Abstract
Background
Segmental resections of the duodenum are uncommonly performed and are technically challenging due to intimate relationships with the biliary tree, pancreas, and superior mesenteric vessels. The objective of this study was to assess indications, operative strategy, and outcomes of duodenal resections and to advocate that this form of resection deserves its own unique Current Procedural Terminology (CPT) and Relative Value Unit (RVU) structure.
Methods
Patients undergoing isolated and partial duodenal resection from 2008-2023 at University of Tennessee Health Science Center affiliated hospitals were retrospectively reviewed. Factors examined included clinical presentation, diagnostic evaluation, operative time, and technique, 90-day morbidity and mortality, and pathologic and survival outcomes.
Results
Thirty-one patients were identified with majority female and a median age of 61. Diagnostic studies included computed tomography and upper (including push) endoscopy. Reconstruction most often involved side-to-side duodenojejunostomy following distal duodenal resection. Intraoperative evaluation (IOE) of the biliary tree was utilized to assess and protect pancreaticobiliary structures in eleven patients. Median operative time was 206 min, increasing to 236 min when IOE was necessary. Procedure-related morbidity was 23% with one 90-day mortality. Median postoperative length of stay was 9 days. Pathology included benign adenoma, adenocarcinoma, GIST, neuroendocrine neoplasms, and erosive metastatic deposit.
Conclusion
Duodenal resections can be effectively employed to safely address diverse pathologies. These procedures are characterized by long operative times, extended hospital stays, and an incidence of postoperative complications that mimics that of pancreatic resection. This work highlights the need for modification to the CPT system to accurately define these distinct procedures for future research endeavors and development of a more accurate valuation unit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Segmental resections of the duodenum are uncommonly performed and have been infrequently characterized in surgical literature. Resections can involve the proximal or distal 2nd portion (D2), as well as the 3rd (D3) and 4th portions (D4) and are technically challenging due to intimate relationships with the biliary tree, the pancreas and the superior mesenteric artery and vein. Protection of vital structures and reconstruction following surgical resection requires deft judgment and adept surgical technique. Surgical strategies, morbidity and mortality, and postoperative outcomes have been uncommonly reported.
Pathology amendable to segmental duodenal resections (DR) include premalignant polyps, adenocarcinoma, gastrointestinal stromal tumors (GIST), and carcinoid tumors. Prior studies regarding these neoplasms have demonstrated that, in the appropriate setting, oncologic outcomes of isolated duodenal resections compare favorably to those with combined pancreatic resections and have created expanded opportunities to utilize this form of resection.1,2,3,4,5,6,7
While duodenal resections are inherently more cumbersome to perform than resections of the jejunum and ileum, the value has unfortunately not been captured accordingly, as Current Procedural Terminology (CPT®) coding is limited to “enterectomy with anastomosis” or 44120 and its associated Relative Value Unit (RVU). Furthermore, research endeavors relative to duodenal resection are encumbered by this shortfall. Thus, it is imperative to establish a specialized CPT and RVU structure designed for segmental duodenal resection in concordance with the complexity involved. The purpose of this study was to assess indications, pathologies, operative strategies, and outcomes of these uncommon resections.
Methods
Patients undergoing DR from 2008 to 2023 by 5 surgeons at the University of Tennessee Health Science Center (UTHSC) affiliated hospitals and Baptist Memorial Medical Center were retrospectively reviewed. Patients were excluded if DR was performed in the traumatic setting, for ulcer disease, or as a wedge resection. Demographic data, clinical history, operative details, and histopathological reports were recorded. The utilization of endoscopic evaluation including esophagogastroduodenoscopy (EGD), push enteroscopy, and endoscopic ultrasound (EUS) was noted. Intraoperative variables included operative time, blood loss, extent of resection, type of reconstruction, and/or drainage procedure and intraoperative interrogation of the biliary tree as part of the surgical strategy. Postoperative outcomes including pathology, hospital length of stay, 90-day morbidity and mortality, and 30-day readmission were captured. Institutional review board (IRB) approval was obtained from each hospital system where deidentified data was collected.
Results
Thirty-one patients underwent isolated or partial duodenal resection during the study period. The median age and BMI were 61 (IQR 55.5–70) and 27.9 (IQR 17.2–30.7), respectively. Thirteen (42%) were male. Common presentations included weight loss, hematochezia, and abdominal pain. Four of the 8 patients presenting with hematochezia were on anti-platelet or anti-coagulant therapy. Four (13%) had incidental findings on CT imaging that lead to further investigation and intervention.
