Abstract
Background
The past 20 years have seen advances in colorectal cancer management. We sought to determine whether survival in patients undergoing resection of colorectal liver metastases (CLM) has improved in association with three landmark advances: introduction of irinotecan- and/or oxaliplatin-containing regimens, molecular targeted therapy, and multigene alteration testing.
Methods
Patients undergoing CLM resection during 1998–2014 were identified and grouped by resection year. The influence of alterations in RAS, TP53, and SMAD4 was evaluated and validated in an external cohort including patients with unresectable metastatic colorectal cancer.
Results
Of 1961 patients, 1599 met the inclusion criteria. Irinotecan- and/or oxaliplatin-containing regimens and molecular targeted therapy were used for more than 50% of patients starting in 2001 and starting in 2006, respectively, so patients were grouped as undergoing resection during 1998–2000, 2001–2005, or 2006–2014. Liver resectability indications expanded over time. The 5-year overall survival (OS) rate was significantly better in 2006–2014, vs. 2001–2005 (56.5% vs. 44.1%, P < 0.001). RAS alteration was associated with worse 5-year OS than RAS wild-type (44.8% vs. 63.3%, P < 0.001). However, OS did not differ significantly between patients with RAS alteration and wild-type TP53 and SMAD4 and patients with RAS wild-type in our cohort (P = 0.899) or the external cohort (P = 0.932). Of 312 patients with genetic sequencing data, 178 (57.1%) had clinically actionable alterations.
Conclusion
OS after CLM resection has improved with advances in medical therapy and surgical technique. Multigene alteration testing is useful for prognostication and identification of potential therapeutic targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The past 20 years have seen a number of advances in the management of colorectal cancer. These include refinement of the techniques for resection of colorectal liver metastases (CLM), 1,2,3,4,5,6 which have expanded indications for CLM resection, and adoption of irinotecan, oxaliplatin, anti-vascular endothelial growth factor (VEGF), and anti-epidermal growth factor receptor (EGFR) for medical treatment of metastatic colorectal cancer on the basis of evidence from phase III trials,7,8,9,10,11 These advance in surgical techniques and medical therapy have in turn led to improvements in survival for patients with metastatic colorectal cancer.12
Another breakthrough in the management of metastatic colorectal cancer is increasing knowledge of molecular biology. RAS alteration status garnered attention because RAS-altered disease is resistant to anti-EGFR therapy.13 BRAF mutation has been consistently shown to be associated with a worse prognosis in patients with metastatic colorectal cancer.14,15 For patients undergoing CLM resection, alterations in RAS, BRAF, TP53, and SMAD4 were demonstrated to have prognostic importance.16,17,18,19,20 With the recent development of next-generation sequencing, testing of multiple gene alterations has become more accessible in clinical practice. Studies have shown that the status of multiple somatic alterations is superior to the status of RAS alteration alone for predicting prognosis in patients undergoing CLM resection.19,21
At present, it remains unknown whether survival in patients undergoing resection of CLM with curative intent has improved in step with the above-described advances in management of metastatic colorectal cancer.
To answer this question, we evaluated the changes in survival over time of patients undergoing CLM resection in relation to three breakthroughs: introduction of irinotecan- and/or oxaliplatin-containing regimens, molecular targeted therapy, and multigene alteration testing. We also investigated the clinical implications of multigene alteration testing.
Materials and Methods
Study Populations
Patients who underwent initial liver resection for CLM with potentially curative intent at MD Anderson Cancer Center from 1998 to 2014 were identified from a prospectively compiled database. To validate the influence of multiple gene alterations, we also analyzed an independent cohort of patients who received non-surgical treatments for metastatic colorectal cancer at Memorial Sloan Kettering Cancer Center from April 2014 through September 2016. The dataset is publicly available through the cBioPortal for Cancer Genomics (http://www.cbioportal.org/study?id=crc_msk_2017).22 The study was approved by the Institutional Review Board at MD Anderson Cancer Center (PA17-0564).
