Abstract
Background
While observation of T1(≤2cm) nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs) is an accepted practice, an ill-defined subgroup of patients with T1 tumors develops metastases. This study aimed to identify those patients via clinical factors.
Methods
Patients from the Surveillance, Epidemiology, and End Results (SEER) registry who were diagnosed with NF-PanNET with size ≤2cm between 1998 and 2014 and who underwent primary tumor resection were identified. Binary logistic regression analyses were performed to evaluate factors associated with pathological nodal and systemic metastatic disease.
Results
A total of 612 patients with T1 NF-PanNETs were identified. Of those, 72 (11.7%) developed nodal metastasis and 35 (5.7%) distant metastasis (M1). In the multivariable analysis, tumor location in the pancreatic body (OR 1.903, p=0.03) (OR 1.407, p=0.038) or tail (OR 1.258, p=0.04) (OR 1.612, p=0.021); tumor grade III–IV (OR 2.042, p=0.022) (OR 5.379, p≤0.001); and younger age (OR 0.963, p=0.01) (OR 0.919, p=0.009) were associated with nodal metastases and the presence of M1 disease, respectively.
Conclusion
While the low metastatic potential of ≤2cm NF-PanNET implies watchful waiting to be an appropriate strategy for most patients, the increased risk of metastatic disease in younger patients with high grade (III–IV) body/tail tumors suggests individualized risk stratification to be optimal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of small PanNETs (T1, size ≤2 cm) has significantly increased in the past few decades due to improvement in diagnostic techniques, frequency of imaging,1 and possibly a true rise in the incidence of small PanNETs.2, 3 In this context, patients with asymptomatic T1 tumors are generally observed due to their presumed low risk of metastasis.4 However, a limited study reported that 7.7% (3/39) of small PanNETs ≤2 cm developed recurrence or metastatic disease and patients eventually die of metastatic progression.4 Moreover, metastatic progression has been observed even in small PanNETs with otherwise low-risk features such as low-grade histology and no other invasive characteristics.4,5,6,7 To this end, a recent collaborative study of 501 surgical resected PanNETs demonstrated that 5% of patients with T1 N0 disease experienced recurrence after resection.6
Due to the overall rarity and heterogeneity of PanNETs, accurate survival prediction and risk stratification have proven to be challenging. Further, the absence of a consistent genetic profile of PanNETs associated with metastatic progression further complicates risk stratification.8 While the apoptotic regulator DAXX (25%), the chromatin modifier ATRX (40%),9 and the mTOR signaling pathway (14%)9, 10 have been implicated in conferring biologic behavior, these alterations have not been found to be reliable PanNET biomarkers.11 Some progress has been made recently with epigenomes and transcriptomes that partially resemble islet α and β cells. Transcription factors ARX and PDX1 specify these normal cells. A recent study demonstrated that relapses in NF-PanNET occurred in patients with ARX+PDX1- tumors and, within this subtype, in cases with alternative lengthening of telomeres.12 Further, a different study showed that within the 2% of small PanNETs that developed distant metastases, each case harbored loss or deletion of at least 1 of DAXX/ATRX, H3K36me3, ARID1A, and CDKN2A markers.13 As these data matures and in the absence of consistent clinically applicable biomarkers, clinical prognosticators remain of critical importance.
A frequently used clinical predictor for metastatic progression in patients with localized PanNET is tumor size.14 However, while there is consensus that resection of PanNETs greater than 2cm is the optimal approach in many patients, there is scarce evidence guiding clinical decision-making for tumors less than 2cm.15 There have been several consensus recommendations addressing the issue of management of T1 NF-PanNETs. The European Neuroendocrine Tumor Society (ENETS)16 and NCCN Clinical Practice Guidelines for Neuroendocrine and Adrenal Tumors17 suggest that patients with tumors ≤2 cm may be selectively observed. This recommendation is stronger for PanNETs <1 cm that are incidentally identified and are of low grade. Observation is also suggested by the North American Neuroendocrine Tumor Society (NANETS), which has recommended an observation period without a plan for immediate surgical resection for tumors measuring < 1 cm and selective observation for patients with tumors 1–2 cm in size.15
Therefore, since tumor biology, molecular markers, and clinical features of T1 NF-PanNETs associated with metastatic spread remain ill-defined, this population-based retrospective study aims at identifying clinical and pathologic factors associated with metastatic progression of NF-PanNETs ≤2 cm. Hence, the hypothesis of this study is that while generally PanNETs ≤2cm are at low risk for metastases, a subgroup of PanNET are at increased risk for metastatic progression and can be identified by clinical-pathologic characteristic. This high-risk subgroup may benefit from surgical resection.
