Abstract
Background
Diabetes mellitus (DM) is known to be a risk factor for postoperative infectious complications (PICs). However, the significance of postoperative hyperglycemia in non-DM cases has not been well investigated. We sought to establish whether postoperative hyperglycemia is associated with PICs and survival among patients with esophageal cancer, with a focus on non-DM cases.
Methods
A total of 430 patients who underwent subtotal esophagectomy for esophageal cancer between 2014 and 2018 were enrolled. Postoperative blood glucose was measured by arterial blood gas test every 8 h from postoperative day (POD) 1 to POD4. The association between hyperglycemia (mean ≥ 200 mg/dl) and PICs or long-term outcomes on each POD was investigated.
Results
There were 53 DM and 377 non-DM cases. PICs occurred in 127 patients. In the multivariate analysis of all cases, PICs were associated with hyperglycemia on POD1 or -2 (odds ratio [OR] = 1.69, 95% CI, 1.05–2.73, P = 0.031 for POD1; OR = 2.55, 95% CI, 1.10–5.93, P = 0.029 for POD 2). Among non-DM cases, the association was more evident, and persisted until POD4 (OR = 1.94, 95% CI, 1.16–3.24, P = 0.012 for POD1; OR = 3.68, 95% CI, 1.28–10.6, P = 0.016 for POD2; OR = 3.07, 95% CI, 1.11–8.51, P = 0.031 for POD4). Survival analyses limited to R0 cases revealed hyperglycemia on POD2 as an independent prognostic factor in all cases (N = 412) [hazard ratio (HR) = 2.61, 95%CI, 1.21–5.63, P = 0.014], with the prognostic impact more evident among non-DM cases (N = 360) (HR = 4.38, 95% CI, 1.82–10.57, P = 0.0010).
Conclusion
Postoperative hyperglycemia is associated with PICs and worse survival after esophagectomy, particularly in patients without DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is an aggressive, malignant tumor, and esophagectomy is the mainstay for curative treatment. However, esophagectomy is associated with a high incidence of postoperative infectious complications (PICs),1, 2 which adversely affect patient postoperative outcomes.3, 4
In clinical practice, patients diagnosed with type 2 diabetes mellitus (DM) frequently experience postoperative hyperglycemia and PICs after esophagectomy.5,6,7 Surgical stress often increases insulin resistance through an elevated production of inflammatory cytokines, resulting in postoperative hyperglycemia.8 This, in turn, causes a functional impairment of neutrophils, bactericidal activity, chemotaxis and phagocytosis,9,10,11 and hyperglycemia itself triggers the activation of inflammatory circuits.
Although postoperative hyperglycemia occurs to various degrees regardless of the presence or absence of DM, it is unclear whether postoperative hyperglycemia in non-DM cases is associated with the PICs or affects prognosis. In this study, we sought to investigate whether postoperative hyperglycemia was associated with the incidence of PICs or long-term outcomes after esophagectomy, with a particular focus on non-DM patients.
Methods
Patients
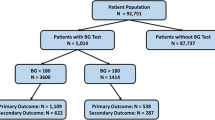
Initially, 445 patients with esophageal cancer, who underwent subtotal esophagectomy with reconstruction with a gastric conduit at the Cancer Institute Hospital between 2014 and 2018, were included in this study. Patients who underwent salvage surgery after chemoradiotherapy (N = 15) were excluded because such cases are at high risk of PICs. As a result, 430 cases were eligible for this study.
Our study was conducted in accordance with the Declaration of Helsinki and the national ethical guidelines of Japan and approved by the human ethics review committee (Institutional Review Board number 2014–1028 for the Cancer Institute Hospital). Informed consent was waived due to the retrospective nature of this study and the analyses used anonymous clinical data.