All patients underwent endoscopic evaluation prior to resection including 4 patients that required push enteroscopy for definitive localization and diagnosis of lesions in D3 and 4 that were not identified on upper endoscopy. D3 was the most common location of underlying pathology (N = 14, 45.2%). Five patients had EUS for staging purposes. Findings on CT imaging included duodenal mass (17/25, 68%), duodenal thickening/stricture (4/25, 16%), or evidence of metastatic disease (3/25, 12%) (Table 1).
Of the 31 patients who underwent DR, 7 had concomitant partial gastrectomy for D1 or proximal D2 disease with subsequent duodenal closure and gastrojejunostomy (GJ) anastomosis. Twenty-four patients had distal duodenal resection. Twenty-one (67.8%) of these distal resections were reconstructed by duodenojejunostomy (DJ) performed with side-to-side handsewn anastomosis to the lateral 2nd portion of the duodenum with careful attention to the ampulla of Vater. One patient underwent end-to-side DJ reconstruction. Two patients who underwent distal DR required GJ reconstruction secondary to insufficient jejunal length to perform an adequate DJ anastomosis. These latter 2 patients had a pyloroplasty performed to allow drainage of pancreatobiliary secretions from the 2nd portion of the duodenum. An open approach was utilized for all operations.
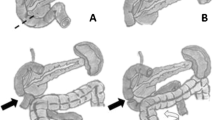
Intraoperative instrumentation of the biliary tree was utilized to assess and protect the location of the ampulla of Vater relative to the site of duodenal transection in 11 (35.5%) patients. The use of a biliary Fogarty catheter was a particularly helpful aid to identify and palpate this critical structure (Fig. 1). One patient had intraoperative EGD to confirm a safe margin from the ampulla before division of the duodenum (Table 2).
Median operative time in patients who underwent duodenal resection was 209 min (IQR 140–237), increasing in those who required intraoperative identification of ampulla of Vater (220 min, IQR 151.5–245.5). Patients who underwent duodenojejunostomy reconstruction had a median operative time of 209 min compared to 190 min for those having gastrojejunostomy. The median time for proximal resections including D1/D2 was 170 min in contrast to operative time of 214.5 min for distal resections involving D2/D3/D4. Average estimated blood loss was 116 mL with only 1 patient requiring intraoperative transfusion.
Seven (22.5%) patients suffered post operative complications, including five Clavien-Dindo class IIIa, one class IIIb, and one class V (Table 3). All complications but one occurred in patients who underwent distal resection. Four patients, including the sole proximal resection, developed intraabdominal abscess managed by percutaneous drainage in 3 and reoperation in 1. One patient had a grade B pancreatic fistula diagnosed on post operative day 16 and required parenteral nutrition and percutaneous drainage. One patient developed a duodenal fistula documented following percutaneous drainage of an abscess that closed spontaneously after 3 months. One patient underwent re-exploration for hemorrhage from a jejunal mesenteric vessel. The sole mortality was a patient with a well-drained intraabdominal abscess that died on postoperative day 28 after continued physiologic decline and cardiac arrest. Of note, this patient had multiple comorbidities including end stage renal disease, diabetes, hypertension, and peripheral vascular disease and underwent resection for an erosive, partially obstructing distal duodenal adenocarcinoma.
The overall median length of stay was 9 days with one 30-day readmission and 1 (3.2%) 90-day mortality. Median length of stay was increased in patients with complications versus those without (14 vs. 8 days).
Final Pathology revealed metastatic ovarian carcinoma in 1 (3.2%), tubulovillous or villous adenomas in 5 (15.6%), adenocarcinoma in 14 (28.1%), GIST in 5 (15.6%), and neuroendocrine neoplasms in 6 (18.8%). All 26 patients with malignant disease underwent R0 resections of the primary lesion.
Four patients (12.5%) underwent resection in the face of metastatic disease due to symptoms related to complete obstruction or clinically ongoing bleeding. Two patients were found to have had miliary disease in the omentum and adjacent jejunum on exploration that was resected en bloc to obtain an R0 resection.
