Abstract
Background
Gastric cancer is the fifth most common malignancy and the fourth most common cause of cancer mortality globally. The role of neoadjuvant chemotherapy in upfront resectable gastric cancer is a subject of ongoing research. In recent meta-analyses, R0 resection rate and superior outcomes were not consistently observed in such regimens.
Aim
To describe the outcomes following phase III randomised control trials; comparing neoadjuvant therapy followed by surgery against upfront surgery with and without adjuvant therapy in resectable gastric cancers.
Methods
The Cochrane Library, CINAHL, EMBASE, PubMed, SCOPUS and Web of Science was searched from January 2002 to September 2022.
Results
13 studies were included (3280 participants). R0 resection rates were in neoadjuvant therapy arms as compared to adjuvant therapy with odds ratio (OR) 1.55[95% CI: 1.13, 2.13](p=0.007) and compared to surgery alone OR 2.49[95% CI: 1.56, 3.96](p=0.0001). 3-year and 5-year progression-, event- and disease-free survival in neoadjuvant therapy as compared to adjuvant therapy were not significantly increased, 3-year OR 0.87[0.71, 1.07](p=0.19). Meanwhile, comparing neoadjuvant therapy to adjuvant therapy, 3-year overall survival (OS) hazard ratio was 0.88[95% CI: 0.70, 1.11](p=0.71) while 3- and 5-year OS OR was 1.18[95% CI: 0.90, 1.55], p=0.22 and 1.27[95% CI: 0.67, 2.42](p=0.47) respectively. Surgical complications were also more common with neoadjuvant therapy.
Conclusion
Neoadjuvant therapy yields higher rates of R0 resection. However, improved long-term survival was not seen as compared to adjuvant therapy. Large multi-centred randomised control trials with D2 lymphadenectomy should be performed to better evaluate the treatment modalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Gastric cancer remains one of the most common malignancies globally according to GLOBOCAN data 2020 and is the fourth most common cause of cancer mortality. Gastric cancers are mostly detected in the advanced stages when they have become symptomatic,1,2 and prognosis remains poor. Radical oncological surgery namely gastrectomy with adequate lymphadenectomy remains as the only curative option in the management of advanced gastric cancer3,4 however recurrence even with R0 resection still occurs frequently.5 To further improve outcomes, multimodality treatments have been suggested for the management of gastric cancer.
The efficacy of neoadjuvant chemotherapy and/or radiotherapy has since been explored as a possible method to improve the outcomes of patients suffering from this malady.6 Several chemotherapy regimens have been trialled thus far. Most notably, the success of the regimen of epirubicin, cisplatin and infused fluorouracil (ECF) in the management of locally advanced gastric cancers7 led to the landmark United Kingdom Medical Research Council Adjuvant Gastric Cancer Infusional Chemotherapy (MAGIC) trial demonstrating the decreased tumour size and stage and improved progression-free and overall survival rates following a perioperative regimen of ECF in the management of resectable gastric cancers, including GEJ tumours.8 Other multimodality management for upper gastrointestinal cancers have been trialled such as in the ChemoRadiotherapy for Esophageal cancer followed by Surgery Study (CROSS) where neoadjuvant chemoradiotherapy versus surgery alone was studied demonstrating survival benefits.9 Arguments have been put forth to consider surgical resection alone, surgery with adjuvant therapy or surgery with neoadjuvant therapy and they are recognised as standard treatments for gastric cancer. In a recent meta-analysis by Yu J,10 neoadjuvant therapy was compared against surgery alone in randomised trials and retrospective studies; despite longer operative times, R0 resection rate was higher in the neoadjuvant therapy groups. However, no significant differences were showed in long-term overall survival, post-operative complications and short-term mortality in the meta-analysis, which was discordant to the findings in the MAGIC trial. Furthermore, in comparison to adjuvant therapy, neoadjuvant therapy may not have improved outcomes as well. In a meta-analysis by Yu X,11 short-term benefits were seen in neoadjuvant chemotherapy as compared to adjuvant therapy, as seen by improved 3-year survival odds. Unfortunately, 5-year survival rates do not enjoy the same improvements with neoadjuvant therapy as compared to their adjuvant counterparts. However, in opposition to the benefits of neoadjuvant therapy, the papers selected for meta-analysis demonstrated significant (>50%) heterogeneity in the analysis of 3-year survival. This was not present for the analysis of 5-year survival; hence this raises some doubts towards the benefits of neoadjuvant therapy. Therefore, the benefits of neoadjuvant chemotherapy in the management of locally advanced gastric cancers remain in question.