DM and hyperglycemia
DM was diagnosed in patients who had a medical history of DM or presented with HbA1c ≥ 6.5% at diagnosis of esophageal cancer, as defined by the National Glycohemoglobin Standardization Program.12, 13 Postoperative blood glucose was measured by arterial blood gas test, which was taken at the intensive care unit (ICU) every 8 h over postoperative days (POD) 1 to POD4. When blood glucose levels were 200 mg/dl or higher, insulin therapy using a sliding scale was administered. Hyperglycemia on each POD was defined as the mean blood glucose level ≥ 200 mg/dl on that day.
Data collection
Medical records were retrospectively reviewed to obtain the following clinical information: age at surgery; body mass index at surgery; sex; physical status, as defined by the American Society of Anesthesiologists (ASA) (ASA I-VI); clinical and pathological disease stage (cStage and pStage), as defined by the Union for International Cancer Control (UICC) 7th TNM classification of esophageal cancer (I-IV); histological type [adenocarcinoma (AC) and squamous cell carcinoma (SCC)]; preoperative neoadjuvant chemotherapy (presence vs. absence); preoperative HbA1c levels (%); surgical procedure (thoracotomy vs. thoracoscopy); preoperative intravenous administration of methylprednisolone 250 mg (present vs. absent); operative time (min); blood loss (g); postoperative blood glucose level (mg/dl); incidence of PICs (presence vs. absence); and patient survival. PICs were defined as “present” when they appeared during the hospital stay and had a Clavien-Dindo grade ≥ II. In the event that a patient presented with more than one PIC, the first PIC recorded was analyzed in this study.
Procedure and perioperative management
Surgical procedures were performed via thoracoscopy or thoracotomy combined with laparoscopy or laparotomy. A feeding catheter was routinely placed in the jejunum through the gastric conduit or the duodenal bulb. Non-DM patients were intravenously administered with 250 mg methylprednisolone 30 min before surgery. An enteral diet of 5 to 10 kcal/kg/day was commenced within 24 h after surgery and gradually increased to the required energy level (30 to 40 kcal/kg/day) within 5 days, according to the conventional clinical pathway in our hospital. MEIN (Meiji, Japan) or ELENTAL (EA pharma, Japan) was used for non-DM cases and a low carbohydrate enteral nutrition (Glucerna, Abbott, Japan) was used for DM cases to minimize blood sugar spikes after surgery.
Endpoints and statistical analysis
The primary endpoint was the identification of an association between postoperative hyperglycemia and the incidence of PICs, and which POD they appeared. As a secondary endpoint, the impact of postoperative hyperglycemia on overall survival (OS) was evaluated among patients who underwent R0 resection.
All P-values were two-sided. Univariate and multivariate analyses were employed to investigate the relation between clinical characteristics and the incidence of PICs. In the univariate analysis, categorical data were assessed using Chi-squared or Fisher’s exact tests, whereas continuous data were assessed using Student’s t-test or the Mann–Whitney U-test. In the multivariate analysis, a logistic regression was performed separately analyses. In survival analysis, the Kaplan–Meier method was used to estimate overall survival distribution, and the log-rank test was performed to compare the overall survival probability according to postoperative hyperglycemia status. The Cox proportional hazards model was used in the multivariate overall survival analyses.
The multivariate model initially included the following clinicopathological variables (without missing information): sex (male vs. female), age (< 75 vs. ≥ 75 years old), BMI (< 25 vs. ≥ 25 kg/m2), physical status (ASA I vs. II-VI), clinical and pathological disease stage (cStage I-II vs. III or IV, pStage 0-II vs. III-IV), histological subtype (AC vs. SCC), neoadjuvant chemotherapy (presence vs. absence), surgical procedure (thoracotomy vs. thoracoscopy), operative time (median split, 550 < vs. ≥ 550 min), blood loss volume (median split, 130 < vs. ≥ 130 g), and the incidence of PICs. A backward elimination was performed with a threshold of P = 0.10 to avoid overfitting in the multivariate analyses. All statistical analyses were performed using the statistical software “R (ver.3.5.2)”.