Analysis of Multivariable Hazard Ratios and Adjusted Overall Survival Curves
Multivariable hazard ratios (HRs) and adjusted overall survival (OS) curves were assessed for the entire cohort and subgroups with special reference to time periods, RAS mutation status, and multigene alteration status. For the assessment of multigene alteration status, we focused on co-existence of somatic alterations in RAS, TP53, and SMAD4, which are associated with prognosis after CLM resection according to our previous studies.16,19,21,23
Surgical Management of CLM
Our institutional approach to surgical management of CLM was detailed previously.24 CLM are deemed resectable when a hepatectomy can achieve a negative margin and preserve more than 30% of the standardized total liver volume.25 Patients with an anticipated insufficient future liver remnant are offered preoperative portal vein embolization and staged hepatectomy. Postoperative chemotherapy is typically administered to complete 12 cycles, including the cycles of preoperative chemotherapy.5 Patients are routinely followed after resection with history, physical examination, laboratory evaluation, and axial imaging every 3 to 4 months for the first 2 years and every 4 to 6 months for the subsequent 3 years.26
Somatic Gene Mutation Profiling
Tumor DNA was isolated from 5-mm-thick unstained sections from tumor tissue blocks or slides. Next-generation sequencing was performed with an AmpliSeq multigene panel (Supplementary Table A.1) using the Ion Torrent Personal Genome Machine (Life Technologies, CA) in a Clinical Laboratory Improvement Amendment–certified molecular diagnostic laboratory.27 Single alterations in the various codons of KRAS and NRAS were analyzed together and reported as RAS mutations.
Clinical Actionability of Genomic Alterations
Genomic alterations were classified using the OncoKB classification system (oncokb.org).28 OncoKB categorize genomic alterations on the basis of published evidence about “clinical actionability” (i.e., the level of evidence that an alteration predicts response or resistance to a particular drug).
Definitions
Liver resection procedures were graded according to a three-level classification (grade I, low complexity; grade II, intermediate complexity; grade III, high complexity).29,30,31,32 Synchronous metastases were defined as metastases diagnosed within 12 months of primary tumor diagnosis. Positive surgical margin was defined as the presence of tumor cells < 1 mm from the transection line. The number and diameter of liver metastases were determined from surgical pathology specimens. Primary tumor T category and N category were classified according to the AJCC Cancer Stating Manual, eighth edition.33 Preoperative chemotherapy regimens were recorded and further categorized based on anti-VEGF or anti-EGFR agent use.
Statistical Analysis
Categorical variables were summarized using frequencies and percentages and were compared among groups using Fisher’s exact test or χ2 test, as appropriate. Continuous variables were summarized using median values with the interquartile range (IQR) and were compared using the Kruskal–Wallis test. Trends of surgical outcomes with a stepwise increase in periods were evaluated using the Cochrane-Armitage trend test.34 For our cohort, OS is defined as the time interval between date of CML resection and the date of death due to any cause. Patients who were alive at the end of 5 years after CLM resection were censored at 5 years. For the external cohort, OS is defined as the time interval between date of diagnosis of metastasis and the date of death due to any cause. Patients who were alive at 5-year follow-up were censored at that time. Using a multivariable Cox proportional hazards model, prognostic factors were assessed and model-adjusted survival rate was estimated. A backward elimination with a threshold P value of 0.05 was used to select variables for the final models. HRs and 95% confidence intervals (CIs) were calculated for each factor. P ≤ 0.05 was considered to indicate statistical significance, and all tests were two-sided. Statistical analysis was conducted with SAS (SAS Institute, Cary, NC).