Internationally, there are varying recommendations for the management of gastric cancer,12 ranging from neoadjuvant therapy being the ideal treatment in western countries to adjuvant being the main treatment in eastern countries. There is an accompanying gradual shift in the perceptions of neoadjuvant therapy in eastern countries as well,13 however the consensus remains that neoadjuvant therapy is not the routine standard in the region.14,15 Adjuvant chemotherapy following gastrectomy remains the gold standard along with D2 gastrectomy.16,17 The need to clarify the utility of neoadjuvant therapy remains given the differences in outcomes noted between large scale trials and meta-analyses.
As more clinical trials are held, more phase II and III trials have begun to report their results. Unfortunately, phase II trials tend to overestimate the intervention effect,18 and statistical analysis of the success of intervention may not be as robust as compared to those in phase III trials.19 Given that the landmark trials are in earlier phases, the benefits of neoadjuvant therapy could have been overestimated.
The purpose of this systematic review is to further describe the outcomes following phase III and randomised control trials; comparing neoadjuvant therapy with surgery against surgery alone or upfront surgery followed by adjuvant therapy in upfront resectable gastric cancers. The gastric cancers are defined as T1-4aN0-3M0, corresponding to stages Ib to IIIc based on the AJCC or type IV linitis plastica tumours.20 The review aims to detail the characteristics of the patients, tumours, interventions, and the associated outcomes to best inform clinicians on the utility of this treatment modality.
Methods
Search Strategy and information sources
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A search of the Cochrane Library, CINAHL, EMBASE, PubMed, SCOPUS and Web of Science was carried out to identify articles published between January 2002 and September 2022. The search terms and results are available in the appendix.
Selection process and eligibility criteria
Three reviewers (Adelina, Alva, Sarah) independently reviewed the studies for inclusion. Studies were considered in the review if they met the following criteria: (1) phase III randomised control trials (RCT); (2) interventions compared neoadjuvant chemotherapy versus surgery alone or versus adjuvant chemotherapy. Studies were excluded if the following were met: (1) articles were not in English; (2) articles were case reports, guidelines, letters, non-comparative studies, protocols; (3) trial was ongoing; (4) patients had metastatic disease preoperatively.
Data collection and analysis
Two reviewers selected and extracted the data from the included studies:
-
1.
Authors, year of publication
-
2.
Patients’ and tumour characteristics
-
3.
Outcomes related to neoadjuvant therapy: patient drop out, pathological response, complications and morbidity, oncological stage pre- and post-neoadjuvant therapy.
-
4.
Outcomes following surgery with or without neoadjuvant therapy: R0 resection, lymph nodes harvested, patient survival, disease progression, and 3-year and 5-year in progression-free survival (PFS), disease-free survival (DFS) or event-free survival (EFS) and overall survival (OS)
The reviewers discussed any differences or omissions in data collection and a third party (TKV, CY) assisted in reaching a consensus.
Meta-analysis for the primary outcomes of 3-year and 5-year PFS, DFS, EFS and OS were conducted in Revman, Version 5.4 (Nordic Cochrane Centre) to obtain the hazard ratio (HR) where possible to determine the likelihood of the outcomes at the 3- or 5-year mark. However, if information provided does not allow for HR to be calculated, odds ratios (OR) would be used instead.21 Secondary outcome for meta-analysis was the incidence of R0 resection. Comparisons and meta-analysis were conducted if 4 or more studies were available, while for comparisons with 3 or less studies, if there was low heterogeneity, analysis would be conducted.
Heterogeneity using I2 was utilized where 25%, 50% and 75% respectively were low, moderate, and high levels of heterogeneity. For low heterogeneity, analysis would be conducted using a fixed effects model, otherwise, a random effects model would be utilized.
Publication bias was assessed using the Egger’s and Begg’s test in MedCalc Statistical Software version 19.2.6 (MedCalc Software bv, Ostend, Belgium), in the events of significant publication bias, the trim and fill method was utilized.22,23
The Cochrane risk-of-bias tool for randomized trials version 2 tool (RoB 2) was utilized for quality and risk-of-bias assessment.24 The tool evaluates biases in randomisation process, deviations from interventions, missing outcome data, measurement of the outcome and selection of the reported result. Two authors (Alva and Adelina) assessed bias within the studies independently and discordance was resolved with third party review.
Results
Study Selection
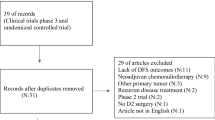
Figure 1 shows the flow chart for the article selection process. A total of thirteen randomised controlled trials (RCTs) were included. A summary of the trial’s patient demographic (table 1), tumour characteristics (table 2), surgery characteristics (table 3), neoadjuvant therapy characteristics (table 4) and outcome parameters (table 5) are included below. RoB 2 analysis results can be found in the appendix.
