibitory role of Osthole in rat MSCs osteogenic differentiation and proliferation via Wnt/beta-catenin and Erk1/2-MAPK pathways. Cell Physiol Biochem 38:2375–2388. https://doi.org/10.1159/000445590
Hua P, Liu J, Tao J, Liu J, Yang S (2015) Influence of caspase-3 silencing on the proliferation and apoptosis of rat bone marrow mesenchymal stem cells under hypoxia. Int J Clin Exp Med 8:1624–1633
Karlsen TA, Mirtaheri P, Shahdadfar A, Floisand Y, Brinchmann JE (2011) Effect of three-dimensional culture and incubator gas concentration on phenotype and differentiation capability of human mesenchymal stem cells. J Cell Biochem 112:684–693. https://doi.org/10.1002/jcb.22978
Kook SH, Son YO, Lee KY, Lee HJ, Chung WT, Choi KC, Lee JC (2008) Hypoxia affects positively the proliferation of bovine satellite cells and their myogenic differentiation through up-regulation of MyoD. Cell Biol Int 32:871–878. https://doi.org/10.1016/j.cellbi.2008.03.017
Kumar S, Vaidya M (2016) Hypoxia inhibits mesenchymal stem cell proliferation through HIF1alpha-dependent regulation of P27. Mol Cell Biochem 415:29–38. https://doi.org/10.1007/s11010-016-2674-5
Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G (2007) Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 25:2118–2127. https://doi.org/10.1634/stemcells.2006-0771
Liang X, Huang X, Zhou Y, Jin R, Li Q (2016) Mechanical stretching promotes skin tissue regeneration via enhancing mesenchymal stem cell homing and Transdifferentiation. Stem Cells Transl Med 5:960–969. https://doi.org/10.5966/sctm.2015-0274
Liu X, Duan B, Cheng Z, Jia X, Mao L, Fu H, Che Y, Ou L, Liu L, Kong D (2011) SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell 2:845–854. https://doi.org/10.1007/s13238-011-1097-z
Lv B, Li F, Fang J, Xu L, Sun C, Han J, Hua T, Zhang Z, Feng Z, Jiang X (2017) Hypoxia inducible factor 1alpha promotes survival of mesenchymal stem cells under hypoxia. Am J Transl Res 9:1521–1529
Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A (2010) Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7:150–161. https://doi.org/10.1016/j.stem.2010.07.007
Nekanti U, Dastidar S, Venugopal P, Totey S, Ta M (2010) Increased proliferation and analysis of differential gene expression in human Wharton's jelly-derived mesenchymal stromal cells under hypoxia. Int J Biol Sci 6:499–512
Ni L, Liu X, Sochacki KR, Ebraheim M, Fahrenkopf M, Shi Q, Liu J, Yang H (2014) Effects of hypoxia on differentiation from human placenta-derived mesenchymal stem cells to nucleus pulposus-like cells. Spine J 14:2451–2458. https://doi.org/10.1016/j.spinee.2014.03.028
Palomaki S, Pietila M, Laitinen S, Pesala J, Sormunen R, Lehenkari P, Koivunen P (2013) HIF-1alpha is upregulated in human mesenchymal stem cells. Stem Cells 31:1902–1909. https://doi.org/10.1002/stem.1435
Park IH, Kim KH, Choi HK, Shim JS, Whang SY, Hahn SJ, Kwon OJ, Oh IH (2013) Constitutive stabilization of hypoxia-inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state. Exp Mol Med 45:e44. https://doi.org/10.1038/emm.2013.87
Pattappa G, Thorpe SD, Jegard NC, Heywood HK, de Bruijn JD, Lee DA (2013) Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng Part C Methods 19:68–79. https://doi.org/10.1089/ten.TEC.2011.0734
Rajaraman G, White J, Tan KS, Ulrich D, Rosamilia A, Werkmeister J, Gargett CE (2013) Optimization and scale-up culture of human endometrial multipotent mesenchymal stromal cells: potential for clinical application. Tissue Eng Part C Methods 19:80–92. https://doi.org/10.1089/ten.TEC.2011.0718
Salem HK, Thiemermann C (2010) Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 28:585–596. https://doi.org/10.1002/stem.269
Sermeus A, Michiels C (2011) Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis 2:e164. https://doi.org/10.1038/cddis.2011.48
Sisakhtnezhad S, Alimoradi E, Akrami H (2016) External factors influencing mesenchymal stem cell fate in vitro. Eur J Cell Biol 96:13–33. https://doi.org/10.1016/j.ejcb.2016.11.003
Sodhi CP, Batlle D, Sahai A (2000) Osteopontin mediates hypoxia-induced proliferation of cultured mesangial cells: role of PKC and p38 MAPK. Kidney Int 58:691–700. https://doi.org/10.1046/j.1523-1755.2000.00215.x
Song SW, Chang W, Song BW, Song H, Lim S, Kim HJ, Cha MJ, Choi E, Im SH, Chang BC, Chung N, Jang Y, Hwang KC (2009) Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells 27:1358–1365. https://doi.org/10.1002/stem.47
Strzalka W, Ziemienowicz A (2011) Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann Bot 107:1127–1140. https://doi.org/10.1093/aob/mcq243
Villani V, Mahadevan KK, Ligorio M, Fernandez-Del Castillo C, Ting DT, Sabbatino F, Zhang I, Vangel M, Ferrone S, Warshaw AL, Lillemoe KD, Wargo J, Deshpande V, Ferrone CR (2016) Phosphorylated histone H3 (PHH3) is a superior proliferation marker for prognosis of pancreatic neuroendocrine tumors. Ann Surg Oncol 23:609–617. https://doi.org/10.1245/s10434-016-5171-x
Wang L, Wu B, Zhang Y, Tian Z (2015) Hypoxia promotes the proliferation of MC3T3-E1 cells via the hypoxia-inducible factor-1alpha signaling pathway. Mol Med Rep 12:5267–5273. https://doi.org/10.3892/mmr.2015.4034
Wang T, Zhang ZX, Xu YJ, Hu QH (2007) 5-Hydroxydecanoate inhibits proliferation of hypoxic human pulmonary artery smooth muscle cells by blocking mitochondrial K(ATP) channels. Acta Pharmacol Sin 28:1531–1540. https://doi.org/10.1111/j.1745-7254.2007.00636.x
Zhang W, Su X, Gao Y, Sun B, Yu Y, Wang X, Zhang F (2009) Berberine protects mesenchymal stem cells against hypoxia-induced apoptosis in vitro. Biol Pharm Bull 32:1335–1342
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal slaughter followed the Ethics Committee of the Institute of Hunan Normal University.
Rights and permissions
About this article
Cite this article
Zhang, J., Xiong, L., Tang, W. et al. Hypoxic culture enhances the expansion of rat bone marrow-derived mesenchymal stem cells via the regulatory pathways of cell division and apoptosis. In Vitro Cell.Dev.Biol.-Animal 54, 666–676 (2018). https://doi.org/10.1007/s11626-018-0281-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-018-0281-3