Of the 5 patients with GIST, all had D3/4 resections with a median tumor size of 35mm (IQR 18–44 mm). One patient received neoadjuvant Imatinib prior to resection that resulted in downstage of the primary lesion from 47 to 39 mm. No patients received adjuvant treatment. At a median follow-up length of 17.3 months, 4 of 5 remained disease-free. One patient had hepatic recurrence approximately 1 year from surgery but was lost to follow up thereafter (Table 4).
Of the 14 with duodenal adenocarcinoma, invasive disease was diagnosed in 10 preoperatively. Eleven underwent D3/4 resections with a median tumor size of 42.5 mm (IQR 13.5–71.5 mm). The median number of lymph nodes harvested was 7 (IQR 4.25–10). Two patients received neoadjuvant chemoradiation prior to resection and 8 received adjuvant therapy. At a median follow-up of 42.3 months, seven patients remain disease-free. Seven patients died at a median of 10.3 months. Of these 7, 3 had palliative resections in the face of metastatic disease for obstruction (n=2) and bleeding (n=1) (Table 5).
Of the 6 patients with neuroendocrine neoplasms, 5 occurred in D1 with a median tumor size of 11 mm (IQR 10–12 mm). Of these 6, 1 with clinically significant bleeding had metastatic disease in the liver at the time of resection and died 9.8 months following surgery. One with D4 involvement developed liver metastases 68 months postoperatively and received adjuvant treatment including Everolimus, 177Lu-DOTA-TATE, and Octreotide. The remainder were disease-free at a median follow-up length of 16.4 months from surgery (Table 6).
Discussion
Segmental duodenal resections are uncommon and have received relatively little focus in surgical literature.1, 5,6,7,8,9 Due to its unique anatomical location relative to other vital structures, resection of the duodenum requires increased resources for diagnosis and operative intervention. We identified the need for close perioperative monitoring as procedure-related morbidity can mimic that of pancreatic resection. The time, effort, and complexity of intra- and postoperative management are not captured by current CPT coding suggesting the need for a unique code and value specific to duodenal resection.
DRs require a high degree of anatomic exposure. Technical approaches include mobilization of the hepatic flexure and ligament of Treitz, wide Kocherization, and access of the pancreaticobiliary tree for identification and protection of the ampulla of Vater. For lesions near the distal second and 3rd portion of the duodenum, careful dissection from the pancreatic head and uncinate process is necessary to seal small vascular and pancreatic duct tributaries. Similarly, short vessels to the superior mesenteric artery and vein require diligent attention and hemostasis. The infectious morbidity noted in this series is not dissimilar to that of pancreatic resection and is likely a result of leakage from small pancreatic duct tributaries as well as spillage of pancreaticobiliary secretions during reconstruction. Concomitant distal gastrectomy (rather than pyloric preservation) is necessary to obtain adequate margins and for reconstruction purposes when managing lesions in D1 or proximal D2 as reconstruction is more easily accomplished by gastrojejunostomy. Furthermore, these proximal resections frequently require a tedious dissection down to the head of the pancreas.
Adding to the complexity of these resection, lymph node harvest necessitates careful skeletonization of the superior mesenteric artery and vein within the retroperitoneum. Given the proximity of the pancreaticobiliary structures, constant vigilance and intraoperative identification of the ampulla are of utmost importance. Liberal use of cholangiography and catheter-based localization of the ampulla can be particularly helpful to protect this vital structure and to determine if the ampulla is spared from the neoplastic process If the primary lesion cannot be separated from the ampulla or if there is perceived invasion into the pancreas, pancreatoduodenectomy or distal pancreatectomy should strongly be considered to provide adequate oncologic clearance.