Patients and Methods
The Surveillance, Epidemiology, and End Results (SEER) database was queried to identify patients with resected pancreatic neuroendocrine tumors (PanNETs) diagnosed between 1998 and 2014. PanNET were classified according to the International Classification of Diseases for Oncology, Third Edition (ICD-O3) codes: islet cell carcinoma (8150), carcinoid (8240), enterochromaffin cell carcinoid (8241), enterochromaffin-like cell tumors (8242), goblet cell carcinoid (8243), composite carcinoid (8244), adenocarcinoid (8245), neuroendocrine carcinoma (8246), and atypical carcinoid (8249). Patients with potentially functioning PanNETs were not included due to the differences in biology and clinical management of functioning tumors compared to nonfunctional tumors.8, 18 According to institutional protocol, this national database study is exempt from institutional review board approval. A waiver of informed consent and a waiver of authorization are requested in this retrospective database review.
Inclusion criteria were patient age over 18, tumor size ≤2 cm, known location (head, body, tail) in the pancreas, known histologic grade, single focus, known nodal status, number of lymph nodes dissected, and complete overall survival and follow-up data. In the SEER database for solid tumor cancers, a four-grade system is utilized: Grade I, also called well-differentiated; Grade II, also called moderately differentiated; Grade III, also called poorly differentiated; and Grade IV, also called undifferentiated or anaplastic. Elements of the WHO grading for neuroendocrine neoplasms, including mitotic rates and Ki67, are not available in the SEER dataset.
Outcome Measurement
The primary outcome measure was to identify the risk factors associated with development of nodal and M1 disease. Secondary outcome measures were disease-specific survival (DSS) and overall survival (OS). DSS was defined as a net survival measure representing survival to death attributable to the primary cancer in the absence of other causes of death from the time of resection and overall survival (OS) defined as a net survival measure representing survival to any cause of death from the time of resection.
Statistical Analyses
Binary logistic regression analyses were performed to analyze factors associated with nodal and systemic metastasis in the entire cohort. Factors included in the analyses were age and tumor size as continuous variables. Tumor size, nodal status (N0, N1, NX), sex (male, female), histological grade (I, II, III, and IV), and location in pancreas (head, body/tail, overlapping, not specified) were analyzed as categorical variables. Categorical variables were compared with χ2 or Fisher’s exact test where appropriate. Continuous variables were analyzed using the Wilcoxon rank sum test. Kaplan-Meier survival analyses and log-rank tests were performed to assess the impact of nodal status on DSS and OS. Nodal status was determined by number of lymph nodes dissected and number of positive lymph nodes recorded in the SEER database (N0, node negative, or N1, node positive). When no lymph nodes were retrieved, or no nodes were found in the pathology specimen, the lymph node status was defined as NX. Mean survival with 95% confidence interval was recorded. Cox proportional hazard regression was used to determine the impact of potential confounders on outcomes. SPSS 21 (IBM corp. Armonk, NY) was used for all statistical analyses. A p value of 0.05 was selected to reject the null hypothesis.
Results
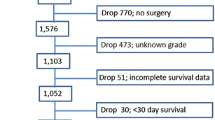
We identified a total of 612 patients who underwent resection for a pancreatic neuroendocrine tumor measuring 2 cm or less and who had complete data (CONSORT diagram, Supplementary Figure 1). Mean age was 55 years (range, 19–85); 294 (48%) were woman. Seventy-two (11.7%) had nodal metastasis (N1), and 35 (5.7%) had M1 disease. Four hundred fifty-one (73.6%) patients had grade I–II disease. Most of the patients had a pancreatic tumor in the head (265, 43.1%). Patient demographic characteristics are presented in Table 1.
