Abstract
Background
Anticholinergic medications may increase risk of dementia and stroke, but prospective studies in healthy older people are lacking.
Objective
Compare risk of incident dementia and stroke by anticholinergic burden among initially healthy older people.
Design
Prospective cohort study.
Setting
Primary care (Australia and USA).
Participants
19,114 community-dwelling participants recruited for the ASPREE trial, aged 70+ years (65+ if US minorities) without major cardiovascular disease, dementia diagnosis, or Modified Mini-Mental State Examination score below 78/100.
Measurements
Baseline anticholinergic exposure was calculated using the Anticholinergic Cognitive Burden (ACB) score. Dementia was adjudicated using Diagnostic and Statistical Manual of Mental Disorders volume IV criteria, and stroke using the World Health Organization definition.
Results
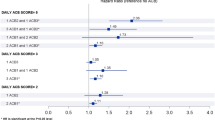
At baseline, 15,000 participants (79%) had an ACB score of zero, 2930 (15%) a score of 1–2, and 1184 (6%) a score of ≥ 3 (indicating higher burden). After a median follow-up of 4.7 years and adjusting for baseline covariates, a baseline ACB score of ≥ 3 was associated with increased risk of ischemic stroke (adjusted HR 1.58, 95% CI 1.06, 2.35), or dementia (adjusted HR 1.36, 95% CI 1.01, 1.82), especially of mixed etiology (adjusted HR 1.53, 95% CI 1.06, 2.21). Results were similar for those exposed to moderate/highly anticholinergic medications.
Limitations
Residual confounding and reverse causality are possible. Assessment of dose or duration was not possible.
Conclusions
High anticholinergic burden in initially healthy older people was associated with increased risk of incident dementia and ischemic stroke. A vascular effect may underlie this association. These findings highlight the importance of minimizing anticholinergic exposure in healthy older people.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rates of dementia and stroke are increasing as populations age.1, 2 Identification and reduction of modifiable risk factors may prevent cognitive decline or stroke. Medications with anticholinergic properties (henceforth anticholinergics) block the neurotransmitter acetylcholine in the central and peripheral nervous systems and have been associated with cognitive decline in several studies.3,4,5,6,7,8,9 However, these studies have been limited by reliance on record linkage rather than prospective cognitive screening and adjudicated outcome ascertainment. Furthermore, previous studies included older people with history of serious cardiovascular disease, such as myocardial infarction, transient ischemic attack, and stroke, which increases the risk of cognitive decline and dementia.10, 11 It is currently unknown whether use of anticholinergics by older people without known major illness is associated with similar undesirable outcomes and whether cumulative anticholinergic burden from exposure to multiple drugs with subclinical anticholinergic burden confers the similar adverse risk.
ASPirin in Reducing Events in the Elderly (ASPREE) was a randomized, placebo-controlled trial of aspirin in 19,114 community-dwelling older adults in Australia and the USA who were initially free of dementia, cardiovascular disease, and significant life-limiting illness.12 ASPREE collected annual prescription medication data, conducted regular cognitive screening, and clinically adjudicated stroke and dementia outcomes. Here, we describe the impact of baseline anticholinergic burden on incident dementia and stroke, and specific subclassifications, using prospective ASPREE data.
METHODS
ASPREE participants at enrolment were aged 70 years or older (65 or older for US minorities) and required to be free of major cardiovascular disease, including myocardial infarction, stroke, transient ischemic attack, or atrial fibrillation. Individuals were also ineligible based on a diagnosis of dementia, prescription of cholinesterase inhibitor, or a Modified Mini-Mental State examination (3MS)13 score less than 78.14 ASPREE was approved by multiple Institutional Review Boards in Australia and the USA prior to data collection, and participants provided written informed consent. Detailed methods and results of ASPREE are described elsewhere.12, 15,16,17,18,19 All ASPREE participants were included in this analysis.
