Abstract
During cold stress, liver cells undergo apoptotic injury as a result of oxidative stress. Heat shock 70 kDa protein (Hsp70) is a protein involved in modulating a variety of physiological processes, including stress responses, proliferation, and apoptosis. In addition, Hsp70 regulates apoptotic signaling pathways in different manners, promoting or suppressing apoptosis. In this study, we investigated the effects of Hsp70 overexpression on hydrogen peroxide (H2O2)-induced apoptosis of Buffalo rat liver (BRL) cells and the underlying mechanisms of these effects. Our results show that in comparison with the control group, Hsp70 overexpression displayed increased protein levels of Bcl-2, and decreased cytochrome c (Cyt c), cleaved caspase 3, and cleaved caspase 8, but no apparent differences were found in levels of Bax. Furthermore, Hsp70 overexpression significantly suppresses the amount of apoptotic cells. Such findings indicate that overexpression of Hsp70 inhibits H2O2-mediated activation of caspase 8 and caspase 3, upregulates the expression of Bcl-2 which is a known anti-apoptotic protein, and decreases the release of Cyt c from the mitochondria into the cytoplasm, collectively decreasing cell apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cold stress affects animal growth, development, and health, which can ultimately harm livestock husbandry. The mechanisms of response to cold stress are complicated; almost all organs and tissues are involved. It has been demonstrated that cold stress causes relatively severe damage to the liver due to decreased levels of glutathione (GSH) and increased levels of protein carbonylation, lipid oxidation, and abundance of intracellular reactive oxygen species (ROS), which collectively leads to oxidative stress-induced apoptosis in the liver (Sahin and Gumuslu 2004, 2007).
Apoptosis can be triggered by a variety of factors, among which ROS-induced oxidative stress is a key step (Ryter et al. 2007). For example, it has been reported that low concentration of hydrogen peroxide (H2O2) can induce apoptosis (Davies 1999; Troyano et al. 2003). The mechanisms of apoptosis are complex, as relevant signaling pathways communicate with one another to collectively regulate the life and death of cells. In death signaling pathways, CD95 (Fas) and tumor necrosis factor (TNF) family proteins cleave and activate downstream target proteins by activating caspase 8, eventually leading to apoptosis (Cohen 1997). In mitochondrial pathways, cytochrome C (Cyt c) released from the mitochondria binds to apoptosis-activating factor 1 (Apaf-1) and activates the caspase 9/caspase 3 cascade. In addition, Cyt c also changes the ratio of apoptosis-related proteins Bcl-2/Bax, thereby triggering apoptosis (Perkins et al. 2000).
Heat shock 70 kDa protein (Hsp70) has multiple biological functions, including protein chaperoning, immune responses, boosting cellular stress tolerance, and participating in the formation and repair of the cytoskeleton. It has been reported that Hsp70 can bind to Apaf-1, c-Jun N-terminal kinase (JNK), and apoptosis-inducing factors to inhibit apoptosis (Beere et al. 2000; Park et al. 2001; Ravagnan et al. 2001). In the hepatocytes of stressed mice, the expression of anti-apoptotic heat shock proteins such as Hsp27, Hsp32, and Hsp70, as well as c-Jun was increased (Vogel et al. 2004). In human colon cancer cells, overexpression of Hsp70 has been shown to suppress TNF-α-mediated NF-κВ signaling pathway activation by interacting with TNF receptor (TNFR) coupling factor 2 (Dai et al. 2010). Therefore, the JNK pathway might modulate TNF-α-mediated apoptosis in colon cancer cells. Furthermore, it has been demonstrated that overexpression of Hsp70 not only alleviated oxidative stress-induced necrosis of muscle cells, but also inhibited apoptosis by preventing the mitochondrial release of Cyt c and Smac (Xiao et al. 2004). Despite evidence from several studies that Hsp70 displays an anti-apoptotic function, the mechanism is still not clear. Therefore, the objective of this study was to investigate the hypothesis that overexpression of Hsp70 could protect rat hepatocytes from H2O2-induced apoptosis and to reveal the mechanism. This study will provide a new insight into the molecular mechanisms of apoptosis inhibition.
Materials and Methods
Cell culture
Buffalo rat liver (BRL) cells were purchased from the Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)/high-glucose medium supplemented with 10% fetal bovine serum and 100 U/mL penicillin-streptomycin (all from Solarbio, Beijing, China). The Hsp70 overexpressed adenovirus was from our lab (Animal Physiology Lab, Heilongjiang Bayi Agricultural University).