Study Characteristics
A total of 3280 patients with ages ranging from 25 to 76, excluding the participants from Xue,25 and Zhao26 (due to unreported age ranges), were involved in the studies. Male patients accounted for the majority of participants at 74.7% of the total while females constituted the remaining 25.3%. In the neoadjuvant chemotherapy compared to surgery alone group, 5-fluorouracil and cisplatin were used in all neoadjuvant regimens, with docetaxel included in the studies by Basi,27 and Hashemzadeh.28 Folinic acid was included in the study by Schuhmacher.29
Comparing adjuvant therapy with surgery to neoadjuvant chemotherapy with surgery, varying combinations of docetaxel, cisplatin, 5-fluorouracil, S-1, oxaliplatin and capecitabine were used in adjuvant therapy. Similar combinations including the same agents were also used in neoadjuvant therapy.
A total of 1534 patients underwent neoadjuvant therapy, 291 underwent surgery alone and 1455 underwent adjuvant therapy only.
Tumour characteristics
In the neoadjuvant therapy compared to surgery alone analysis, 372 patients (66.9% of 556 patients) were diagnosed at T3 or T4 stage, 99 (17.8%) at T2 or T1 stage, and 8 (1.4%) at T0 stage. Nodal metastases (N1-3) were detected in 360 (64.7%) patients and no nodal metastases (N0) were detected in 93 (16.7%) patients.
In the adjuvant compared to neoadjuvant group, 1402 patients (51.46% of 2724) were diagnosed at T4 stage, 393 (14.4%) at T3, 80 (2.9%) at T2, 0 (0%) at T1 and 0 (0%) at T0. Nodal metastases (N1-3) were detected in 1722 (63.2%) patients and no nodal metastases (N0) were detected in 180 (6.6%) patients. Raw values for TMN staging were not reported in the papers by Fazio,30 Biffi,31 and Zhao.26
Surgery characteristics and outcomes
Among the patients who underwent surgery in the neoadjuvant compared to the surgery alone group, a majority of them underwent D2 lymphadenectomy. In the surgery alone group, the rate of D2 lymphadenectomy ranged from 11.5% to 100% in the neoadjuvant group (Table 3). Additionally, the rate of D1 lymphadenectomy ranged from 7.4% to 48.1% in the surgery only group and 2.9% to 86.4% in the neoadjuvant group. This does not include patients reported by Basi,27 and Ychou,32 as the degree of lymphadenectomy was unreported. The number of lymph nodes removed by Ramachandra,33 Schuhmacher,29 and Ychou,32 ranged from 2 to 88 in the surgery alone group and 1 to 80 in the neoadjuvant group. Meta-analysis of rates of R0 in the neoadjuvant versus adjuvant comparisons shows that the OR of R0 resection was 1.55 [1.13, 2.13], p=0.007, with neoadjuvant chemotherapy resulting in higher rates of R0 (Fig. 2). Comparing neoadjuvant therapy to surgery alone, the OR of R0 resection was 2.49 [1.56, 3.96], p=0.0001, with higher rates of R0 resection attained in neoadjuvant therapy groups (Fig. 3).
Among the patients who underwent surgery in the adjuvant compared to neoadjuvant group, almost all of them underwent D2 lymphadenectomy, with D2 rates ranging from 91% to 100% in the adjuvant group and 90.6% to 100% in the neoadjuvant group. Fazio,30 Biffi,31 Kang,34 and Xue,25 reported a range of 9 to 69 nodes removed in the adjuvant group and 13 to 76 in the neoadjuvant group. Rate of R0 resection averaged at 85.6% in the adjuvant group and 92.3% in the neoadjuvant group. Iwasaki,35 and Terashima36 did not report the degree of lymphadenectomy performed, number of nodes resected or rate of R0 resection (Fig. 4).
In neoadjuvant therapy patient arms, a total of 325 surgical complications were observed as compared to 69 in patients treated with surgery alone and 324 in patients treated with adjuvant chemotherapy and surgery. However, there was significant heterogeneity seen in reporting of adverse events, ranging from naming conventions of events to grouping of complications in intervention and control arms, hence meta-analysis was not conducted.
Disease free progression was reported in the trials by Ychou32 and Zhao.26 In Ychou’s study,32 neoadjuvant therapy sees better 5-year DFS as compared to surgery alone (34% vs 19%). In Zhao’s study,26 2-year DFS was greater in neoadjuvant arms as compared to adjuvant therapy arms, p=0.000, while there is no significant difference in DFS between the neoadjuvant therapy regimens p=0.189.
Chemotherapy characteristics and outcomes
Complete pathological response in neoadjuvant arms was noted in 8 studies; the rate of complete pathological response ranged from 1.7% to 18.5%. Partial pathological response was described to varying degrees in the papers, with papers describing response to the neoadjuvant therapy as shrinkage, regression or partial response; the rates of which ranged depending on the extent of the response as seen in Table 4.