Results
The baseline characteristics of the eligible 430 patients are shown in Table 1. There were 53 DM and 377 non-DM patients. R0 resection was achieved in 412 cases (95.8%). DM cases more frequently experienced hyperglycemia through POD1-4 as compared to non-DM cases (Supplementary Fig. 1).
PICs occurred in 127/430 cases (29.5%) including 112/377 cases of non-DM (29.7%) and 15/53 cases with DM (28.3%). Among the PICs observed, pneumonia was most common (51.1%), followed by anastomotic leakage (21.2%), surgical site infection (14.1%), abdominal abscess (2.4%), pyothorax (2.4%), mediastinitis (1.6%), pancreatic fistula (1.6%), liver abscess (0.8%), cholangitis (0.8%), catheter-related bloodstream infection (0.8%), infections endocarditis (0.8%), bacteremia (0.8%), and infection from unknown focus (1.6%) (Table 2). These descriptors are shown according to DM status in Table 2.
PIC timing of occurrence in all cases showed a bimodal distribution, with the first and second peaks at POD3 and POD7, respectively (Fig. 1A). Among the 127 cases with PICs, 38 cases (29.9%) experienced infectious events early, during POD1-4 [two patients at POD1, seven patients at POD2, 16 patients at POD3, and 13 patients at POD4]. Pneumoniae was the most frequent PIC on POD3. These trends were the same when the data was split for observations in non-DM cases only (Fig. 1B). Comparatively, in DM cases, PICs most frequently occurred on POD9; albeit the number of events was limited (Supplementary Fig. 2).
Incidence of postoperative infectious complications (PICs) detected during the hospital stay and recorded on a specific postoperative day (POD). a A bimodal distribution of infections (N = 127) in all cases (including DM and non-DM cases), with the first peak at POD3 and the second one at POD7. b Distribution limited to non-DM cases (N = 112). DM, diabetes mellitus
Association between hyperglycemia and PICs on each POD
The association between mean hyperglycemia and PIC incidence on each POD was examined in all cases (including DM and non-DM cases) and then separately for non-DM cases. In the univariate analysis of all cases (N = 430), the presence of PICs was significantly associated with hyperglycemia on POD1 [univariate OR = 1.88, 95% confidence interval (CI), 1.17–3.00, P = 0.0068]. The multivariate analysis revealed a significant positive relation between PICs and hyperglycemia on POD1 and -2 (multivariate OR = 1.69, 95%CI, 1.05–2.73, P = 0.031 for POD1; multivariate OR = 2.55, 95%CI, 1.10–5.93, P = 0.029 for POD2; Table 3a). When limited to non-DM cases (N = 377), the association was more evident, with significant associations observed on POD1, -2, and -4 in the multivariate analyses (multivariate OR = 1.94, 95%CI, 1.16–3.24, P = 0.012 for POD1; multivariate OR = 3.68, 95%CI, 1.28–10.6, P = 0.016 for POD2; multivariate OR = 3.07, 95%CI, 1.11–8.51, P = 0.031 for POD 4; Table 3b). Because surgical stress is one of the well-known causes of postoperative hyperglycemia, we also examined the association between operation time, blood loss volume and postoperative hyperglycemia in non-DM cases. The non-DM patients with hyperglycemia on POD1 significantly longer operative time, as compared to those without (P = 0.017; 564 ± 84 min in the patients with hyperglycemia, and 534 ± 98 min in the patients without hyperglycemia). Such association was limited to POD1. Similar association was observed between hyperglycemia on POD1 and blood loss volume in non-DM cases, however, it did not reach a statistical significance (P = 0.059; 150 ± 247 min in the patients with hyperglycemia, and 130 ± 178 min in the patients without hyperglycemia).
Overall survival impact of postoperative hyperglycemia in R0 resected cases
We also tested the prognostic impact of postoperative hyperglycemia among R0 resected cases (N = 412) in univariate and multivariate analysis. The median follow-up for these 412 cases was 919 days. There was no surgery-related death during the research period, in spite that 14 patients were categorized into ASA class III.