Cell model establishment
The working concentration of H2O2 was determined by cell viability, oxidative damage, and apoptosis after exposure to different concentrations of H2O2 (0, 50, 100, 150, 200, and 250 μmol/L) for 2 h. After determining the working concentration of H2O2, in the following experiment, cells were divided into six groups. Cells in groups 1–3 were cultured in normal medium; however, groups 4–6 were cultured in medium with certain concentration (150 μmol/L) of H2O2: group 1 (BRL cells), group 2 (BRL cells infected with an empty recombinant adenovirus, Ad-CMV-Null), group 3 (BRL cells infected with an Hsp70-encoding recombinant adenovirus, Ad-CMV-Hsp70), group 4 (BRL cells treated with H2O2), group 5 (BRL cells treated with H2O2 and infected with the empty recombinant adenovirus), and group 6 (BRL cells treated with H2O2 and infected with an Hsp70-encoding recombinant adenovirus).
Evaluation of cell viability
BRL cells (1.5 × 103/well) were seeded into 96-well plate, then incubated at 37°C for 48 h. Afterwards, cells were treated with different concentrations of H2O2 (H3410, Sigma, St. Loius, MO) according to the experimental requirements. Each group was composed of five replicates. WST-1 (C0036, Beyotime, Jiangsu, China, Cell Proliferation and Cytotoxicity Assay Kit) detection was performed after 2 h of H2O2 treatment. This experiment was repeated at least three times.
Assessment of cell oxidative damage
Each group of BRL cell was harvested and washed twice with pre-cold PBS. Cells were scraped and transferred into a 1.5-mL centrifuge tube. After centrifugation, the supernatant was discarded and pellet was resuspended in cold saline and sonicated at 40% magnitude for 10 cycles of 5 s on and 30 s off. A total of 450 μL homogenate was used to determine the protein carbonyl concentration (Protein Carbonyl assay kit, A087, Jiancheng, Nanjing, China) and total superoxide dismutase (SOD) activity (Total Superoxide Dismutase Assay Kit with NBT, S0107, Beyotime, Jaingsu, China). This experiment was repeated at least three times.
Flow cytometry analysis
Cells (1 × 106) were harvested for apoptosis assessment by Annexin-V/FITC-P cell apoptosis detection kit (C1063, Beyotime) and gently resuspended in 195 μL of Annexin V-FITC binding buffer. Then, 5 μL Annexin V-FITC was added, gently mixed, and incubated at room temperature 15 min in the dark. Subsequently, 5 μL of propidium iodide (PI) staining solution was added and gently mixed. The samples were placed in an ice bath in the dark before being examined by flow cytometry. This experiment was repeated three times.
Separation of mitochondria
Mitochondria were isolated from cells using the Cell Mitochondria Isolation Kit (C3601, Beyotime) according to the manufacturer’s instructions. The lysates were centrifuged at 3000g for 5 min, and then, the supernatants were centrifuged at 15,000g for 10 min. The supernatant contained the cytosolic fraction. The pellet was dissolved in 0.1% sodium dodecyl sulfate (SDS; L3771, Sigma) and centrifuged at 15,000g for 20 min. The supernatant contained the mitochondrial fraction.
Western blot
The cells were directly lysed in gel sample buffer, separated under reducing conditions on 12% SDS-polyacrylamide gels, and subjected to immunoblotting using primary antibodies against Hsp70 (1:1000), cleaved caspase 8 (1:800), Bcl-2 (1:200), Bax (1:2000), Cyt c (1:500), cleaved caspase3 (1:500), and β-actin (1:1000), and detection was made using HRP-conjugated goat anti-mouse IgG (1:10,000). The anti-Hsp70 antibody (ab47455) was purchased from Abcam (Cambridge, MA). The anti-β-actin (AA128-1), anti-cleaved caspase 3 (AC003), and HRP-conjugated goat anti-mouse IgG antibodies (A0216) were purchased from Beyotime. The anti-cleaved caspase 8 (13423-1-AP), anti-Bax (60267-1-LG), and anti-Cyt c (10993-1-AP) antibodies were purchased from Proteintech (Chicago, IL). The anti-Bcl-2 (BA0412) antibody was purchased from BOSTER (Wuhan, China). Signals were generated using chemiluminescence, and the blots were imaged using a Bio-Rad gel imaging system (molecular imager). Densitometry values of the bands were calculated. Target protein levels are expressed as ratios of the band density compared with that of β-actin. This experiment was repeated three times at least.