In the neoadjuvant groups by Fazio,30 Biffi,31 and Xue,25 comparing the neoadjuvant to adjuvant trials, stage reduction was noted in 3 trials with reduction rates ranging from 56.5% to 71.4%. In the adjuvant groups however, stage reduction was not stated.
Across the studies, a total of 7217 events were recorded following chemotherapy, of which 4968 events occurred in the neoadjuvant chemotherapy group. A total of 950 grade III or grade IV events were reported; 609 occurred in the neoadjuvant therapy arms in the analysis of neoadjuvant therapy versus chemotherapy trials.
Survival outcomes
PFS, EFS and DFS in neoadjuvant therapy and surgery versus surgery alone
In the neoadjuvant treatment arm studied by Schuhmacher,29 3-year PFS was higher at 43.1% compared to 38.9% in the surgery alone group. The 5-year PFS in the neoadjuvant group was also higher at 18.1% compared to 15.3% in the surgery alone group. However, the HR comparing chemotherapy and surgery versus surgery alone was not statistically significant(p=0.20). In Ychou’s study, disease free survival was defined from 6 months after random assignment, and neoadjuvant therapy with surgery saw higher DFS with HR 0.69[95% CI: 0.48, 0.89](p=0.003). At 5 years, DFS rates were 34%[95% CI: 26%, 44%] for neoadjuvant therapy with surgery patients and 19%[95% CI, 13%,28%] in surgery alone patients.
OS in neoadjuvant therapy and surgery versus surgery alone
The 5-year OS rates in the neoadjuvant arms were reported by Basi27 and Ychou.32 In Basi’s trial,27 5-year OS rates in the neoadjuvant arms were 36% as compared to 23% in surgery alone arms. While in Ychou’s trial,32 5-year OS rates were 38% in the neoadjuvant arms as compared to 24% in the surgery alone arm. For YChou, OS was found to be significantly higher in neoadjuvant therapy groups as compared to surgery alone arms with HR for death being 0.69[95% CI: 0.50, 0.95](p=0.02). Hashemzadeh,28 and Ramachandra33 reported neither PFS, DFS, EFS nor OS while Schuhmacher29 reported OS at 4.1-years and 4.7 years for the surgery and neoadjuvant arms. Due to the low number of studies and significant heterogeneity, a meta-analysis was not conducted for PFS, EFS, DFS and OS in neoadjuvant therapy versus surgery alone trials.
PFS, EFS and DFS in neoadjuvant therapy and surgery versus adjuvant therapy and surgery
Neoadjuvant therapy was compared to adjuvant therapy. For Fazio and Biffi,30,31 event-free survival was defined by time to relapse, progression, death from any cause starting from randomization. The EFS at 5 years was 44.1% [95% CI: 27.3%, 59.7%] in adjuvant therapy arms compared to 43.5% [95% CI: 26.5%, 59.4%] in neoadjuvant therapy arms. At 10 years, the EFS was 44.1% [95% CI: 27.3%, 59.7%] in adjuvant therapy arms versus 29.4% [95% CI: 14.7%, 45.8%] in neoadjuvant arms. There was no significant difference in EFS at 2.5 years in both arms(p=0.5). For Iwasaki and Terashima,35,36 progression-free survival was defined as the time from randomization to the first occurrence of disease progression, diagnosis of being able to undergo R0 or R1 or death from any cause. The 3-year PFS was 47.7% in both arms, HR 0.976[95% CI: 0.738, 1.292](p=0.87). Kang’s PRODIGY trial34 defined PFS as progression of disease or death, definition of progression involved distant metastasis, persistence of cancer at resection margins or recurrence. In the PRODIGY trial, PFS was better in the patients treated with neoadjuvant therapy HR 0.70[95% CI: 0.52, 0.95](p=0.023). The 3-year PFS was 66.3%[95% CI: 59.6, 72.1] in the neoadjuvant therapy arm compared to 60.2%[95% CI: 53.6, 66.3] in the adjuvant therapy arms. Disease-free survival was defined as time from randomisation to recurrence of primary cancer, new gastric cancer, distant metastases or death from any cause seen in Zhang’s RESOLVE trials.37 Comparing perioperative SOX to adjuvant CapOx, DFS was higher, HR 0.77[95% CI: 0.61, 0.97](p=0.028). As the definitions of EFS, DFS and PFS were similar, a meta-analysis for HR was attempted but only 2 studies34,35,36 provided sufficient data and there was significant heterogeneity, hence OR analysis was conducted.