Factors Associated with N1 Disease
Among the 72 patients with nodal metastases, the mean age was 55 years (range 19–85); 33 were woman (45.4%), 9 patients (13.2%) also had distant metastatic disease, and 22 patients (31%) had grade III–IV disease. Tumor location in the body (odds ratio [OR] 1.903; 95% confidence interval [CI] 1.155–3.148; p=0.030) or tail (OR 1.258; 95% CI 1.009–2.623; p=0.040) of pancreas, histological grade III–IV (OR 2.042; 95% CI 1.106–3.768; p=0.022), and age (OR 0.963; 95% CI 0.936–0.991; p=0.010) were associated with the presence of nodal metastases (Table 2). In histological grade I–II PanNETs, tumor location in the tail (odds ratio [OR] 1.460; 95% confidence interval [CI] 1.02–2.87; p=0.040) and younger age (OR 0.981; 95% CI 0.963–0.999; p=0.040) were associated with the presence of nodal metastases. In both cohorts, tumor size when modeled as a continuous variable was not associated with the presence of nodal metastases.
Factors Associated with M1 Disease
Among the 35 patients with nodal metastases, the mean age was 53 years (range 22–84); 18 were woman (51.4%). Eighteen patients (51.4%) had grade III–IV disease. Patient age (OR 0.919; 95% CI 0.863–0.979; p=0.009), tumor location in the body (OR 1.407; 95% CI 1.105–2.006; p=0.038) and tail (OR 1.612; 95% CI 1.074–2.420; p=0.021) of pancreas, and histological grade III–IV (OR 5.379; 95% CI 2.083–13.891; p<0.001) were associated with the presence of M1 disease (Table 2). In the sub analysis of the histological grade I-II patients. Tumor location in the tail (odds ratio [OR] 1.541; 95% confidence interval [CI] 1.05–2.10; p=0.033) and age (OR 0.921; 95% CI 0.88–0.961; p=0.012) were associated with the presence of M1 disease.
Disease-Specific Survival and Overall Survival
The mean DSS among all patients was 141.022 months (95% CI, 133.4–148.5). Mean OS was 142.186 months (95% CI, 134.6–149.7). Cox multivariate regression models demonstrated that age (OS hazard ratio [HR] 1.045 95% CI [1.023–1.067], p=0.001, DSS HR 1.047 95% CI [1.018–1.076], p=0.001), woman (OS HR 0.749 95% CI [0.531–0.968], p=0.007, DSS HR 0.635 95% CI [0.407–0.863], p=0.006), grade I–II disease (OS HR 0.328 95% CI [0.158–0.497], p<0.001, DSS HR 0.252 95% CI [0.102–0.402], p<0.001), and N1 disease (OS HR 2.269 95% CI [1.161–3.773], p<0.001; DSS HR 3.529 95% CI [1.375–5.682], p= 0.005) were significant factors affecting overall survival and disease-specific survival (Supplementary Table 2). Using the same Cox multivariate regression for histological grade I–II, only age (OS HR 1.076 95% CI [1.031–1.0123], p=0.001, DSS HR 1.029 95% CI [1.003–1.055], p=0.028) and N1 disease (OS HR 1.011 95% CI [1.005–1.018], p=0.001; DSS HR 2.603 95% CI [1.248–5.432], p= 0.011) were significant factors affecting overall survival and disease-specific survival. Tumor size was only significant for OS (HR 1.012 95% CI [1.003–1.012], p=0.001).