Kaplan–Meier curves according to hyperglycemic status at POD1, 2, 3, and 4 for all cases are provided in Supplementary Fig. 3. The 3-year overall survival rates of those with and without hyperglycemia were 75.1% and 87.2% on POD1 (P = 0.0050), 68.2% and 84.9% on POD2 (P = 0.0017), 74.2% and 84.5% on POD3 (P = 0.023), and 83.8% and 84.0% on POD4 (P = 0.25), respectively (Supplementary Fig. 3). Limiting to non-DM cases, the 3-year overall survival rates with and without hyperglycemia were 76.8% and 87.2% on POD1 (P = 0.018), 70.1% and 85.5% on POD2 (P = 0.0022), 88.2% and 84.4% on POD3 (P = 0.80), and 93.3% and 84.5% on POD4 (P = 0.96), respectively (Supplementary Fig. 4).
In multivariate analysis utilizing all cases, hyperglycemia on POD2 was an independent prognostic factor of worse overall survival in the multivariate analysis [multivariate hazard ratio (HR) = 2.61, 95%CI, 1.21–5.63, P = 0.014, Table 4a]. When focusing on the non-DM cases with R0 resection (N = 360), the prognostic impact of hyperglycemia at POD2 was more evident (multivariate HR = 4.38, 95%CI 1.82–10.57, P = 0.0010 for POD2, Table 4b). Similar trends were observed for POD1 in the analyses of all cases and non-DM only cases (Table 4a, b); albeit these findings did not reach statistical significance in the multivariate analyses.
Discussion
Esophageal cancer surgery is an invasive treatment and frequently causes varying degrees of postoperative hyperglycemia. DM is widely accepted to be associated with the presentation of infectious complications after esophagectomy.5,6,7 However, the significance of such complications among patients without DM has not been well examined. This study revealed hyperglycemia on POD1, -2, or -4, defined as the mean blood glucose level ≥ 200 mg/dl on that day, to be an independent risk factor for PICs. In particular, evidence of hyperglycemia on POD2 was determined as being an independent prognostic factor among non-DM esophageal cancer patients who underwent esophagectomy.
Previous reports have investigated the association between early-postoperative hyperglycemia and PICs in patients with esophageal cancer; however, the results from these studies are controversial.14, 15 Specifically, Vriesendorp et al. reported that early postoperative hyperglycemia was not a risk factor for PICs in patients undergoing esophagectomy (N = 151), the authors divided the patients into four groups according to quartiles of the mean blood glucose levels during the 48 h after surgery, and analyzed the quartiles as ordinal variables.14 The authors observed a higher PIC frequency in the 3rd and 4th quartiles (higher blood glucose quartiles) than in the 1st and 2nd ones (lower quartiles); although there was no statistical significance. A positive association may have been observed if the patients were dichotomized as hyperglycemia “present” or “absent” at the specific blood glucose level, as in our current study. Another study by Ito et al. examined the mean blood glucose levels on POD1, -3, -5, and -7 after esophagectomy (N = 109) in relation to PICs, with subgroup analyses performed according to three types of glucose tolerance levels: i.e., DM, pre-DM, and normal, as defined by the American Diabetes Association criteria.15 Among the normal cases, PICs were significantly associated with the relatively higher blood glucose levels on POD3 or POD5, but, in the multivariate analysis, adjustments for the clinical factors could not be undertaken due to a limited number of cases. Both reports examined relative blood glucose levels rather than setting a specific cutoff value for hyperglycemia; such a strategy is less useful in clinical postoperative patient management. To date, in the field of general surgery, perioperative hyperglycemia is usually examined in relation to PICs by setting a specific cutoff value of hyperglycemia at 125, 180 or 200 mg/dl.16,17,18,19,20,21,22 Consistent with our findings, some studies have identified blood glucose levels ≥ 200 mg/dl as being associated with a high risk of PICs.17, 20, 22 In the present study, we observed mean blood glucose levels ≥ 200 mg/dl on early POD1 or -2 as being a significant risk factor of PICs, with the association more evident among non-DM cases.