OS in neoadjuvant therapy and surgery versus adjuvant therapy and surgery
For 3- and 5- year OS, 3-year OS was reported in 2 papers while for 5-year OS, 2 papers report these outcomes. The 3-year OS between the two treatment arms analysed had an HR of 0.88[95% CI: 0.70, 1.11], p=0.71 (Fig. 5). For OR calculation, 3-year and 5-year data were analysed. The 3-year OS between the treatment arms were analysed and an OR of 1.18 [95% CI:0.90, 1.55], p=0.22 while 5-year OS had an OR of 1.27 [95% CI:0.67, 2.42], p=0.47 (Fig. 6).
Discussion
Neoadjuvant therapy demonstrates utility in attaining R0 resections in patients treated with this modality. Attaining R0 resection has been shown to increase the chance of cure for the patients.38 The higher rates of R0 resection also seem to predict the increased rate of survival achieved in the paper by Basi.27 This, combined with the pathological response noted in the papers, hint towards the possibility of utilising neoadjuvant chemotherapy to facilitate subsequent surgery of advanced gastric cancers that would normally pose difficulties in resection due to size or spread. The idea of utilising neoadjuvant therapy to downstage tumours is not novel and has been used frequently in monitoring the response of other cancers.39,40 However, surgical complications occurred more frequently in the neoadjuvant therapy groups which suggests an increased surgical technically difficulty or perhaps more vigilance in post-operative care in neoadjuvant therapy patients who had previously suffered from adverse effects in the chemotherapy.41 Nonetheless, it is well known that neoadjuvant chemotherapy is considered a personalised in-vivo drug test to assess chemotherapy efficacy and to further guide treatment planning.42
Comparing 3-year and 5-year PFS, DFS, EFS and OS to that in adjuvant therapy regimens, our analysis demonstrates a lack of evidence supporting the utility of neoadjuvant therapy in providing patients with long term benefits. Furthermore, given the notable adverse effects following chemotherapy treatments, the benefits may not outweigh the harms experienced by the patient. In Kang’s study,34 we noted that there were already 704 adverse events, of which 74 (10.5%) were grade III or IV toxicities reported in response to chemotherapy before surgery was performed. The harms brought to the patient through the neoadjuvant chemotherapy regimen were compounded by the 343 events, where 31 events were grade III or IV adverse events in the adjuvant therapy stage. The significant rates of dropouts following neoadjuvant chemotherapy, intervals between treatment and adverse events that may have resulted in a small percentage of patients facing progression of their conditions before receiving the necessary surgery. Given the inability to conduct a meta-analysis for neoadjuvant therapy as compared to surgery alone trials, this paper is unable to commit to the efficacy of neoadjuvant therapy as compared to surgery alone. However, there appears to be higher rates of survival in patients receiving neoadjuvant therapy and surgery as compared to having surgery alone.
In the landmark MAGIC trial, it was found that neoadjuvant therapy was associated with improved long-term outcomes. This is also corroborated by the meta-analysis by Xiong,43 where similar findings supported improved outcomes for neoadjuvant chemotherapy. In the same paper, 1820 patients were included from 12 RCTs which showed that neoadjuvant chemotherapy slightly improved survival rates [OR = 1.32, 95% confidence interval (CI): 1.07-1.64, P=0.01]. There was also significantly improved three-year progression-free survival (PFS) rates [OR: 1.85 (1.39, 2.46), p < .0001], tumour down-staging rates [OR: 1.71 (1.26, 2.33), p=.0006] and R0 resection rates [OR: 1.38 (1.08, 1.78) p=.01] in these patients. This was also seen in other meta-analyses by Xu44 and Cai.45 In the paper by Xu,44 the nine RCTs with a total of 1056 participants illustrated higher R0 rates in neoadjuvant therapy subjects as compared to those in the surgery alone intervention group. [25.68% vs 16.95%, RR: 1.92, 95% CI: 1.20–3.06, P = 0.006]. In the network meta-analysis by Cai,45 the eight trials with 2434 participants who underwent multiple regimens of neoadjuvant chemotherapy showed significantly improved survival. However, discordant findings were noted within the meta-analyses. In the meta-analysis by Yu J,10 and the paper by Liao,46 neoadjuvant therapy was reported to not be associated with improved long-term outcomes. For Yu J,10 the team found that in the 20 studies with 3362 total participants, neoadjuvant chemotherapy led to significantly increased R0 resection rates (p=0.003) but no significant differences in overall survival (p=0.240). The lack of improved survival was similar in Liao’s46 study of six trials with 781 patients, however R0 resection in this paper was not significantly improved in the neoadjuvant therapy study arm.
Possible reasons for the varying findings could be due to the different regimens used in the interventions. For example, in the paper by Xiong,43 regimens included 5-fluorouracil (5-FU) + cisplatin, paclitaxel (PTX) + 5-fluorouracil/leucovorin/oxalipatin (FOLFOX), docetaxel/cisplatin/5-fluorouracil (TCF), 5-FU/Adriamycin/methotrexate (FAMTX) and epirubicin + cisplatin + fluorouracil (ECF). This difference in regimens was seen throughout the other meta-analyses as well.10,44,45 Furthermore, whether D2 lymphadenectomy was performed was also a variable that could have possibly contributed to the incongruous findings. In the MAGIC trial, only 43% of cases underwent D2 lymphadenectomies while a larger proportion of the patients in this study completed D2 (Table 3).