Collection of Medication and Calculation of AC burden
Participants presented their medications (or medication list) at their baseline data collection visit for staff review. Medication lists were also reviewed where access to medical records was available. The majority of participants (96.5%) were connected with a primary care provider who was enrolled as an associate investigator (Australia) or clinical trial center (USA) to facilitate access to medical records. Data for prescribed medications were transcribed into the ASPREE data system20 and coded according to the World Health Organization (WHO) Anatomical and Therapeutic Chemical (ATC) coding system.21 Detailed methods for the coding process are published elsewhere.22
Several scales exist for calculating burden from anticholinergic medications. We assigned an anticholinergic burden score using the Anticholinergic Cognitive Burden (ACB) scale.23 This scale was selected because it was formulated specifically for cognitive outcomes relevant to this analysis, and previous studies have demonstrated its utility for quantifying anticholinergic exposure from medications.24, 25 The majority of medications included in the ACB scale are also included in the Anticholinergic Risk Scale26 and the Anticholinergic Drug Scale,27 and hence the ACB scale was considered a robust scale for this analysis. Medications with possible anticholinergic effects but without linked evidence of clinically negative cognitive effects were assigned an ACB score of 1, and medications with established, clinically relevant cognitive effects were assigned a score of 2 or 3 based on blood-brain permeability23 (see Table S1 for a complete list). Participants’ total baseline anticholinergic burden scores were calculated by multiplying the number of anticholinergics prescribed at baseline, by their respective medication ACB score and summing the results. Participants were divided into 3 groups according to their total ACB score at baseline: those with an ACB score of 0 (no anticholinergic burden), 1–2 (likely subclinical anticholinergic burden), or 3 or more (likely clinical anticholinergic burden).
Outcome Measures
Data were gathered through quarterly telephone contact with participants and annual in-person assessments. Cognitive assessments were administered at baseline, year 1, and biennially throughout follow-up. The 3MS was used to measure global cognition13, 28 and screen for cognitive decline. The process for confirmation of a suspected dementia diagnosis and subclassification of Alzheimer’s dementia (AD) has been previously published.29 In brief, a 3MS score of < 78,14 a drop of 10.15 points or more from the 5-year predicted score at baseline,30 a documented clinical diagnosis of dementia, or the prescription of cholinesterase inhibitors (Australia only) triggered conduct of additional cognitive assessments and collection of supporting documentation such as laboratory tests, brain imaging, and clinical notes. This information was reviewed by an expert adjudication committee who determined if Diagnostic and Statistical Manual for Mental Disorders, American Psychiatric Association (DSM-IV) criteria for dementia were reached.31
Subclassification of probable AD was made according to the National Institute on Aging-Alzheimer’s Association 2011 (NIA-AA) core clinical criteria.32 This includes insidious onset worsening over time and either amnestic or non-amnestic presentation. Possible AD was classified as individuals who met core AD criteria but with an atypical course or etiologic mixed presentation including evidence of other pathology such as cerebrovascular, Lewy body, and frontotemporal disease.32
The definition of stroke was based on the WHO definition of rapidly developing clinical signs of focal or global disturbance of cerebral function lasting more than 24 h, with no apparent cause other than ischemic or hemorrhagic cerebrovascular disease.33 Stroke reports were presented to a committee of experts who adjudicated whether the event met the WHO criteria.34
Statistical Analysis
We compared participants of the ASPREE clinical trial exposed to anticholinergics at baseline with participants not exposed. All analyses were restricted to events occurring during the ASPREE clinical trial, between 1 March 2010 and 12 June 2017. Participant characteristics were represented as means and standard deviations for numeric variables, and counts and percentages for categorical variables. Ordinal logistic regression was used to examine associations of these characteristics across ACB score groups (i.e., 0, 1–2, and 3+). Models were adjusted for sex and baseline age (Table 1). Brant tests35 of the proportional odds assumption were significant in some models, but further inspection of cut-point-specific ORs suggested that this statistical significance was due in part to the large sample size, since the overall ORs provide a reasonable summary of the cut-point-specific counterparts.