The presence of regional lymph node spread was associated with reduced DSS and OS. Mean DSS was 151.5 months for patients with T1N0M0 disease and 125.6 months for those with T1N1M0 disease (log-rank test, p=0.002) (Fig. 1a). Mean OS was 148.2 months for patients with T1N0 disease and 120.5 months for those with T1N1 disease (log-rank test, p=0.002) (Table 3) (Fig. 1b). As expected, presence of distant metastasis was associated with worse survival. Patients with T1 Nany M1 disease had a mean DSS of 94.7 months, compared with 139.7 months for patients with T1NanyM0 disease (log-rank test, p<0.001) (Supplementary Figure 2A); mean OS was 98.1 months in patients with T1 Nany M1disease versus 148.2 months for T1NanyM0 disease (log-rank test, p<0.001) (Supplementary Figure 2B), respectively.
Survival analysis stratified by the clinical risk factor for metastatic disease showed that patients with high-risk features including location in the body and tail and histologic grade III–IV had worse survival than patients without these features [DSS mean 109.5 vs. 143.5 months; p=0.031 and OS 106.7 vs. 144.4 months, p=0.037] (Table 3) (Fig. 2).
Discussion
In this population-based study, we found that tumor location in the body or tail, histologic grade III–IV, and younger age were associated with presence of nodal and distant metastasis. Further, DSS and OS were significantly shorter in patients with N1 disease compared to patients with N0 disease. These easily identifiable clinical factors (age, tumor location, and grade) were associated with an increased risk of recurrence and death in patients with T1 PanNETs. In the appropriate clinical context, this increased risk may prompt stratification towards resection rather than observation.
Pancreatic neuroendocrine tumors of ≤2 cm are an increasingly encountered clinical problem. They are often considered biologically indolent and safe to be observed.4, 19,20,21 Nonetheless, our study showed that 17.4% of patients with NF-T1 PanNETs presented with metastatic disease (N1 or M1) at time of resection. This implies watchful waiting to be an appropriate strategy for most patients, but not all. Identification of at-risk patients is crucial because indiscriminate resection would lead to overtreatment and unnecessary pancreatic resection. Undertreatment would lead to missing the opportunity of resection while still curable. Undertreatment of NF-T1 PanNETs is a serious concern, because, as reported here, once metastasized, even small PanNETs are uncurable in most patients.
In this study of patients with small PanNETs who underwent resection of the primary tumor, 11.7% of patients had N1 disease, and 5.7% patient had M1 disease. The impact of metastases on survival of patients with PanNET is an area of active research. NCCN guidelines for T1 PanNETs do not recommend routine lymphadenectomy, which seems appropriate considering the low frequency of N1 diseases in T1 PanNETs.17 However, based on this study, N1 disease was associated with worse DSS and OS. Basing the decision for lymphadenectomy on only radiographic suspicion may not suffice, because cross-sectional and nuclear imaging have been demonstrated to be frequently inaccurate in predicting metastases preoperatively.2, 19, 22, 23 Therefore, the present study aims at providing clinical factors that can aid in conjunction with imaging in the decision-making for lymphadenectomy. This study demonstrates that tumor location in the body or tail, histologic grade III–IV, and younger age are associated with nodal and distant metastasis and indeed worse DSS and OS compared to the rest of the cohort. Therefore, an individualized treatment approach that includes (a) the clinical prognosticators identified here, (b) surgical risk, and (c) the extent of resection may be a superior to indiscriminate watchful waiting for all patients with small NF-PanNET.
The relationship of metastases and histologic grade identified here is consistent with previous studies. A study from 16 European centers with 210 patients undergoing resection for T1 NF-PanNETs found 10.6% patients to have lymph node metastases. Only 3% of patients with WHO grade 1 tumors had positive nodes, while 16% of grade 2 and 100% of grade 3 tumors had N1 disease. Of those with grade 3, 11 patients developed recurrence at a median of 8 months.23 Hence, our study highlights the importance of understanding grade and differentiation even in small PanNETs. Although biopsy is not necessarily needed in all patients with small PanNETs, the lesion should have classic CT or MRI imaging characteristics of a well-differentiated low-grade PanNET if observation is recommended. Additionally, if 68Ga-DOTATATE PET/CT is performed, disease should be strongly avid. As the recent NANETS Consensus Guidelines for Surveillance and Medical Management of PanNETs stated, grade III (poorly differentiated) and grade IV (undifferentiated) neuroendocrine carcinomas represent a different clinical, pathologic, and genetic entity than well-differentiated lower grade neuroendocrine tumors.24 This is confirmed in this study by the observation that grade III–IV disease was associated with the highest risk of nodal and distant metastases.