As noted above, the morbidity of these operations was not insignificant and occurred in approximately one-fourth of our patients. Compared to patients who have more distant enteric anastomosis, those who undergo duodenal resection are more likely to experience complications such as duodenal leak, pancreatic fistula, intraabdominal infection, and hemorrhage.4,5,6, 10, 11 These complications result in an extended period of hospitalization and recovery time and have associated morbidity rates comparable to that observed with pancreatic resections.12, 13
One factor influencing disease-free and overall survival in those with duodenal malignancies is obtaining an R0 resection.1,2,3,4, 14,15,16,17 This study reported a 100% negative margin rate for malignant pathologies, which is consistent with findings from similar studies.5,6,7, 10, 18 Nodal retrieval in this series of duodenal resection was acceptable and comparable to other series.7, 10, 18 Finally, while complex, duodenal resections may offer excellent palliation in the face of known metastatic disease particularly when faced with obstruction or clinically relevant bleeding.7, 9, 19
The CPT® is utilized for coding of procedures and reimbursement and refers to a medical code set created and maintained by the American Medical Association. Unfortunately, the available codes (44120–44130) do not accurately reflect the work effort, complexity, and postoperative intensity of care associated with duodenal resections. Adjunct procedures are often necessary with duodenal resection as well as complex reconstructions. The median operative time for both proximal and distal duodenal resection in this series was over 3 h, comparable to studies previously published.1, 4, 8,9,10 In a review of patients with small bowel obstruction with subsequent resection and anastomosis, the average operative time was 148.6 min with an open approach.20 Since duodenal resections do not have a distinct CPT code and RVU, both the surgeon and hospital are not likely reimbursed for the increased time and cost investment. Furthermore, future research endeavors relative to DR may be hindered by a coding structure that does not allow for differentiation between DR and more common and less arduous small bowel resections.
Limitations of this study are largely secondary to the retrospective nature of this work. Data relative to long-term disease-free and overall survival were not often possible. We did not perform a specific comparison between duodenal and more distal small bowel resections. Future study might include a retrospective comparison perhaps utilizing a national database. Finally, all patients in this series had segmental duodenectomy via an open approach. These operations could potentially be performed in a minimally invasive fashion. Given the retroperitoneal location of these resections, the close relationships to the pancreas and mesenteric vasculature and the risk of morbidity not dissimilar to pancreatic resection, conduct, and formal training and mentorship should be limited to experienced centers.21
In conclusion, duodenal resection is a complex and unique procedure that is associated with prolonged operative times and significant procedure-related morbidity. Utilization of adjunct procedures to complete resection near the pancreaticobiliary structures is often necessary. Margin negative resection and lymph node sampling compare favorably to more aggressive en bloc procedures including the pancreas suggesting DR follows safe oncologic guidelines. Revision of current CPT coding to separate DR from other small bowel resections as a distinct entity would more accurately reflect the enhanced effort involved and better capture this unique operation for future research endeavors.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. Raw data that suport the findings of this study are available from the corresponding author, upon reasonable request.
References
Bakaeen FG, Murr MM, Sarr MG, et al. What prognostic factors are important in duodenal adenocarcinoma? Arch Surg. Jun 2000;135(6):635-41; discussion 641-2. https://doi.org/10.1001/archsurg.135.6.635
Cloyd JM, Norton JA, Visser BC, Poultsides GA. Does the extent of resection impact survival for duodenal adenocarcinoma? Analysis of 1,611 cases. Ann Surg Oncol. Feb 2015;22(2):573-80. https://doi.org/10.1245/s10434-014-4020-z
Kaklamanos IG, Bathe OF, Franceschi D, Camarda C, Levi J, Livingstone AS. Extent of resection in the management of duodenal adenocarcinoma. Am J Surg. Jan 2000;179(1):37-41. https://doi.org/10.1016/s0002-9610(99)00269-x