Cox proportional hazards regression models were used to estimate the association between ACB score and time to incident dementia, and stroke, adjusting for sex, age, years of education, ethnicity, smoking status, allocated treatment group, presence of depression (CESD-10 ≥ 8),36 hypertension, diabetes, and baseline 3MS. The proportional hazards assumptions were inspected visually by plotting Schoenfeld residuals against time, which suggested that the assumptions were adequate. Subclassification of dementia and stroke outcomes were also assessed (Table 2). Sensitivity analyses pertaining to medications with high AC score were conducted by analyzing only individuals with exposure to an AC medication with a score or 2 or 3 (Table 3).
Polypharmacy, defined as concurrent use of 5 or more medications, and high anticholinergic burden are known to be co-prevalent.37, 38 High anticholinergic burden produced through the prescription of multiple low anticholinergic property medications is likely to occur in the presence of polypharmacy. Previous studies have not explicitly explored the relationship between polypharmacy, anticholinergic burden, and clinical outcomes,7,8,9 and hence it is unknown whether polypharmacy mediates the effect of anticholinergics. We divided the cohort into those with and without polypharmacy in order to explore these relationships (Table 4).
Supplemental analyses were undertaken to assess variability in AC score throughout follow-up (Table S2), the role of specific medication classes (Table S3), and subclassification of ischemic stroke (Table S5). Additional sensitivity analysis focussed only on individuals exposed to cardiovascular medications, and those with uncontrolled hypertension (SBP of ≥ 140mmHg or DBP ≥ 90mmHg) (Table S4).
RESULTS
At baseline, 15,000 (79% of total) had an ACB score of 0, 2930 (15%) had an ACB score of 1–2, and 1184 (6%) had ACB score of 3 or more at baseline (Figure 1). The proportion of participants who became lost to follow-up or died was similar between these groups, as was median follow-up time. Overall, 75% of participants remained in the same ACB group as baseline throughout the follow-up period (Table S2).
Participants who were female, older in age, from the USA, had lower education, current or former smokers, or had diabetes, chronic kidney disease, depression, or hypertension, were more likely to have a higher ACB score (Table 1). Participants with lower cognitive scores were more likely to have a higher ACB score. The most frequently prescribed anticholinergics were primary prevention cardiovascular medications with AC properties (e.g., atenolol, metoprolol, furosemide, and nifedipine) (9.6% of the cohort), followed by antidepressants (4.2%), corticosteroids (2.4%), opioids (1.9%), benzodiazepines (1.7%), H2-receptor antagonists (1.5%), and bladder antimuscarinics (1.4%) (Table S1).
Dementia
Compared to participants with an ACB score of 0, rates were higher in participants with an ACB score of ≥ 3 (adjusted HR 1.36, 95% CI 1.01, 1.82), but similar in participants with an ACB score of 1–2 (adjusted HR 1.03, 95% CI 0.82, 1.29) (Table 2). With regard to dementia subclassification, participants with an ACB score of ≥ 3 were more likely to be diagnosed with possible AD dementia (e.g., mixed etiology and vascular dementia) compared to participants with an ACB score of 0 (adjusted HR 1.53, 95% CI 1.06, 2.21). There were no significant differences between ACB score groups and probable dementia (ACB 0 vs ≥ 3 adjusted HR 1.15, 95% CI 0.70, 1.87). Compared to those without exposure to a highly anticholinergic medication (ACB score of 2 or 3), participants with exposure had a higher rate of incident dementia (adjusted HR 1.35, 95% CI 1.01, 1.80) (Table 3). Sensitivity analyses (Table S3) included limiting the population to only those participants taking psychoactive medications, but event numbers were too small to draw conclusions.