Previous reports25, 26 have suggested that size of 1.5cm should be considered a size cut-off for surgical resection due to an incrementally increased risk of lymph node metastases. This finding contrasts with the results of our analysis that did not show an association between tumor size when modeled as a continuous variable and presence of nodal metastases. This difference in result may be due to a larger cohort of patients (612 vs 249 or 392) analyzed here. In addition, previous studies dichotomized the continuous variable of tumor size, which is associated with a loss of information, statistical power,27 and inflation of type I error.28 Therefore, careful consideration of the risk factors identified here (high histologic grade, tumor localization in the body or tail of pancreas, younger age) and growth over time (rather than the absolute size itself) should guide the decision-making for resections of small PanNET.
While this is the largest population-based study on small PanNET to date, this study has limitations beyond its retrospective nature. This is an observational study subject to unmeasured confounders. Additionally, some degree of selection bias is inherent in the analysis of patient whom underwent resection. Not all patients with small, arterially enhancing pancreatic lesion undergo biopsy and therefore are not entered into the registry. SEER represents a surgical cohort, and all patients received surgical management, possibly underestimating the true denominator. Nonetheless, this large, population-based study did identify important clinical prognosticators (high histologic grade, tumor localization in the body or tail of pancreas, and younger age) for small PanNETs applicable to daily practice. Third, SEER does not include elements of the WHO grading for neuroendocrine neoplasms, including proliferation index (Ki-67), mitotic rate, and lymphovascular invasion or perineural invasion, which further aid in prognostication.
In summary, this study suggests that individualized decision-making with the aid of the simple clinical parameters identified here may be the optimal approach to the management of patients with T1 NF-PanNET. PanNETs are a heterogeneous group of tumors characterized by clinical features and ill-defined genetic alterations. While new precise biologic prognosticators including genetic alterations are being identified in the future, the clinical parameters identified here can aid in risk stratification and clinical decision-making, today.
Conclusion
Our results suggest that a well-selected subgroup of young T1 NF-PanNET patients with concern for higher grade histology as indicated by biopsy, imaging features, or growth may benefit from a surgical approach. Moreover, while recognizing the limitations of the study cohort investigated here, the significant difference in OS/DSS in T1, N1 NF-PanNET suggests that inclusion of a lymphadenectomy should be considered in select patient when T1 PanNETs are being resected for purpose of prognostication.
References
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017; 3(10):1335-42.
Zerbi A, Falconi M, Rindi G, Delle Fave G, Tomassetti P, Pasquali C, et al. Clinicopathological features of pancreatic endocrine tumors: a prospective multicenter study in Italy of 297 sporadic cases. American Journal of Gastroenterology. 2010;105(6):1421-9.
Vagefi PA, Razo O, Deshpande V, McGrath DJ, Lauwers GY, Thayer SP, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Archives of Surgery. 2007;142(4):347-54.
Sadot E, Reidy-Lagunes DL, Tang LH, Do RK, Gonen M, D'Angelica MI, et al. Observation versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case-Control Study. Ann Surg Oncol. 2016;23(4):1361-70.
Gaujoux S, Partelli S, Maire F, D'Onofrio M, Larroque B, Tamburrino D, et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2013;98(12):4784-9.
Rosenblum RE, Harris CK, Baeg KJ, Starr JA, Brais LK, Stashek KM, et al. Predictors of Recurrence and Survival in Patients With Surgically Resected Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49(2):249-54.
Bettini R, Partelli S, Boninsegna L, Capelli P, Crippa S, Pederzoli P, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150(1):75-82.
Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14(12):3492-500.
Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199-203.
Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M, et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28(2):245-55.
Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543(7643):65-71.