In the evaluation of adjuvant therapy as compared to neoadjuvant therapy, the large PRODIGY trial involving 18 centres, demonstrated improved PFS(p=0.0227) but OS was not significantly improved(p=0.3383). The findings of the PRODIGY trial disagree with what our meta-analysis has demonstrated. Possible reasons include the small number of trials available for meta-analysis as well as the PRODIGY trial involving patients who were at earlier stages of their disease. Furthermore, the PRODIGY trial also only utilized adjuvant S1 as compared to the other trials utilizing a combination other than JCOG0501 trial by Iwasaki and Terashima.35,36 Furthermore, the evaluation of improvement of PFS was conducted using the results following 38.6 months, while we evaluated PFS at the 36 months. Looking at another large trial with a total of 749 patients, Zhao26 demonstrated improved short-term disease-free survival rates in patients treated with neoadjuvant therapy.
Our findings are that outcomes are not improved by neoadjuvant therapy as compared to adjuvant therapy. However, as compared to surgery alone, there may be benefits in PFS, DFS and OS as compared to neoadjuvant therapy; but, more large-scale trials need to be conducted for viability of meta-analysis. Furthermore, given the smaller number of phase III trials available for analysis, there remains a need for more large scale multicentred randomised control trials studying the effects of neoadjuvant chemotherapy versus adjuvant chemotherapy or surgery alone, allowing for more subgroup analyses that take the above into account.47 With multiple phase III trials ongoing comparing different regimens of neoadjuvant therapy,48 neoadjuvant chemoradiotherapy,49 neoadjuvant versus adjuvant therapy,50 and immunotherapy,51 a final consensus on the best management would eventually arise and a large-scale trial can then be conducted to fully evaluate if neoadjuvant therapy is indeed superior to surgery alone.
Limitations of the current study
There exists significant heterogeneity in data reporting for some outcomes such as pathological downstaging, morbidity from chemotherapy or surgery. Comparison of data from the various papers were limited by the lack of standardised parameters used in documentation and analysis. Although other outcomes would be served better with statistical validation, they were unable to be accorded with the analysis due to this heterogeneity. Therefore, this limits the generalizability of this study towards future trials.
Conclusion
Neoadjuvant therapy yields higher rates of R0 resection but improved long-term survival following neoadjuvant therapy was not observed comparing neoadjuvant to adjuvant therapy. Given that survival appears to be improved as compared to surgery alone, it may be put forth that outcomes of ongoing randomised controlled trials comparing different neoadjuvant regimens to adjuvant regimens would further inform on the survival outcomes regarding neoadjuvant therapy for gastric cancers. However, more large multi-centre randomised control trials should be performed to validate this finding. Furthermore, standard D2 lymphadenectomy should also be performed for better utility of findings. As patients face more side effects with more cycles of chemotherapy, it may be put forth that adjuvant therapy is sufficient for improving long term survival in patients with locally advanced gastric cancers.
References
Takahashi T, Saikawa Y, Kitagawa Y. Gastric Cancer: Current Status of Diagnosis and Treatment. Cancers (Basel). 2013;5:48–63.
Sitarz R, Skierucha M, Mielko J, Offerhaus JA, Maciejewski R, Polkowski WP. Cancer Management and Research Dovepress Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10 239.
Coccolini F, Montori G, Ceresoli M, Nita GE, Ansaloni L, Cima S, et al. Advanced gastric cancer: what we know and what we still have to learn peritoneal; Surgery; Definition. World J Gastroenterol. 2016;22:1139–59.
Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–24.
Johnston FM, Beckman M. Updates on Management of Gastric Cancer. Curr Oncol Rep. 2019;21:67.
Proserpio I, Rausei S, Barzaghi S, Frattini F, Galli F, Iovino D, et al. Multimodal treatment of gastric cancer. World J Gastrointest Surg. 2014;6:55–8.
Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–7.
Cunningham D, Allum WH, Stenning SP, Thompson JN, van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer Engl J Med. 2006;355(1), 11-20.
Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8.
Yu J-H, Wang Z-Z, Fan Y-C, Liu M-X, Xu K, Zhang N, et al. Comparison of neoadjuvant chemotherapy followed by surgery vs. surgery alone for locally advanced gastric cancer: a meta-analysis. Chin Med J. 2021;134(14), 1669-1680
Yu X, Hu Y, Hu D, Li W. Neoadjuvant chemotherapy brings more survival benefits than postoperative chemotherapy for resectable gastric cancer: a Meta-analysis of randomized controlled trials. JBUON. 2019;24:201–14.