Stroke
In the overall cohort, absolute rate of incident stroke was higher among participants with ACB scores of ≥ 3 (6.5 events per 1000 person-years) compared with participants with scores of 0 (4.4 events per 1000 person-years), but this difference was explained by adjustment for baseline covariates. However, compared to participants with an ACB score of 0, ischemic stroke rates were higher in participants with an ACB score of ≥ 3 (rate 3.4 vs 5.3, adjusted HR 1.58, 95% CI 1.06, 2.35) (Table 2). Similar results for ischemic stroke were observed in sensitivity analyses, which included limiting the population to only those participants taking cardiovascular medications, and those with uncontrolled hypertension (Table S4). While subclassification of ischemic strokes produced groups with small numbers, some increased risks were observed for cardioembolic stroke and strokes with undetermined etiology in sensitivity analysis (Table S5). Of the 939 participants who experienced either stroke or dementia, only 34 participants experienced both events (4%). Compared to participants without exposure to moderate or highly anticholinergic medications, those with exposure had a higher rate of incident ischemic stroke (rate of 3.5 vs 5.4; adjusted HR 1.54, 95% CI 1.04, 2.26) (Table 3).
Polypharmacy
In those with polypharmacy, the rate of dementia was 9.9 events per 1000 person-years in those with an ACB score of ≥ 3, compared with 7.0 events per 1000 person-years in those without exposure to anticholinergics (adjusted HR 1.18, 95% CI 0.78, 1.78). In those without polypharmacy, the rate of dementia was 9.8 events per 1000 person-years in participants with an ACB score of ≥ 3 compared with 6.2 events per 1000 person-years in those with a score of 0 (adjusted HR 1.51, 95% CI 0.96, 2.38). In those with polypharmacy, the rate of stroke was 5.3 events per 1000 person-years in those with an ACB score of > 3, compared with 4.6 events in those without exposure (adjusted HR 1.10, 95% CI 0.63, 1.90). In those without polypharmacy, the rate of stroke was 8.3 events per 1000 person-years in participants with an ACB score of ≥ 3 compared with 4.4 events per 1000 person-years in those with a score of 0 (adjusted HR 1.91, 95% CI 1.16, 3.13). The test of interaction did not suggest that the presence of polypharmacy mediated the association between high anticholinergic burden and dementia (p for interaction 0.71) or stroke (p for interaction 0.11) (Table 4).
DISCUSSION
In healthy older people without evidence of major cardiovascular disease or physical or cognitive disability, we found higher baseline anticholinergic use (ACB score of ≥3) was associated with increased risk of dementia and, separately, ischemic stroke during 4.7 years of follow-up.
Use of anticholinergics (21% of cohort) was higher in ASPREE than in the French, community-based PAQUID, and 3C studies (13.7% and 7.5% respectively),3, 4 but similar prevalence to the younger EPIC-Norfolk cohort (19.6% of cohort with median age 58.9 years).5 This can be attributed to differences in medication classification, as both the PAQUID and 3C studies excluded certain cardiovascular medications, opioids, or benzodiazepines, which have an ACB score of 1.23 The overall burden of anticholinergics in our cohort was much lower than previously reported for nursing home cohorts (48–82% prevalence),25, 39, 40 as expected given the lower comorbidity burden and community-dwelling status.
The correlation between ACB score and dementia risk is consistent with studies in older cohorts that included people with serious cardiovascular comorbidity and in nursing home care.4, 7,8,9, 41 However, in contrast to a previous report,9 no association between anticholinergics and probable Alzheimer’s dementia (AD) was observed. This novel finding may be explained both by active baseline cognitive screening of the cohort and the rigorous clinical validation of dementia diagnosis and subclassification. Anticholinergics are often prescribed in response to prodromal symptoms of dementia,42, 43 and hence it is difficult to completely avoid indication bias in case-controlled and cohort studies that do not screen cognitive performance at baseline. Furthermore, given that dementia is known to be under-diagnosed in the community,44 analyses based on record linkage are likely to misclassify some participants as controls. Participation in ASPREE required not only the absence of a dementia diagnosis but also achieving a minimum of 78/100 on the 3MS. Thus, although indication bias cannot be excluded, ASPREE is likely to be cleaner than previous case-controlled and cohort studies with relatively limited protopathic bias.