Cejas P, Drier Y, Dreijerink KMA, Brosens LAA, Deshpande V, Epstein CB, et al. Enhancer signatures stratify and predict outcomes of non-functional pancreatic neuroendocrine tumors. Nat Med. 2019;25(8):1260-5.
Roy S, LaFramboise WA, Liu TC, Cao D, Luvison A, Miller C, et al. Loss of Chromatin-Remodeling Proteins and/or CDKN2A Associates With Metastasis of Pancreatic Neuroendocrine Tumors and Reduced Patient Survival Times. Gastroenterology. 2018;154(8):2060-3.e8.
Ro C, Chai W, Yu VE, Yu R. Pancreatic neuroendocrine tumors: biology, diagnosis,and treatment. Chin J Cancer. 2013;32(6):312-24.
Howe JR, Merchant NB, Conrad C, Keutgen XM, Hallet J, Drebin JA, et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49(1):1-33.
Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103(2):153-71.
Shah MH, Goldner WS, Halfdanarson TR, Bergsland E, Berlin JD, Halperin D, et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018 J Natl Compr Canc Netw. 2018;16(6):693-702.
Strosberg JR, Cheema A, Weber J, Han G, Coppola D, Kvols LK. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol. 2011;29(22):3044-9.
Lee LC, Grant CS, Salomao DR, Fletcher JG, Takahashi N, Fidler JL, et al. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152(6):965-74.
Sharpe SM, In H, Winchester DJ, Talamonti MS, Baker MS. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J Gastrointest Surg. 2015;19(1):117-23; discussion 23.
Finkelstein P, Sharma R, Picado O, Gadde R, Stuart H, Ripat C, et al. Pancreatic Neuroendocrine Tumors (panNETs): Analysis of Overall Survival of Nonsurgical Management Versus Surgical Resection. J Gastrointest Surg. 2017;21(5):855-66.
Kim MJ, Choi DW, Choi SH, Heo JS, Park HJ, Choi KK, et al. Surgical strategies for non-functioning pancreatic neuroendocrine tumours. Br J Surg. 2012;99(11):1562-8.
Sallinen VJ, Le Large TYS, Tieftrunk E, Galeev S, Kovalenko Z, Haugvik SP, et al. Prognosis of sporadic resected small (</=2 cm) nonfunctional pancreatic neuroendocrine tumors - a multi-institutional study. HPB (Oxford). 2018;20(3):251-9.
Halfdanarson TR, Strosberg JR, Tang L, Bellizzi AM, Bergsland EK, O'Dorisio TM, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49(7):863-81.
Dong DH, Zhang XF, Poultsides G, Rocha F, Weber S, Fields R, et al. Impact of tumor size and nodal status on recurrence of nonfunctional pancreatic neuroendocrine tumors </=2 cm after curative resection: A multi-institutional study of 392 cases. J Surg Oncol. 2019;120(7):1071-9.
Zhang IY, Zhao J, Fernandez-Del Castillo C, Braun Y, Razmdjou S, Warshaw AL, et al. Operative Versus Nonoperative Management of Nonfunctioning Pancreatic Neuroendocrine Tumors. J Gastrointest Surg. 2016;20(2):277-83.
Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127-41.
Austin PC, Brunner LJ. Inflation of the type I error rate when a continuous confounding variable is categorized in logistic regression analyses. Stat Med. 2004;23(7):1159-78.
Author information
Authors and Affiliations
Contributions
Substantial contributions to:
- The conception or design of the work: all authors.
- The acquisition, analysis, or interpretation of data for the work: EAV, OCK, OS, SVA, and CC.
- Drafting the work or revising it critically for important intellectual content: all authors.
- Final approval of the version to be published: all authors.
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vega, E.A., Kutlu, O.C., Alarcon, S.V. et al. Clinical Prognosticators of Metastatic Potential in Patients with Small Pancreatic Neuroendocrine Tumors. J Gastrointest Surg 25, 2593–2599 (2021). https://doi.org/10.1007/s11605-021-04946-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-021-04946-x