Results
Study Population
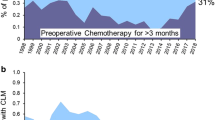
Of 1961 patients who underwent CLM resection during the study period, 1599 patients met the inclusion criteria (Supplementary Figure A.1). Figure 1 shows chronological trends in perioperative chemotherapy regimens, molecular targeted therapy, RAS alteration testing, and next-generation sequencing. Regimens including irinotecan and/or oxaliplatin were used in more than 50% of patients starting in 2001 (Fig. 1A), and anti-VEGF and/or anti-EGFR agents were used in more than 50% of patients starting in 2006 (Fig. 1B). As such, we divided the 1599 patients into 3 groups: 149 patients who underwent liver resection in 1998–2000, 443 who underwent liver resection in 2001–2005, and 1007 who underwent liver resection in 2006–2014 (Supplementary Figure A.1). Demographic and clinicopathologic characteristics of the patients by group are summarized in Table 1. The proportion of patients with American Society of Anesthesiologists score ≥ 3 differed by group. Primary lesion factors did not differ significantly by group. In contrast, all liver metastasis factors differed significantly among the three groups. Synchronous metastasis and concomitant extrahepatic metastasis were more frequent in patients who underwent resection in 2006–2014, compared to those who underwent resection in 1998–2000.
During the follow-up period, 946 patients (59.2%) died. The median duration of follow-up was 4.2 years (IQR 2.3–7.0 years) for the entire cohort, 4.3 years (IQR 1.9–10.8 years) for patients undergoing CLM resection during 1998–2000, 4.1 years (IQR 2.2–10.1 years) for patients with CLM resection during 2001–2005, and 4.2 years (IQR 2.3–6.3 years) for patients with CLM resection during 2006–2014.
Changes in Morphologic Factors and Surgical Techniques
Figure 2 shows the changes in morphologic factors and surgical techniques over time. The rates of multiple CLM and largest CLM diameter < 2 cm were significantly associated with a stepwise increase from 1998–2000 to 2006–2014 (both, P < 0.001) (Fig. 2A A and 2B). The rate of grade III (high-complexity) procedures did not differ significantly between the time periods (P = 0.205) (Fig. 2C), whereas the rate of a parenchymal-sparing approach increased significantly over time (P < 0.001) (Fig. 2D). The rate of resection with use of intraoperative ablation decreased significantly (Fig. 2E) and the rate of two-stage hepatectomy increased significantly (Fig. 2F) in a stepwise fashion from 1998–2000 to 2006–2014 (both, P < 0.001).
Changes over time in A number of CLM, B largest CLM diameter, C complexity of liver resection, D use of a parenchymal-sparing approach, E concomitant use of intraoperative ablation, and F two-stage hepatectomy. *Grade I, low complexity; grade II, intermediate complexity; grade III, high complexity.29 †Defined as frequency of multiple resections (≤ 1 Couinaud segment) for multiple CLM
Predictors of OS After CLM Resection
A multivariable Cox proportional hazards model analysis revealed that CLM resection in 2006–2014 was significantly associated with lower risk for OS than CLM resection in 2001–2005, whereas HR for OS did not differ significantly between CLM resection in 1998–2000 and 2001–2005 (Supplementary Table A.2). Also associated with OS were age, primary tumor location, T category, primary lymph node metastasis, prehepatectomy chemotherapy, extrahepatic disease, number of CLM, largest liver metastasis diameter, and surgical margin status (Supplementary Table A.2).
For patients undergoing CLM resection during 2006–2014, a multivariable Cox proportional hazards model analysis was repeated including alteration status of RAS and BRAF (Supplementary Table A.3). RAS alteration and undetermined RAS alteration status were associated with higher risk for OS than RAS wild-type.
Adjusted OS Estimates by Time Period and by RAS Mutation Status During 2006–2014
OS curves after adjustment for other prognostic factors are shown in Fig. 3. CLM resection in 2006–2014 was associated with better OS than CLM resection in 2001–2005, whereas OS did not differ significantly between CLM resection in 1998–2000 and 2001–2005 (Fig. 3A). In patients undergoing CLM resection during 2006–2014, the OS rates were significantly better in patients with RAS wild-type than in patients with RAS alteration, and the OS rate for patients with RAS alteration status undetermined was intermediate (Fig. 3B).