Use of anticholinergics at baseline is associated with an increased risk of dementia subclassified as “Possible AD,” which is applied to cases of mixed or atypical etiology, including cerebrovascular disease.32 Our findings lend strength to Coupland et al.8’s conclusion that anticholinergics are associated with increased risk of mixed dementia with a vascular component. To our knowledge, this is the first analysis to explore polypharmacy as a mediator of the association between anticholinergics and dementia. ASPREE participants with polypharmacy were five times more likely to be concurrently prescribed anticholinergics compared to those without polypharmacy. However, polypharmacy did not mediate the associations observed, suggesting that anticholinergic burden may be an independent risk factor for dementia.
Stroke
Higher baseline anticholinergic burden was associated with increased risk of ischemic stroke in our study of initially healthy older people without major cardiovascular disease. EPIC-Norfolk5 examined the association between medications with AC properties and stroke, reporting an association between overall stroke, ischemic stroke, and stroke death, but no association for hemorrhagic stroke. The EPIC-Norfolk cohort was slightly younger (mean age 58.9) than ASPREE, and included those with significant cardiovascular disease, including previous myocardial infarction (11% prevalence in patients with an ACB of > 3). The differential risk profile of the two cohorts may explain why an association with stroke death was not observed in our older but healthier cohort.
Few participants in our study experienced both stroke and dementia (n = 34), suggesting the events observed and analyzed in this study were unlikely to be part of the same disease continuum (i.e., stroke leading to dementia or vice versa) but may share an underlying pathway affected by exposure to anticholinergics. Our finding of increased risk of ischemic stroke, together with the results for possible AD, suggests that, if these associations do share a common pathway, vascular mechanisms may be involved. Hypothetical explanatory mechanisms for this observation include loss of acetylcholine-mediated vasodilation contributing to loss of cerebral autoregulation,45 suppression of parasympathetic heart rate control contributing to tachyarrhythmias that may increase the risk of ischemic stroke,46, 47 adrenergic stimulation leading to drug-induced atrial fibrillation,48 and reduced glucose metabolism and cerebral atrophy.49 Risacher et al.49 demonstrated an association with anticholinergics and changes in brain structure, with those with highest total anticholinergic burden scores demonstrating the most atrophy. With regard to stroke and vascular dementia, others have suggested that inhaled anticholinergics could have pro-arrhythmic and pro-ischemic properties potentially contributing to cerebral ischemia.50, 51 Muscarinic receptors have also been shown to increase immune/inflammatory responses in rat models,52 and although this process has not been demonstrated in humans, it is possible that anticholinergics may contribute to inflammatory responses that increase the risk of stroke and dementia.6, 53, 54 Nonetheless, the mechanisms discussed above are largely speculative and additional studies are needed to understand the underlying mechanism.
Finally, we find that those with high cumulative anticholinergic exposure (i.e., cumulative ACB score of ≥ 3—Table 2) demonstrated similar associations to those exposed to a moderate or highly anticholinergic medication for both dementia and ischemic stroke (Table 3). This suggests that reduction of overall anticholinergic burden is an important clinical target for risk reduction in addition to minimizing specific highly anticholinergic medications.
Strengths and Limitations
A key strength of our study is the prospective design with regular cognitive screening and robust event adjudication, which minimized ascertainment bias. We used a well validated ACB score. We used a large sample of healthy older adults and were able to control for a wide range of demographic, lifestyle, and known risk factors. Reverse causality is a major factor in measuring the association between medications and health outcomes. However, our participants were clinically free of dementia, myocardial infarction, and stroke or TIA at baseline. Furthermore, we screened and excluded participants with a baseline 3MS score that indicated possible early mild cognitive impairment. Overall data quality was high with limited missing data.20
Given the observational design, it is not possible to evaluate causality. This analysis accounted for a wide range of potential confounding variables, but potential for residual confounding remains. Although this population was certified to be free from dementia and major cardiovascular disease at baseline, indication bias cannot be excluded. We did not collect medication dose or length of exposure, nor did we collect non-prescription medications. In our cohort, the total number of participants with polypharmacy was limited. Thus, it is possible that the true associations between anticholinergic exposure and dementia do differ between those with polypharmacy and those without, but we were unable to detect that difference. Finally, although anticholinergic burden remained stable throughout the study for the majority of participants, 25% of participants changed categories and further investigation is required to understand the impact of these transitions on risk of outcomes.