Eom SS, Choi W, Eom BW, Park SH, Kim SJ, Kim YI, et al. A Comprehensive and Comparative Review of Global Gastric Cancer Treatment Guidelines. J Gastric Cancer. Korean Gastric Cancer Association; 2022;22:3.
Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. Wiley-Blackwell; 2021;41:747.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018. Gastric Cancer 2021;24 1:121.
Ryu KW, Park YS, Kwon OK, Oh J, Lee HH, Kong SH, et al. Korean Practice Guideline for Gastric Cancer 2018: an Evidence-based, Multi-disciplinary Approach. J Gastric Cancer. Korean Gastric Cancer Association; 2019;19:1.
Okines A, Verheij M, Allum W, Cunningham D, Cervantes A. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology. Elsevier; 2010;21:v50–4.
Songun I, Putter H, Kranenbarg EMK, Sasako M, van de Velde CJH. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol.; 2010;11:439–49.
Burke DL, Billingham LJ, Girling AJ, Riley RD. Meta-analysis of randomized phase II trials to inform subsequent phase III decisions. Trials. BioMed Central Ltd.; 2014;15:1–15.
Liang F, Wu Z, Mo M, Zhou C, Shen J, Wang Z, et al. Comparison of treatment effect from randomised controlled phase II trials and subsequent phase III trials using identical regimens in the same treatment setting. Eur J Cancer. Pergamon; 2019;121:19–28.
Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. American Medical Association; 2019;321:1983.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. BioMed Central; 2007;8:1–16.
Duval S, Tweedie R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J Am Stat Assoc. ; 2012;95:89–98.
Lin L, Chu H. Quantifying Publication Bias in Meta-Analysis. Biometrics.; 2018;74:785.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. BMJ; 2019;366.
Xue K, Ying X, Bu Z, Wu A, Li Z, Tang L, et al. Oxaliplatin plus S-1 or capecitabine as neoadjuvant or adjuvant chemotherapy for locally advanced gastric cancer with D2 lymphadenectomy: 5-year follow-up results of a phase II - III randomized trial. Chin J Cancer Res.; 2018;30:516–25.
Zhao Q, Lian C, Huo Z, Li M, Liu Y, Fan L, et al. The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: A multicenter randomized clinical trial. Cancer Med.2020;9:5731–45.
Basi A, Sohrabkhani S, Zamani F, Baghai-Wadji M, Rabiei N, Razavi S-M, et al. Comparing Efficacy of Preoperative neo-Adjuvant Chemotherapy and Surgery versus Surgery Alone in Patients with Resectable Gastroesophageal Cancer. Int J Hematol Oncol Stem Cell Res. 2013;7:24–8.
Hashemzadeh S, Pourzand A, Somi MH, Zarrintan S, Javad-Rashid R, Esfahani A. The effects of neoadjuvant chemotherapy on resectability of locally-advanced gastric adenocarcinoma: a clinical trial. Int J Surg. Int J Surg; 2014;12:1061–9.
Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210–8.
Fazio N, Biffi R, Maibach R, Hayoz S, Thierstein S, Brauchli P, et al. Preoperative versus postoperative docetaxel-cisplatin-fluorouracil (TCF) chemotherapy in locally advanced resectable gastric carcinoma: 10-year follow-up of the SAKK 43/99 phase III trial. Ann Oncol. Ann Oncol; 2016;27:668–73.
Biffi R, Fazio N, Luca F, Chiappa A, Andreoni B, Zampino MG, et al. Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. . World J. Gastroenterol.: WJG; 2010;16:868.
Ychou M, Boige V, Pignon J-P, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21.
Ramachandra, Goel V, Raju K, Rao TS, Patnaik, Nusrath, et al. Prospective Randomized Controlled Study Comparing Primary Surgery Versus Neoadjuvant Chemotherapy Followed by Surgery in Gastric Carcinoma. Indian J Surg Oncol. 2019;10:245–50.
Kang YK, Yook JH, Park YK, Lee JS, Kim YW, Kim JY, et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J Clin Oncol. J Clin Oncol; 2021;39:2903–13.
Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer. Gastric Cancer; 2021;24:492–502.
Terashima M, Iwasaki Y, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer. Gastric Cancer; 2019;22:1044–52.
Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol.; 2021;22:1081–92.
Biondi A, Persiani R, Cananzi F, Zoccali M, Vigorita V, Tufo A, et al. R0 resection in the treatment of gastric cancer: Room for improvement resection in the treatment of gastric cancer: Room for improvement. World J Gastroenterol. 2010;16:3358–70.