Zhou B, Zhang M, Wu J, Yan S, Zhou J, Zheng S. Pancreaticoduodenectomy versus local resection in the treatment of gastrointestinal stromal tumors of the duodenum. World J Surg Oncol. Aug 2013;11:196. https://doi.org/10.1186/1477-7819-11-196
Blanco-Fernandez G, Rojas-Holguin A, De-Armas-Conde N, Gallarin-Salamanca I, Lopez-Guerra D, Jaen-Torrejimeno I. Side-to-side duodenojejunostomy after resection of third and fourth duodenal portions with pancreatic preservation. Updat Surg. Dec 2020;72(4):1105-1113. https://doi.org/10.1007/s13304-020-00823-5
Dorcaratto D, Heneghan HM, Fiore B, et al. Segmental duodenal resection: indications, surgical techniques and postoperative outcomes. J Gastrointest Surg. Apr 2015;19(4):736-42. https://doi.org/10.1007/s11605-015-2744-0
Mitchell WK, Thomas PF, Zaitoun AM, Brooks AJ, Lobo DN. Pancreas preserving distal duodenectomy: A versatile operation for a range of infra-papillary pathologies. World J Gastroenterol. Jun 21 2017;23(23):4252-4261. https://doi.org/10.3748/wjg.v23.i23.4252
Bourgouin S, Hornez E, Guiramand J, et al. Duodenal gastrointestinal stromal tumors (GISTs): arguments for conservative surgery. J Gastrointest Surg. Mar 2013;17(3):482-7. https://doi.org/10.1007/s11605-012-2075-3
Garcia-Molina FJ, Mateo-Vallejo F, Franco-Osorio Jde D, Esteban-Ramos JL, Rivero-Henandez I. Surgical approach for tumours of the third and fourth part of the duodenum. Distal pancreas-sparing duodenectomy. Int J Surg. Jun 2015;18:143-8. https://doi.org/10.1016/j.ijsu.2015.04.051
Golhar A, Mangla V, Mehrotra S, Lalwani S, Mehta N, Nundy S. Limited distal duodenal resection: Surgical approach and outcomes. A case series. Ann Med Surg (Lond). Jun 2018;30:36-41. https://doi.org/10.1016/j.amsu.2018.04.005
Sakr A, Emile SH, Abdallah E, Thabet W, Khafagy W. Predictive Factors for Small Intestinal and Colonic Anastomotic Leak: a Multivariate Analysis. Indian J Surg. Dec 2017;79(6):555-562. https://doi.org/10.1007/s12262-016-1556-0
Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. Jul 2006;244(1):10-5. https://doi.org/10.1097/01.sla.0000217673.04165.ea
Penumadu P, Barreto SG, Goel M, Shrikhande SV. Pancreatoduodenectomy - preventing complications. Indian J Surg Oncol. Mar 2015;6(1):6-15. https://doi.org/10.1007/s13193-013-0286-z
Kim MJ, Choi SB, Han HJ, et al. Clinicopathological analysis and survival outcome of duodenal adenocarcinoma. Kaohsiung J Med Sci. May 2014;30(5):254-9. https://doi.org/10.1016/j.kjms.2013.12.006
Sun H, Liu Y, Lv L, Li J, Liao X, Gong W. Prognostic Factors and Clinical Characteristics of Duodenal Adenocarcinoma With Survival: A Retrospective Study. Front Oncol 2021;11:795891. https://doi.org/10.3389/fonc.2021.795891
Burch J, Ahmad I. Gastrointestinal Stromal Cancer. StatPearls. 2022.
Chok AY, Koh YX, Ow MY, Allen JC, Jr., Goh BK. A systematic review and meta-analysis comparing pancreaticoduodenectomy versus limited resection for duodenal gastrointestinal stromal tumors. Ann Surg Oncol. Oct 2014;21(11):3429-38. https://doi.org/10.1245/s10434-014-3788-1
Onkendi EO, Boostrom SY, Sarr MG, et al. 15-year experience with surgical treatment of duodenal carcinoma: a comparison of periampullary and extra-ampullary duodenal carcinomas. J Gastrointest Surg. Apr 2012;16(4):682-91. https://doi.org/10.1007/s11605-011-1808-z
Spalding DR, Isla AM, Thompson JN, Williamson RC. Pancreas-sparing distal duodenectomy for infrapapillary neoplasms. Ann R Coll Surg Engl. Mar 2007;89(2):130-5. https://doi.org/10.1308/003588407X155815
Otani K, Ishihara S, Nozawa H, et al. A retrospective study of laparoscopic surgery for small bowel obstruction. Ann Med Surg (Lond). Apr 2017;16:34-39. https://doi.org/10.1016/j.amsu.2017.02.045
Al Abbas AI, Wang C, Hamad AB, et al. Mentorship and formal robotic proficiency skills curriculum improve subsequent generations’ learning curve for the robotic distal pancreatectomy. HPB. Dec 2021;23(12):1849-55.
Author information
Authors and Affiliations
Contributions
DP and SB: conceptualization, methodology, data curation, writing and editing of the final manuscript. AA: writing and editing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, D.D., Abdulkarim, A.B. & Behrman, S.W. Segmental Duodenal Resections: Toward Defining Indications, Complexity, and Coding. J Gastrointest Surg 27, 2373–2379 (2023). https://doi.org/10.1007/s11605-023-05837-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-023-05837-z