CONCLUSION
We provide evidence that high anticholinergic burden in older adults without pre-existing major cardiovascular disease or cognitive impairment is associated with an increased risk of dementia and ischemic stroke, which may share an underlying vascular pathway. Our findings emphasize the importance of minimizing cumulative anticholinergic burden in health older adults.
References
Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019 May 1;18(5):439–58.
Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019 Jan 1;18(1):88–106.
Lechevallier-Michel N, Molimard M, Dartigues J-F, Fabrigoule C, Fourrier-Réglat A. Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID Study. Br J Clin Pharmacol. 2005 Feb;59(2):143–51.
Carrière I, Fourrier-Reglat A, Dartigues J-F, Rouaud O, Pasquier F, Ritchie K, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009 Jul;169(14):1317–24.
Gamble DT, Clark AB, Luben RN, Wareham NJ, Khaw K-T, Myint PK. Baseline anticholinergic burden from medications predicts incident fatal and non-fatal stroke in the EPIC-Norfolk general population. Int J Epidemiol. 2018 Apr 1;47(2):625–33.
Tan ECK, Eriksdotter M, Garcia-Ptacek S, Fastbom J, Johnell K. Anticholinergic burden and risk of stroke and death in people with different types of dementia. J Alzheimers Dis JAD. 2018;65(2):589–96.
Richardson K, Fox C, Maidment I, Steel N, Loke YK, Arthur A, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ [Internet]. 2018 Apr 25 [cited 2019 Dec 1];361. Available from: https://www.bmj.com/content/361/bmj.k1315
Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019 Aug 1;179(8):1084–93.
Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015 Mar 1;175(3):401–7.
Justin BN, Turek M, Hakim AM. Heart disease as a risk factor for dementia. Clin Epidemiol. 2013 Apr 26;5:135–45.
Haring B, Leng X, Robinson J, Johnson KC, Jackson RD, Beyth R, et al. Cardiovascular disease and cognitive decline in postmenopausal women: results from the Women’s Health Initiative Memory Study. J Am Heart Assoc. 2013 Dec 18;2(6):e000369.
Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013 Nov 1;36(2):555–64.
Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987 Aug;48(8):314–8.
Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini-Mental State examination (3MS) as a screen for dementia. Can J Psychiatry Rev Can Psychiatr. 2001 Aug;46(6):506–10.
McNeil JJ, Woods RL, Nelson MR, Murray AM, Reid CM, Kirpach B, et al. Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) Study. J Gerontol A Biol Sci Med Sci. 2017 Oct;72(11):1586–93.
McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018 Sep 16;0(0):null.
McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018 Oct 18;379(16):1519–28.
McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018 Oct 18;379(16):1509–18.
Lockery JE, Collyer TA, Abhayaratna WP, Fitzgerald SM, McNeil JJ, Nelson MR, et al. Recruiting general practice patients for large clinical trials: lessons from the Aspirin in Reducing Events in the Elderly (ASPREE) study. Med J Aust. 2019 Jan 28;210(4):168–73.
Lockery JE, Collyer TA, Reid CM, Ernst ME, Gilbertson D, Hay N, et al. Overcoming challenges to data quality in the ASPREE clinical trial. Trials. 2019 Dec 9;20(1):686.
WHOCC - ATC/DDD Index [Internet]. [cited 2019 Oct 3]. Available from: https://www.whocc.no/atc_ddd_index/
Lockery JE, Rigby J, Collyer TA, Stewart AC, Woods RL, McNeil JJ, et al. Optimising medication data collection in a large-scale clinical trial. PLOS ONE. 2019 Dec 27;14(12):e0226868.
Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008 Jun 1;4(3):311–20.
Campbell N, Boustani M, Limbil T, Ott C, Fox C, Maidment I, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225–33.
Fox C, Richardson K, Maidment ID, Savva GM, Matthews FE, Smithard D, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011 Aug;59(8):1477–83.
Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008 Mar 10;168(5):508–13.
Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006 Dec;46(12):1481–6.
Ryan J, Woods RL, Britt C, Murray AM, Shah RC, Reid CM, et al. Normative performance of healthy older individuals on the Modified Mini-Mental State (3MS) examination according to ethno-racial group, gender, age, and education level. Clin Neuropsychol [Internet]. 2019 May 19 [cited 2019 Dec 23];33(4). Available from: https://research.monash.edu/en/publications/normative-performance-of-healthy-older-individuals-on-the-modifie
Ryan J, Storey E, Murray A, Woods RL, Wolfe R, Reid CM, et al. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline.
Tombaugh TN. Test-retest reliable coefficients and 5-year change scores for the MMSE and 3MS. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol. 2005 Jun;20(4):485–503.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Washington, DC: American Psychiatric Association; 1994.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2011 May;7(3):263–9.
Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989 Oct;20(10):1407–31.
Wolfe R, Murray AM, Woods RL, Kirpach B, Gilbertson D, Shah RC, et al. The ASPirin in Reducing Events in the Elderly trial: statistical analysis plan. Int J Stroke Off J Int Stroke Soc. 2018 Apr;13(3):335–8.
Brant R. Assessing proportionality in the proportional odds model for ordinal logistic regression. Biometrics. 1990 Dec;46(4):1171–8.
Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977 Jun 1;1(3):385–401.
Joung K, Shin J-Y, Cho S. Features of anticholinergic prescriptions and predictors of high use in the elderly: population-based study. Pharmacoepidemiol Drug Saf. 2019 Dec 1;28(12):1591–600.
Sevilla-Sánchez D, Molist-Brunet N, González-Bueno J, Solà-Bonada N, Espaulella-Panicot J, Codina-Jané C. Prevalence, risk factors and adverse outcomes of anticholinergic burden in patients with advanced chronic conditions at hospital admission. Geriatr Gerontol Int. 2018 Aug;18(8):1159–65.
Kolanowski A, Fick DM, Campbell J, Litaker M, Boustani M. A Preliminary study of anticholinergic burden and relationship to a quality of life indicator, engagement in activities, in nursing home residents with dementia. J Am Med Dir Assoc. 2009 May 1;10(4):252–7.
Landi F, Dell’Aquila G, Collamati A, Martone AM, Zuliani G, Gasperini B, et al. Anticholinergic drug use and negative outcomes among the frail elderly population living in a nursing home. J Am Med Dir Assoc. 2014 Nov 1;15(11):825–9.
Chatterjee S, Mehta S, Sherer JT, Aparasu RR. Prevalence and predictors of anticholinergic medication use in elderly nursing home residents with dementia. Drugs Aging. 2010 Dec 1;27(12):987–97.
Amieva H, Le Goff M, Millet X, Orgogozo JM, Pérès K, Barberger-Gateau P, et al. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. 2008 Nov;64(5):492–8.
Singh-Manoux A, Dugravot A, Fournier A, Abell J, Ebmeier K, Kivimäki M, et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry. 2017 01;74(7):712–8.
Lang L, Clifford A, Wei L, Zhang D, Leung D, Augustine G, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open [Internet]. 2017 Feb 3 [cited 2019 Dec 1];7(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5293981/
Roher AE, Kuo YM, Potter PE, Emmerling MR, Durham RA, Walker DG, et al. Cortical cholinergic denervation elicits vascular A beta deposition. Ann N Y Acad Sci. 2000 Apr;903:366–73.
van Vlymen JM, Parlow JL. The effects of reversal of neuromuscular blockade on autonomic control in the perioperative period. Anesth Analg. 1997 Jan;84(1):148–54.