Overall survival (OS) by A year of resection and B RAS alteration status in patients with CLM resection during 2006–2014. (A) OS curves after adjustment for age, primary tumor location, T category, lymph node metastasis, prehepatectomy chemotherapy, extrahepatic disease, number of CLM, largest liver metastasis diameter, and surgical margin status. (B) OS curves after adjustment for age, lymph node metastasis, prehepatectomy chemotherapy, extrahepatic disease, number of CLM, largest liver metastasis diameter, and surgical margin status
Adjusted OS Estimates by Alteration Status of RAS, TP53, and SMAD4 in Patients Undergoing CLM Resection and Patients Receiving Nonsurgical Treatments
OS curves after adjustment for other prognostic factors were further stratified by alteration status of RAS, TP53, and SMAD4 in patients undergoing CLM resection during 2006–2014. Patients with RAS alteration with wild-type TP53 and SMAD4 and patients with RAS wild-type had similar 5-year OS rates, but patients with RAS alteration and TP53 and/or SMAD4 alteration had a significantly worse 5-year OS rate than patients with RAS wild-type (Supplementary Table A.4).
A similar analysis was repeated for the external validation cohort including 499 patients who underwent nonsurgical treatments for metastatic colorectal cancer and did not have missing data. Demographic and clinicopathologic characteristics of the cohort are summarized in Supplementary Table A.5. Median (IQR) age was 56 (46–65) years. The number of sites involved at first diagnosis of metastasis was 1 for 294 patients (58.9%), 2 for 138 patients (27.7%), and 3 or more for 67 patients (13.4%). The median duration of follow-up was 1.7 years (IQR, 1.1–2.6 years). During the follow-up period, 254 patients (50.9%) died. Similar to the results of our cohort of resected patients, the 5-year OS rates for these unresectable metastatic patients did not differ significantly between patients with RAS alteration and wild-type TP53 and SMAD4 and patients with RAS wild-type, whereas the 5-year OS rate was significantly worse in patients with RAS alteration and TP53 and/or SMAD4 alteration than in patients with RAS wild-type (Supplementary Table A.4).
Changes in 5-Year OS Rates by Year of CLM Resection, RAS Alteration Status, and Multiple Gene Alteration Status
The changes in adjusted 5-year OS rates after CLM resection in our cohort and after the diagnosis of metastasis from colorectal cancer in the external cohort by year of CLM resection and by gene alteration status are shown in Fig. 4.
Clinical Actionability of 312 Patients with the Next-Generation Sequencing Data
Of the 312 patients with the next-generation sequencing data, 178 (57.1%) had clinically actionable gene alterations. Of these 178 patients, 77 (24.7%) had one or more actionable genes other than RAS or BRAF (Fig. 5).
Discussion
Our study demonstrates that OS after adjustment for risk factors was significantly better in patients undergoing CLM resection during 2006–2014 (a period when irinotecan- and/or oxaliplatin-containing chemotherapy regimens and molecular targeted therapy were each used in more than 50% of patients) than in patients undergoing CLM resection during 1998–2000 (before the introduction of irinotecan/oxaliplatin chemotherapy) and patients undergoing CLM resection during 2001–2005 (after the introduction of irinotecan/oxaliplatin chemotherapy but before the introduction of molecular targeted therapy). Over the years of our study, indications for CLM resection were expanded to patients with worse performance status, multiple CLM, and extrahepatic disease. The survival gain that we observed despite these expanding indications for CLM resection may be attributable to the concomitant advances in medical therapy and refinements of surgical technique.