Quantitative RT-PCR
Total RNA was isolated from cells with Trizol reagent (T9424, Sigma) and reverse-transcribed with the RT kit (D6110A, Takara, Dalian, China) following the manufacturer’s instructions. Quantitative RT-PCRs were carried out in the presence of SYBR Green (QPK-201, Takara) and the reactions were monitored using a Bioer Real-time instrument ((FQD-48A, Hangzhou Bioer Technology Co., Ltd., Zhejiang, China). Reactions were carried out in triplicate. The resulting data were used to calculate the relative quantity using the maximum second derivative method, which was accomplished with the built-in software of the Line-Gene K fluorescence quantitative PCR device. The following primers (5′ to 3′) were used: Hsp70: GCTCGAGTCCTACGCCTCAATA/TCCTGGCACTTGTCCAGCAC; β-actin: TCACCAACTGGGACG/GCATACAGGGACAACA. This experiment was repeated three times at least.
Statistical analyses
The SPSS 19.0 (IBM, New York, NY) was used to perform the relevant statistical analyses. All experimental data were expressed as the means ± standard deviations (x ± s). Univariate analysis of variance and t test were used as the statistical methods. P < 0.05 was considered statistically significant.
Results
Effect of H2O2 on the viability of BRL cells
As shown in Fig. 1, cells treated with 50 μmol/L H2O2 for 2 h displayed elevated cell viability (P < 0.05), whereas cells treated with 100 μmol/L H2O2 exhibited a slight reduction in viability, compared with the control group which received no H2O2 treatment. Cells treated with over 150 μmol/L H2O2 showed a significant decrease in cell viability (P < 0.01), with a trend of higher H2O2 concentrations correlating with lower cell viabilities.
Effect of H2O2 on the apoptosis of BRL cells
As shown in Fig. 2, apoptosis occurred at a very low frequency in the control group. However, after 2 h treatment of H2O2, the percentage of apoptotic cells increased and positively correlated with H2O2 concentrations. Specifically, the percentage of apoptotic cells for each group was as follows: 50 μmol/L H2O2, 8.09 ± 0.57%; 100 μmol/L H2O2, 14.37 ± 0.52%; 150 μmol/L H2O2, 25.41 ± 0.54%; 200 μmol/L H2O2, 40.74 ± 0.45%; and 250 μmol/L H2O2, 56.51 ± 0.54%. In addition, H2O2 also caused cell death. The percentage of dead cells for each group was as follows: 100 μmol/L H2O2, 4.85 ± 0.55%; 150 μmol/L H2O2, 7.89 ± 0.61%; 200 μmol/L H2O2, 16.17 ± 0.63%; and 250 μmol/L, 15.94 ± 0.65%, respectively. Hence, we chose 150 μmol/L H2O2 treatment for the following experiments.
Effect of H2O2 on the protein carbonyl content and SOD activity of BRL cells
As shown in Table 1, cells treated with 150 μmol/L H2O2 for 2 h displayed increased protein carbonyl content and decreased total SOD activity, and both parameters were significantly different from the corresponding values of the control group (P < 0.05). This revealed that exposure to 150 μmol/L of H2O2 resulted in oxidative stress to cells.
Overexpression of Hsp70 in BRL cells
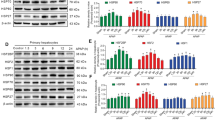
As shown in Fig. 3A, cells infected with an adenovirus expressing Hsp70 showed a significantly higher level of Hsp70 mRNA than that of the control groups (P < 0.01). Likewise, cells infected with an adenovirus expressing Hsp70 showed a significantly higher abundance of Hsp70 protein levels than that of the control groups (P < 0.01) (Fig. 3B). Hsp70/β-actin ratios determined by densitometry are shown in Fig. 3C.
Hsp70 mRNA and protein levels. (A) Hsp70 mRNA levels. (B) Hsp70 protein levels. (C) Hsp70/β-actin ratios determined by densitometry. Untreated groups 1, 2, and 3. H 2 O 2 treated groups 4, 5, and 6. Control group 1 and group 4. Ad-CMV-Null group 2 and group 5. Ad-CMV-Hsp70 group 3 and group 6. **P < 0.01.