In this study, despite that postoperative hyperglycemia less frequently occurred in non-DM patients than DM cases on POD1-4 (Supplementary Fig. 1), we observed that the incidence of PIC was similar regardless of the presence or absence of DM. Positive association between longer operative time and hyperglycemia on POD1 in non-DM patients supported that surgical stress causes hyperglycemia, resulting in higher incidence rate of PICs. However, we observed such causal relation was limited to POD1 in this study, and the reason why hyperglycemia on POD2 was the most significant relation to PIC still remains unclear. Further prospective interventional or experimental studies are warranted, particularly studies that control for blood glucose through continuous glucose monitoring or provision of low carbohydrate enteral nutrition during the early PODs, focusing on non-DM patients who experienced severe surgical stress.
DM is a dismal prognostic factor for various types of cancer. 23,24,25 In the present study, we showed that hyperglycemia onset during the early PODs was associated with worse prognosis among non-DM cases. Fiorillo et al., also reported worse oncologic-related mortality among non-diabetic gastric cancer patients who experienced hyperglycemia (defined as blood glucose levels ≥ 140 mg/dl) within 72 h after gastrectomy.26 Surgical stress causes an excess production of hyperglycemic hormones and insulin resistance, which, in turn, triggers the production of inflammatory cytokines, such as TNF-α and IL-6. These cytokines impair anti-tumor immune function, thereby promoting micrometastasis and leading to worse outcomes.27,28,29,30 Further basic research is necessary to clarify the mechanism underlying the link between early postoperative hyperglycemia and worse overall survival.
There are some potential limitations in our work. First, it is plausible that our observations might have been influenced by postoperative hyperglycemia caused by a preceding yet inapparent incidence of PICs, and thus the cause-and-effect of the findings remains unclear. However, more than 70% of PICs appeared on POD5 or after, and we assume that these results did not significantly impact our observations. Another limitation of this study is that it is a single-center, retrospective study, utilizing intermittent blood glucose monitoring. Furthermore, due to the limited statistical power of DM cases (N = 53), their dismal effect of hyperglycemia on PICs could not be compared to that in non-DM cases. Nonetheless, the results of this study are based on the largest cohort to date of patients treated with esophagectomy using standardized monitoring of postoperative blood glucose.
In conclusion, hyperglycemia within the early postoperative period after esophagectomy among patients with esophageal cancer is associated with a high risk of PICs and poor long-term prognosis, particularly among non-DM cases.
References
Ohkura Y, Miyata H, Konno H, Udagawa H, Ueno M, Shindoh J, et al. Development of a model predicting the risk of eight major postoperative complications after esophagectomy based on 10 826 cases in the Japan National Clinical Database. J Surg Oncol. 2019.
Takeuchi H, Miyata H, Gotoh M, Kitagawa Y, Baba H, Kimura W, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg. 2014;260:259-66.
Nathan H, Yin H, Wong SL. Postoperative Complications and Long-Term Survival After Complex Cancer Resection. Ann Surg Oncol. 2017;24:638-44.
Yamamoto M, Shimokawa M, Yoshida D, Yamaguchi S, Ohta M, Egashira A, et al. The survival impact of postoperative complications after curative resection in patients with esophageal squamous cell carcinoma: propensity score-matching analysis. J Cancer Res Clin Oncol. 2020;146:1351-60.
Li SJ, Wang ZQ, Li YJ, Fan J, Zhang WB, Che GW, et al. Diabetes mellitus and risk of anastomotic leakage after esophagectomy: a systematic review and meta-analysis. Dis Esophagus. 2017;30:1-12.
Okamura A, Watanabe M, Imamura Y, Hayami M, Yamashita K, Kurogochi T, et al. Glycemic Status and Prognosis of Patients with Squamous Cell Carcinoma of the Esophagus. World J Surg. 2017;41:2591-7.
van Kooten RT, Voeten DM, Steyerberg EW, Hartgrink HH, van Berge Henegouwen MI, van Hillegersberg R, et al. Patient-Related Prognostic Factors for Anastomotic Leakage, Major Complications, and Short-Term Mortality Following Esophagectomy for Cancer: A Systematic Review and Meta-Analyses. Ann Surg Oncol. 2022;29:1358-73.