RAS alteration testing is another development in the management of metastatic colorectal cancer that occurred during the study period. However, the findings of our study show that RAS mutation testing alone is not sufficient for prognostication. Instead, testing of multiple gene alterations is essential for accurately predicting prognosis in both patients undergoing CLM resection and patients with unresectable metastatic colorectal cancer.
Improvements in survival after CLM resection were multifactorial. The gain in OS during 2006–2014 correlated with an increasing use of oxaliplatin and anti-VEGF agent for prehepatectomy chemotherapy (Figs. 1 and 3). Patients undergoing CLM resection during 2006–2014 had higher number of CLM but smaller largest CLM diameter than patients undergoing CLM resection during 2001–2005 and during 1998–2000 (Fig. 2). The smaller diameter of CLM during 2006–2014 is most likely attributable to effective prehepatectomy chemotherapy regimens. Downsizing of CLM diameter can facilitate tumor detachment from hepatic vasculature to preserve parenchyma. These effects of prehepatectomy chemotherapy may have expanded surgical indications for patients who had multiple CLM. Another important change between the earlier years of the study and 2006–2014 was refinement of surgical technique, which was reflected by increased use of a parenchymal-sparing approach and decreased use of intraoperative ablation. Our group previously demonstrated that use of a parenchymal-sparing approach6 and lack of use of intraoperative ablation2 were associated with better prognosis in patients undergoing CLM resection. Development of two-stage hepatectomy has also improved resectability of bilateral CLM.35 Taken together, our findings from the current study indicate that advances in medical therapy and refinements of surgical technique were associated with improved OS in patients undergoing CLM resection despite expanding eligibility for CLM resection. These findings emphasize the importance of a multimodality approach to achieve optimal long-term outcomes.36
Another important finding of our study was the clinical implications of multigene alteration testing. RAS alteration status is clearly important for predicting response to anti-EGFR therapy37,38 and OS after CLM resection (Fig. 3). However, RAS alteration status with coexisting alterations in TP53 and SMAD4 may better stratify prognosis not only in patients undergoing CLM resection but also in patients with unresectable colorectal metastases. Importantly, we found that patients with RAS alteration and wild-type TP53 and SMAD4 did not have significantly shorter OS than patients with wild-type RAS. Patients with RAS alteration and wild-type TP53 and SMAD4 accounted for 31.7% (45 of 142) of the patients with RAS alteration in our cohort and 28.6% (74 of 259) of the patients with RAS alteration in the external cohort (Supplementary Table 4). Patients with RAS alteration with wild-type TP53 and SMAD4 and patients with RAS wild-type had the most favorable survival probability: the 5-year OS rate after adjustment was approximately 73% in patients undergoing CLM resection and 26% in patients with unresectable colorectal liver metastases (Fig. 4). In contrast, patients with triple alterations in RAS, TP53, and SMAD4 had the worst prognosis.