Effect of Hsp70 overexpression on Bcl-2, caspase 3, caspase 8, Cyt c, and Bax levels in BRL cells
As shown in Fig. 4, cells treated with H2O2 (groups 4, 5) displayed significantly lower protein levels of Bcl-2 (P < 0.01) than their equivalent group without H2O2 treatment (groups 1, 2). However, in group 6, where Hsp70 was overexpressed, Bcl-2 levels were not significantly decreased after H2O2 treatment compared to the untreated cells (group 3) (P > 0.05). Similarly, cleaved caspase 3 (Fig. 5) displayed lower levels in the H2O2 treatment groups (4, 5) compared to the untreated groups (1, 2). Whereas, caspase 8 (Fig. 5) displayed significantly higher levels in the H2O2 treatment groups (4, 5) compared to the untreated groups (1, 2). However, compared to the untreated cells, those infected with the Hsp70 overexpressing adenovirus (group 6) had a significant reduction in cleaved caspase 3 and caspase 8 (P < 0.01), even after H2O2 treatment (group 3).
Effect of Hsp70 overexpression on Bcl-2 protein levels. (A) Bcl-2 protein expression measured by western blotting. (B) Bcl-2/β-actin ratios determined by densitometry. Untreated groups 1, 2, and 3. H 2 O 2 treated groups 4, 5, and 6. Control group 1 and group 4. Ad-CMV-Null group 2 and group 5. Ad-CMV-Hsp70 group 3 and group 6. **P < 0.01.
As shown in Fig. 6, cells treated with H2O2 (groups 4, 5) exhibited a significant release of Cyt c from the mitochondria into the cytoplasm, compared with cells without H2O2 exposure (groups 1, 2, 3). However, in the Hsp70 overexpressed group (group 6), the amount of Cyt c released was significantly reduced (P < 0.05). Nevertheless, no difference was found in Bax protein levels (Fig. 7) in all the groups of BRL cells.
Effect of Hsp70 overexpression on Bax protein levels. (A) Bax protein expression measured by western blotting. (B) Bax/β-actin ratios determined by densitometry are shown above the western blots. Untreated groups 1, 2, and 3. H 2 O 2 treated groups 4, 5, and 6. Control group 1 and group 4. Ad-CMV-Null group 2 and group 5. Ad-CMV-Hsp70 group 3 and group 6.
Effect of Hsp70 overexpression on H2O2-induced apoptosis in BRL cells
As shown in Fig. 8, cells treated with H2O2 (Fig. 8B, D, F, groups 4, 5, 6) all exhibited a higher percentage of apoptotic cells than the untreated cells (Fig. 8A, C, E, groups 1, 2, 3). However, cells infected by the adenovirus overexpressing Hsp70 (Fig. 8F, group 6) showed a markedly lower percentage of apoptotic cells than the control groups (Fig. 8B, D, group 4, 5) (P < 0.01).
Discussion
Oxidative stress can cause apoptosis through multiple pathways, such as the mitochondria, the death receptor, or ER stress (Huang et al. 2003; Singh and Singh 2008; Li et al. 2010). Alternatively, oxidative stress also activates the mitogen-activated protein kinase (MAPK) pathway and the caspase cascade to induce apoptosis (Chen et al. 2001; Zhuang et al. 2007). The expression of Hsps is closely linked to cell growth and differentiation. These proteins play pivotal regulatory roles in the aforementioned apoptosis pathways. Hence, in this study, 150 μmol/L H2O2 was used to induce apoptosis of rat BRL cells to explore the mechanisms how Hsp70 modulates H2O2-induced apoptosis.
A large body of evidence suggests that the Bcl-2 family members play important regulatory roles in the mitochondrial pathway of apoptosis. Bcl-2 is a critical anti-apoptotic protein that stabilizes the permeability of the mitochondrial membrane (Chen et al. 2001). In the mitochondrial pathway, Cyt c is the key mediator and is released from the mitochondria into the cytoplasm when cells are under stress. Our results revealed that after cells are exposed to H2O2, the amount of Cyt c released from the mitochondria into the cytoplasm was significantly increased, and this was accompanied with reduced expression of the Bcl-2 protein. Correspondingly, cells displayed apoptotic damage. Although Bax levels were not significantly changed, the ratio of apoptosis-related proteins Bcl-2/Bax was changed, as we expected. This indicates that oxidative stress may induce apoptosis by multiple routes, such as affecting mitochondrial membrane permeability, promoting the release of Cyt c into the cytoplasm, and regulating Bcl-2 levels.