Matuschek C, Jazmati D, Bölke E, Tamaskovics B, Corradini S, Budach W, et al. Post-Neoadjuvant Treatment Strategies in Breast Cancer. Cancers (Basel).; 2022:14(5)1246.
Rosenblatt R, Sherif A, Rintala E, Wahlqvist R, Ullén A, Nilsson S, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61:1229–38.
Cui H, Cui J-X, Wang Y-N, Cao B, Deng H, Zhang K-C, et al. Could neoadjuvant chemotherapy increase postoperative complication risk of laparoscopic total gastrectomy? A mono-institutional propensity score-matched study in China. World J Gastrointest Surg. 2021;13:429–42.
Imyanitov EN, Yanus GA. Neoadjuvant therapy: theoretical, biological and medical consideration. Chin Clin Oncol. Chin Clin Oncol; 2018;7.
Xiong B-H, Cheng Y, Ma L, Zhang C-Q. An updated meta-analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer. Cancer Invest. 2014;32:272–84.
Xu AM, Huang L, Liu W, Gao S, Han WX, Wei ZJ. Neoadjuvant Chemotherapy Followed by Surgery versus Surgery Alone for Gastric Carcinoma: Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One.; 2014;9:e86941.
Cai Z, Yin Y, Zhao Z, Xin C, Cai Z, Yin Y, et al. Comparative Effectiveness of Neoadjuvant Treatments for Resectable Gastroesophageal Cancer: A Network Meta-Analysis. Front Pharmacol. 2018;9:872.
Liao Y, Yang Z, Peng J, Xiang J, Wang J. Neoadjuvant chemotherapy for gastric cancer: a meta-analysis of randomized, controlled trials. J Gastroenterol Hepatol. 2013;28:777–82.
Sevdalis N, Jacklin R. Interaction effects and subgroup analyses in clinical trials: more than meets the eye? J Eval Clin Pract. 2008;14:919–22.
Wang X, Li S, Sun Y, Li K, Shen X, Xue Y, et al. The protocol of a prospective, multicenter, randomized, controlled phase III study evaluating different cycles of oxaliplatin combined with S-1 (SOX) as neoadjuvant chemotherapy for patients with locally advanced gastric cancer: RESONANCE-II trial. BMC Cancer. 2021;21:20.
Liu X, Jin J, Cai H, Huang H, Zhao G, Zhou Y, et al. Study protocol of a randomized phase III trial of comparing preoperative chemoradiation with preoperative chemotherapy in patients with locally advanced gastric cancer or esophagogastric junction adenocarcinoma: PREACT. BMC Cancer. 2019;19:606: https://doi.org/10.1186/s12885-019-5728-8
Tokunaga M, Mizusawa J, Machida N, Fukagawa T, Katai H, Nishida Y, et al. Phase III trial to evaluate the efficacy of neoadjuvant chemotherapy with S-1 plus oxaliplatin followed by D2 gastrectomy with adjuvant S-1 in locally advanced gastric cancer: Japan Clinical Oncology Group study JCOG1509 (NAGISA trial). J Clin Oncol.; 2017;35:TPS4134–TPS4134.
Tabernero J, Bang Y-J, van Cutsem E, Fuchs CS, Janjigian YY, Bhagia P, et al. KEYNOTE-859: a Phase III study of pembrolizumab plus chemotherapy in gastric/gastroesophageal junction adenocarcinoma. Future Oncol. 2021;17:2847–55.
Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg. 2004;240:205.
Acknowledgements
This study was not funded, we would like to thank Lee Kong Chian School of Medicine’s Library for their assistance with the search strategy.
Author information
Authors and Affiliations
Contributions
Ho Si Ying, Adelina: acquisition, analysis and interpretation of data, manuscript drafting and approval, agreement for accountability for all aspects of the work
Lim Khai Shin, Alva: project development, acquisition, analysis and interpretation of data, manuscript drafting and approval, agreement for accountability for all aspects of the work
Sarah Neo Hui Wen: acquisition of data, manuscript drafting and approval, agreement for accountability for all aspects of the work
Charleen Yeo Shan Wen: project development, interpretation of data, manuscript drafting and approval, agreement for accountability for all aspects of the work
Tay Kon Voi: project development, interpretation of data, manuscript drafting and approval, agreement for accountability for all aspects of the work
Corresponding author
Ethics declarations
Registration and protocol
Review was not registered.
Conflict of interests
The authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Exempt from ethics board approval
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lim Khai Shin, A., Ho Si Ying, A., Neo Hui Wen, S. et al. Systematic review and meta-analysis of the outcomes following neoadjuvant therapy in upfront resectable gastric cancers compared to surgery alone in phase III randomised controlled trials. J Gastrointest Surg 27, 1261–1276 (2023). https://doi.org/10.1007/s11605-023-05641-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-023-05641-9