Mathew J, Hunsberger S, Fleg J, Sherry FM, Williford W, Yusuf S. Incidence, predictive factors, and prognostic significance of supraventricular tachyarrhythmias in congestive heart failure. CHEST. 2000 Oct 1;118(4):914–22.
Hooft CS van der, Heeringa J, Herpen G van, Kors JA, Kingma JH, Stricker BHC. Drug-induced atrial fibrillation. J Am Coll Cardiol. 2004 Dec 7;44(11):2117–24.
Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016 Jun 1;73(6):721–32.
Singh S, Loke YK, Enright P, Furberg CD. Pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications. Thorax. 2013 Jan 1;68(1):114–6.
Yuan W, Nie S, Wang H, Xu Q, Jia N. Anticholinergics aggravate the imbalance of the autonomic nervous system in stable chronic obstructive pulmonary disease. BMC Pulm Med [Internet]. 2019 May 9 [cited 2019 Dec 1];19. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6506959/
Razani-Boroujerdi S, Behl M, Hahn FF, Pena-Philippides JC, Hutt J, Sopori ML. Role of muscarinic receptors in the regulation of immune and inflammatory responses. J Neuroimmunol. 2008 Feb;194(1–2):83–8.
McGeer PL, Rogers J, McGeer EG. Inflammation, antiinflammatory agents, and Alzheimer’s disease: the last 22 years. J Alzheimers Dis JAD. 2016 04;54(3):853–7.
Guerriero F, Sgarlata C, Francis M, Maurizi N, Faragli A, Perna S, et al. Neuroinflammation, immune system and Alzheimer disease: searching for the missing link. Aging Clin Exp Res. 2017 Oct;29(5):821–31.
Smith A, Services (Firm) WP. Symbol digit modalities test : manual [Internet]. Los Angeles, Calif. : Western Psychological Corporation; 2002 [cited 2018 Nov 1]. Available from: https://trove.nla.gov.au/version/42656069
Ross TP. The reliability of cluster and switch scores for the Controlled Oral Word Association Test. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol. 2003 Mar;18(2):153–64.
Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998 Feb 1;12(1):43–55.
Acknowledgments
A. G. Bayer provided aspirin and matching placebo. The authors acknowledge the dedicated and skilled staff in Australia and the USA for the conduct of the trial. The authors also are most grateful to the ASPREE participants, who so willingly volunteered for this study, and the general practitioners and medical clinics who support the participants in the ASPREE study. Trial Registration: International Standard Randomized Controlled Trial Number Register (ISRCTN83772183) and clinicaltrials.gov (NCT01038583).
Funding
The work was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (grant U01AG029824); the National Health and Medical Research Council of Australia (grant numbers 334047 and 1127060); Monash University (Australia); and the Victorian Cancer Agency (Australia).
Author information
Authors and Affiliations
Consortia
Contributions
JEL and MEE were responsible for the concept and design of work. JEL was responsible for data analysis and interpretation of data and preparation of the first draft of the manuscript. JCB completed the data analysis, and contributed to interpretation of data, drafting, and critical revision of the manuscript. JR, ACS, RLW, TC, GCC, AM, JDR, RS, ES, SW, RW and CMR, and MEE interpreted the data, and critically reviewed and revised the manuscript. All authors approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Ethics Approvals
Ethics approval was obtained for all study sites. In the USA, ethics approval was granted by the relevant Institutional Review Board at study sites. In Australia, primary ethics approval was granted by Monash University Human Research Ethics Committee [CF07/3730 - 2006/745MC]. All participants provided informed consent.
Data Availability
The datasets used and/or analyzed for this publication are available via the ASPREE Principal Investigators. Requests for data access can be directed to aspree.ams@monash.edu
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Lockery, J.E., Broder, J.C., Ryan, J. et al. A Cohort Study of Anticholinergic Medication Burden and Incident Dementia and Stroke in Older Adults. J GEN INTERN MED 36, 1629–1637 (2021). https://doi.org/10.1007/s11606-020-06550-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-020-06550-2