Caspase 3 and caspase 8 are the main executors of apoptosis, and their activation is a central event in apoptosis. Our results indicate that H2O2-induced apoptosis of rat BRL cells was stimulated by the activation of caspase 3 and caspase 8, which is consistent with a previous report (Zhu et al. 2006). Our data also reveals that cells infected with an adenovirus overexpressing Hsp70 exhibited a decrease in the activation of caspase 3 and caspase 8, resulting in reduced apoptosis after exposure to H2O2. Together, this indicates that Hsp70 may regulate the activation of caspase 3 and caspase 8 to protect cells from apoptosis.
Conclusions
In this study, cellular oxidative stress was induced by H2O2, which regulates apoptosis via multiple pathways. Overexpression of Hsp70 inhibits the activation of caspase 3 and caspase 8, and also modulates the release of Cyt c into the cytoplasm. These events enhance the expression of the anti-apoptotic protein Bcl-2 and suppress the activation of caspase 3. As a consequence, BRL cells are protected from apoptosis.
References
Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR (2000) Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2:469–475
Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH (2001) Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol 280:H2313–H2320
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326(Pt 1):1–16
Dai S, Jiang L, Wang G, Zhou X, Wei X, Cheng H, Wu Z, Wei D (2010) HSP70 interacts with TRAF2 and differentially regulates TNFalpha signalling in human colon cancer cells. J Cell Mol Med 14:710–725
Davies KJ (1999) The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB Life 48:41–47
Huang HL, Fang LW, Lu SP, Chou CK, Luh TY, Lai MZ (2003) DNA-damaging reagents induce apoptosis through reactive oxygen species-dependent Fas aggregation. Oncogene 22:8168–8177
Li G, Scull C, Ozcan L, Tabas I (2010) NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol 191:1113–1125
Park HS, Lee JS, Huh SH, Seo JS, Choi EJ (2001) Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J 20:446–456
Perkins CL, Fang G, Kim CN, Bhalla KN (2000) The role of Apaf-1, caspase-9, and bid proteins in etoposide- or paclitaxel-induced mitochondrial events during apoptosis. Cancer Res 60:1645–1653
Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G (2001) Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 3:839–843
Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM (2007) Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9:49–89
Sahin E, Gumuslu S (2004) Cold-stress-induced modulation of antioxidant defence: role of stressed conditions in tissue injury followed by protein oxidation and lipid peroxidation. Int J Biometeorol 48:165–171
Sahin E, Gumuslu S (2007) Stress-dependent induction of protein oxidation, lipid peroxidation and anti-oxidants in peripheral tissues of rats: comparison of three stress models (immobilization, cold and immobilization-cold). Clin Exp Pharmacol Physiol 34:425–431
Singh M, Singh N (2008) Induction of apoptosis by hydrogen peroxide in HPV 16 positive human cervical cancer cells: involvement of mitochondrial pathway. Mol Cell Biochem 310:57–65
Troyano A, Sancho P, Fernandez C, de Blas E, Bernardi P, Aller P (2003) The selection between apoptosis and necrosis is differentially regulated in hydrogen peroxide-treated and glutathione-depleted human promonocytic cells. Cell Death Differ 10:889–898
Vogel A, van Den Berg IE, Al-Dhalimy M, Groopman J, Ou CN, Ryabinina O, Iordanov MS, Finegold M, Grompe M (2004) Chronic liver disease in murine hereditary tyrosinemia type 1 induces resistance to cell death. Hepatology 39:433–443
Xiao WM, Jiang BM, Shi YZ (2004) Mechanisms of heat shock proteins inhibiting C2C12 cell apoptosis induced by hydrogen peroxide. Zhong Nan Da Xue Xue Bao Yi Xue Ban 29:6–10
Zhu, T., R. Chen, A. Li, J. Liu, D. Gu, Q. Liu, C. C. H, and J. Zhou (2006) JWA as a novel molecule involved in oxidative stress-associated signal pathway in myelogenous leukemia cells. J Toxicol Environ Health A 69:1399–411
Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG (2007) ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol 292:F440–F447
Acknowledgments
This work was supported by the Natural Science Foundation of Heilongjiang Province (Grant No. C2015041), National Natural Science Foundation of China (Grant No. 31372398), and Scientific Research Foundation for Returned Overseas Chinese Scholars, the State Educational Commission of Heilongjiang Province of China (No. 1253HQ013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Editor: Tetsuji Okamoto
Fanzhi Kong and Hui Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kong, F., Wang, H., Guo, J. et al. Hsp70 suppresses apoptosis of BRL cells by regulating the expression of Bcl-2, cytochrome C, and caspase 8/3. In Vitro Cell.Dev.Biol.-Animal 52, 568–575 (2016). https://doi.org/10.1007/s11626-016-0005-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-016-0005-5