The findings of our study showed that advances in chemotherapy and molecular targeted therapy were associated with improved survival in patients undergoing CLM resection. Our findings yield three main messages regarding care for patients with resectable CLM and unresectable metastatic colorectal cancer. First, curative resection of CLM remains associated with better survival than unresectable metastatic colorectal cancer. Eligibility criteria for liver resection have evolved over time. With advances in medical therapy and surgical technique, patients with non-ideal performance status, multiple CLM, synchronous metastases, and extrahepatic disease may be considered for resection when the eligibility criteria for liver resection is determined by specialized liver surgeons. Second, it is important for liver surgeons to keep up to date with knowledge regarding the current optimal chemotherapy regimens, novel agents, and advances in surgical technique. Further development of novel agents and refinement of surgical technique may lead to further improvement in survival for patients with metastatic colorectal cancer. Third, multigene alteration testing is useful for better prognostication and may change clinical decision making, for example, by leading to a decision to extend the period of posthepatectomy chemotherapy or to adjust surveillance frequency and intensity. Because the prognosis of patients with stage IV colorectal cancer may be influenced by alterations in multiple somatic genes, independent from clinicopathologic factors, information about gene alteration status may also be important to balance the background of groups studied in future clinical trials. Finally, multigene alteration testing is expected to provide potentially clinically actionable therapeutic information.28 We found that approximately 60% of patients in our cohort had clinically actionable alterations in genes and 25% had actionable alterations in genes other than RAS or BRAF. RAS mutation status is established as a predictive molecular biomarker for response to anti-EGFR therapy for colorectal cancer.13 Targeted therapies for BRAF, PIK3CA, and ERBB2 are expected to become potential therapeutic options for colorectal cancer.39,40,41
Our study should be understood in the context of potential limitations. First, it was a retrospective cohort study in a single institution. The selection process for liver resection is complex and considers patient factors and tumors factors including tumor biology. We do not exclude patients based on specific genetic mutations. Patients who underwent CLM resection during each of the three periods had sufficient follow-up, and the results showed improvement of survival with advances in medical therapy and surgical technique, and expanding surgical indications. Furthermore, prognostication based on RAS, TP53, and SMAD4 was reproduced in an independent external cohort including patients with unresectable colorectal metastases. A secondary limitation is that barriers may exist to incorporation of medical therapy and surgical resection because close coordination among the medical oncologist, radiologist, pathologist, and surgeon is needed for each patient.
Conclusions
In conclusion, in the past 20 years, advances in medical therapy and surgical technique have improved survival after CLM resection such that 5-year OS rates are approximately 70% for patients with favorable tumor biology despite expanding surgical indications. Multigene alteration testing allows better prognostication than RAS mutation testing alone and provides information on clinically actionable genes for patients with metastatic colorectal cancer.
Abbreviations
- CLM:
-
Colorectal liver metastases
- VEGF:
-
Vascular endothelial growth factor
- EGFR:
-
Epidermal growth factor receptor
- HR:
-
Hazard ratio
- OS:
-
Overall survival
- IQR:
-
Interquartile range
References
Kawasaki S, Makuuchi M, Kakazu T, Miyagawa S, Takayama T, Kosuge T et al. Resection for multiple metastatic liver tumors after portal embolization. Surgery. 1994;115(6):674-7.
Abdalla EK, Vauthey J-N, Ellis LM, Ellis V, Pollock R, Broglio KR et al. Recurrence and Outcomes Following Hepatic Resection, Radiofrequency Ablation, and Combined Resection/Ablation for Colorectal Liver Metastases. Annals of surgery. 2004;239(6):818–27. doi:https://doi.org/10.1097/01.sla.0000128305.90650.71.
Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Annals of surgery. 2000;232(6):777-85.
Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber J-C, Bachellier P. A Two-Stage Hepatectomy Procedure Combined With Portal Vein Embolization to Achieve Curative Resection for Initially Unresectable Multiple and Bilobar Colorectal Liver Metastases. Annals of surgery. 2004;240(6):1037-51. doi:https://doi.org/10.1097/01.sla.0000145965.86383.89.
Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(8):1083–90. doi:https://doi.org/10.1200/JCO.2010.32.6132.
Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis: Improves Salvageability and Survival. Annals of surgery. 2015. doi:https://doi.org/10.1097/SLA.0000000000001194.
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. The New England journal of medicine. 2000;343(13):905–14. doi:https://doi.org/10.1056/NEJM200009283431302.
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. The Lancet. 2000;355(9209):1041-7. doi:https://doi.org/10.1016/s0140-6736(00)02034-1.
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(1):23-30. doi:https://doi.org/10.1200/JCO.2004.09.046.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. The New England journal of medicine. 2004;350(23):2335-42. doi:https://doi.org/10.1056/NEJMoa032691.
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. The New England journal of medicine. 2009;360(14):1408-17. doi:https://doi.org/10.1056/NEJMoa0805019.
Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(22):3677-83. doi:https://doi.org/10.1200/JCO.2008.20.5278.
Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. Journal of the National Cancer Institute. 2009;101(19):1308-24. doi:https://doi.org/10.1093/jnci/djp280.
Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(3):466-74. doi:https://doi.org/10.1200/JCO.2009.23.3452.
Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(15):2011-9. doi:https://doi.org/10.1200/JCO.2010.33.5091.
Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Annals of surgery. 2013;258(4):619–26; discussion 26–7. doi:https://doi.org/10.1097/SLA.0b013e3182a5025a.
Margonis GA, Buettner S, Andreatos N, Kim Y, Wagner D, Sasaki K et al. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA surgery. 2018;153(7):e180996. doi:https://doi.org/10.1001/jamasurg.2018.0996.
Mizuno T, Cloyd JM, Vicente D, Omichi K, Chun YS, Kopetz SE et al. SMAD4 gene mutation predicts poor prognosis in patients undergoing resection for colorectal liver metastases. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2018;44(5):684-92. doi:https://doi.org/10.1016/j.ejso.2018.02.247.
Chun YS, Passot G, Yamashita S, Nusrat M, Katsonis P, Loree JM et al. Deleterious Effect of RAS and Evolutionary High-risk TP53 Double Mutation in Colorectal Liver Metastases. Annals of surgery. 2019;269(5):917-23. doi:https://doi.org/10.1097/SLA.0000000000002450.
Kawaguchi Y, Kopetz S, Tran Cao HS, Panettieri E, De Bellis M, Nishioka Y et al. Contour prognostic model for predicting survival after resection of colorectal liver metastases: development and multicentre validation study using largest diameter and number of metastases with RAS mutation status. The British journal of surgery. 2021. doi:https://doi.org/10.1093/bjs/znab086.
Kawaguchi Y, Kopetz S, Newhook TE, De Bellis M, Chun YS, Tzeng CD et al. Mutation Status of RAS, TP53, and SMAD4 is Superior to Mutation Status of RAS Alone for Predicting Prognosis after Resection of Colorectal Liver Metastases. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25(19):5843-51. doi:https://doi.org/10.1158/1078-0432.CCR-19-0863.
Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer cell. 2018;33(1):125–36 e3. doi:https://doi.org/10.1016/j.ccell.2017.12.004.
Mizuno T, Cloyd JM, Vicente D, Omichi K, Chun YS, Kopetz SE et al. SMAD4 gene mutation predicts poor prognosis in patients undergoing resection for colorectal liver metastases. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2018. doi:https://doi.org/10.1016/j.ejso.2018.02.247.
Kawaguchi Y, Lillemoe HA, Panettieri E, Chun YS, Tzeng CD, Aloia TA et al. Conditional Recurrence-Free Survival after Resection of Colorectal Liver Metastases: Persistent Deleterious Association with RAS and TP53 Co-Mutation. Journal of the American College of Surgeons. 2019;229(3):286–94 e1. doi:https://doi.org/10.1016/j.jamcollsurg.2019.04.027.
Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Annals of surgery. 2009;250(4):540–8. doi:https://doi.org/10.1097/SLA.0b013e3181b674df.
Kawaguchi Y, Kopetz S, Lillemoe HA, Hwang H, Wang X, Tzeng CD et al. A New Surveillance Algorithm After Resection of Colorectal Liver Metastases Based on Changes in Recurrence Risk and RAS Mutation Status. Journal of the National Comprehensive Cancer Network : JNCCN. 2020;18(11):1500-8. doi:https://doi.org/10.6004/jnccn.2020.7596.
Singh RR, Patel KP, Routbort MJ, Reddy NG, Barkoh BA, Handal B et al. Clinical validation of a next-generation sequencing screen for mutational hotspots in 46 cancer-related genes. The Journal of molecular diagnostics : JMD. 2013;15(5):607-22. doi:https://doi.org/10.1016/j.jmoldx.2013.05.003.
Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J et al. OncoKB: A Precision Oncology Knowledge Base. JCO precision oncology. 2017;2017. doi:https://doi.org/10.1200/PO.17.00011.
Kawaguchi Y, Fuks D, Kokudo N, Gayet B. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Annals of surgery. 2018;267(1):13-7. doi:https://doi.org/10.1097/SLA.0000000000002176.
Kawaguchi Y, Hasegawa K, Tzeng CD, Mizuno T, Arita J, Sakamoto Y et al. Performance of a modified three-level classification in stratifying open liver resection procedures in terms of complexity and postoperative morbidity. The British journal of surgery. 2019. doi:https://doi.org/10.1002/bjs.11351.
Kawaguchi Y, Tanaka S, Fuks D, Kanazawa A, Takeda Y, Hirokawa F et al. Validation and performance of three-level procedure-based classification for laparoscopic liver resection. Surgical endoscopy. 2019. doi:https://doi.org/10.1007/s00464-019-06986-6.
Kawaguchi Y, Lillemoe HA, Vauthey J-N. Surgical Resection. Clinics in liver disease. 2020;24(4):637-55. doi:https://doi.org/10.1016/j.cld.2020.07.004.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA: a cancer journal for clinicians. 2017;67(2):93–9. doi:https://doi.org/10.3322/caac.21388.
Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA : the journal of the American Medical Association. 2007;298(3):309-16. doi:https://doi.org/10.1001/jama.298.3.309.
Kawaguchi Y, Lillemoe HA, Vauthey JN. Dealing with an insufficient future liver remnant: Portal vein embolization and two-stage hepatectomy. Journal of surgical oncology. 2019;119(5):594-603. doi:https://doi.org/10.1002/jso.25430.
Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. The oncologist. 2012;17(10):1225-39. doi:https://doi.org/10.1634/theoncologist.2012-0121.
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(13):1658-64. doi:https://doi.org/10.1200/JCO.2006.08.1620.
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(10):1626-34. doi:https://doi.org/10.1200/JCO.2007.14.7116.
Yaeger R, Cercek A, O'Reilly EM, Reidy DL, Kemeny N, Wolinsky T et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(6):1313-20. doi:https://doi.org/10.1158/1078-0432.CCR-14-2779.
Juric D, Rodon J, Tabernero J, Janku F, Burris HA, Schellens JHM et al. Phosphatidylinositol 3-Kinase alpha-Selective Inhibition With Alpelisib (BYL719) in PIK3CA-Altered Solid Tumors: Results From the First-in-Human Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(13):1291-9. doi:https://doi.org/10.1200/JCO.2017.72.7107.
Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. The lancet oncology. 2016;17(6):738-46. doi:https://doi.org/10.1016/s1470-2045(16)00150-9.
Acknowledgements
The authors thank Mario De Bellis for reviewing the data used in the study, Ms. Ruth Haynes for administrative support in the preparation of this manuscript, and Ms. Stephanie Deming, an employee of the Research Medical Library at MD Anderson Cancer Center, for copyediting the manuscript.
Funding
This research was supported in part by the National Institutes of Health through MD Anderson Cancer Center Support Grant, CA016672.
Author information
Authors and Affiliations
Contributions
Substantial contributions to:
The conception or design of the work: YK and JNV.
The acquisition, analysis, or interpretation of data for the work: YK, SK, EP, HH, XW, HT, CWT, YSH, TA, and JNV.
Drafting the work or revising it critically for important intellectual content: All authors.
Final approval of the version to be published: All authors.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kawaguchi, Y., Kopetz, S., Panettieri, E. et al. Improved Survival over Time After Resection of Colorectal Liver Metastases and Clinical Impact of Multigene Alteration Testing in Patients with Metastatic Colorectal Cancer. J Gastrointest Surg 26, 583–593 (2022). https://doi.org/10.1007/s11605-021-05110-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-021-05110-1