May AK, Kauffmann RM, Collier BR. The place for glycemic control in the surgical patient. Surg Infect (Larchmt). 2011;12:405-18.
Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067-72.
McManus LM, Bloodworth RC, Prihoda TJ, Blodgett JL, Pinckard RN. Agonist-dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia. J Leukoc Biol. 2001;70:395-404.
Turina M, Fry DE, Polk HC, Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33:1624-33.
International Expert C. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-34.
Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE, et al. The national glycohemoglobin standardization program: a five-year progress report. Clin Chem. 2001;47:1985-92.
Vriesendorp TM, DeVries JH, Hulscher JB, Holleman F, van Lanschot JJ, Hoekstra JB. Early postoperative hyperglycaemia is not a risk factor for infectious complications and prolonged in-hospital stay in patients undergoing oesophagectomy: a retrospective analysis of a prospective trial. Crit Care. 2004;8:R437-42.
Ito N, Iwaya T, Ikeda K, Kimura Y, Akiyama Y, Konosu M, et al. Hyperglycemia 3 days after esophageal cancer surgery is associated with an increased risk of postoperative infection. J Gastrointest Surg. 2014;18:1547-56.
Chen EB, Nooromid MJ, Helenowski IB, Soper NJ, Halverson AL. The relationship of preoperative versus postoperative hyperglycemia on clinical outcomes after elective colorectal surgery. Surgery. 2019;166:655-62.
Kiran RP, Turina M, Hammel J, Fazio V. The clinical significance of an elevated postoperative glucose value in nondiabetic patients after colorectal surgery: evidence for the need for tight glucose control? Ann Surg. 2013;258:599-604; discussion -5.
Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, Farrokhi ET, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261:97-103.
Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257:8-14.
Meister KM, Hufford T, Tu C, Khorgami Z, Schauer PR, Brethauer SA, et al. Clinical significance of perioperative hyperglycemia in bariatric surgery: evidence for better perioperative glucose management. Surg Obes Relat Dis. 2018;14:1725-31.
Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Tokumaru T, Iiyama T, et al. Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care. 2014;37:1516-24.
Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, Zinner M, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248:585-91.
Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. Jama. 2008;300:2754-64.
Cai H, Xu Z, Xu T, Yu B, Zou Q. Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: a meta-analysis of 11 cohort studies. Diabetes Metab Res Rev. 2015;31:336-43.
Lee J, Giovannucci E, Jeon JY. Diabetes and mortality in patients with prostate cancer: a meta-analysis. Springerplus. 2016;5:1548.
Fiorillo C, Quero G, Laterza V, Mascagni P, Longo F, Menghi R, et al. Postoperative hyperglycemia affects survival after gastrectomy for cancer: A single-center analysis using propensity score matching. Surgery. 2020;167:815-20.
Demicheli R, Retsky MW, Hrushesky WJ, Baum M. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat Clin Pract Oncol. 2007;4:699-710.
Forget P, Simonet O, De Kock M. Cancer surgery induces inflammation, immunosuppression and neo-angiogenesis, but is it influenced by analgesics? F1000Res. 2013;2:102.
Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2004;164:2005-11.
Smiley D, Umpierrez GE. Management of hyperglycemia in hospitalized patients. Ann N Y Acad Sci. 2010;1212:1-11.
Acknowledgements
We would like to express our sincere appreciation for data acquisition to Ikumi Haraguchi (Cancer Institute).
Funding
There was no funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hori, S., Imamura, Y., Kanie, Y. et al. Early postoperative hyperglycemia as a predictor of postoperative infectious complications and overall survival in non-diabetic patients with esophageal cancer. J Gastrointest Surg 27, 2743–2751 (2023). https://doi.org/10.1007/s11605-023-05869-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-023-05869-5