Abstract
Objectives
Organ-sparing endoscopic submucosal dissection (ESD) is an acceptable treatment strategy for superficial neoplastic lesions of the esophagus and stomach. The adoption of this technique has lagged in North America compared with Asia, and we sought to report on our experiences with ESD for upper GI neoplasia.
Methods
A prospectively entered database of all patients undergoing endoscopic resection of esophageal and gastric neoplasia at McGill University from 2009 to 2019 was queried for those who received ESD.
Results
A total of 103 consecutive ESDs were identified from 2009 to 2019. Seventy-one (69%) patients were male and the median age was 72 (range: 38–90). Sixty-one (59%) cases were esophageal and 42 (41%) gastric. Forty-nine (48%) were performed in the endoscopy suite under local sedation only. Perforation occurred in 9 patients (7 esophageal and 2 stomach), of which 3 required operative repair. Histology was principally invasive carcinoma (79, 77%), with 17 (16%) dysplastic lesions (e.g., HGD), 1 (1%) neuroendocrine tumor, and 7 (7%) benign lesions. En bloc resection was achieved in 90 (87%), and the complete resection rate was 74 (72%), with 51 (50%) of procedures fulfilling the criteria for curative resection. At medium of 23-month (2–199) follow-up of these 51 curative resections, one case of recurrent carcinoma was found at follow-up and was managed with repeat endoscopic resection. Non-curative ESDs were found 45 (R1 resection = 29: risk of lymph node metastasis = 16), 21 had active surveillance, and 24 were resected.
Conclusion
ESD is a viable, effective, and safe therapeutic and staging modality for superficial lesions of the stomach and esophagus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancers of the esophagus and stomach represent a significant health burden worldwide. Combined, they have an estimated annual incidence of 1.4 million cases, with over 1.1 million deaths in 2012.1 Management of superficial esophageal and gastric neoplasms has seen striking advances in the last few decades; approaches have shifted from higher risk surgical resection to endoscopic, organ-sparing resectional techniques. Initially, endoscopic mucosal resection (EMR) was introduced in Japan, a technically facile technique enabling en bloc removal of smaller lesions (up to 1 cm), and this approach has experienced wide adoption in the West.2,3,4,5 However, larger lesions require a piecemeal approach with EMR to achieve complete resection, complicating pathologic assessment of margins, and leading to a high local rate of recurrence in some studies.6
Endoscopic mucosal dissection (ESD) is another technique originating from Japan7 which allows for the en bloc removal of larger and deeper lesions.8,9 While ESD is more technically challenging for the practitioner, this technique has been shown in Japanese gastric cancer patients to be associated with lower local recurrence rates and greater curative resection rates, while maintaining similar rates of serious intra-operative complication, such as emergency surgery for bleeding.9,10 Similar findings were reported regarding ESD as compared with EMR in the management of superficial esophageal neoplasms.6,11
The higher en bloc resection rate seen with ESD also allows for a more consistent pathological assessment of the resected specimen. Lymphovascular invasion, tumor depth, and tumor involvement at the resection margin are all critical in assessing the curability of a procedure as well as planning post-operative patient management.12,13 Indeed, according to standard guidelines, the en bloc nature itself of a particular resection, as compared with piecemeal, is an important factor in determining curability.13
Despite the advantages of ESD over EMR for the removal of upper GI lesions, the adoption of ESD in North America has been limited, and most of the data that have been collected originate from East Asian countries. We report on our experience with ESD, one of the largest in a North American setting, for the treatment of superficial neoplastic lesions of the upper GI tract to assess its viability, safety, and efficacy from a Western perspective.
Materials and Methods
Data Collection
A local, prospectively maintained gastric and esophageal cancer database from a single university-associated North American hospital was reviewed for patients undergoing endoscopic submucosal dissection from 2010 to 2019 irrespective of histology or indications for endoscopic resection (curative vs diagnostic/staging). Patients were selected for ESD as either a therapeutic, diagnostic, or staging procedure. All patients consented to the study through an IRB-approved protocol. Patient demographics, lesion characteristics and histology, procedural approach, and variables, length of stay, and post-operative outcomes were collected and reported. Details were supplemented by hospital charts. An en bloc dissection is defined as complete removal of the tumor in one piece. Histological complete resection (R0) is defined as having margins negative for malignancy and designated as either deep or circumferential (mucosal). Curative and non-curative resections were determined according to the Japanese Gastric Cancer Treatment Guidelines13: a curative resection must be en bloc; the tumor must be less than or equal to 2 cm, histologically differentiated, and pT1a; and there must be no pathological evidence of margin involvement or lymphovascular invasion. Resections were classified as curative under expanded indications if the above criteria were met, but the tumor was larger than 2 cm; the tumor was histologically undifferentiated; or the tumor was less than or equal to 3 cm and pT1b (SM1). Non-curative resections were further subclassified as incomplete margins (R1), or non-curative based on the relatively higher risk of occult lymph node metastasis (T1b(SM2) and/or LVI+). In the absence of similarly well-defined criteria on the curability of esophageal adenocarcinoma resections, and noting that these guidelines apply equally to gastro-esophageal junction cancers, these criteria were extrapolated to the esophageal tumors in this study, supplemented by previous work on the risk of lymph node metastases in these cases.14
ESD Technique
Esophageal and gastric ESD procedures were performed in two different settings: in the operating room under general anesthetic and in the endoscopy suite under conscious sedation.
A single thoracic surgeon/surgical endoscopist performed all procedures with a standard high-definition gastroscope. Various attachments were used including an electrosurgical device with the insulated tip ITknife2 (KD-611 Olympus Co. Ltd., Japan), electrosurgical dual knife (KD650 L Olympus Co. Ltd., Japan), and/or Coagrasper (FD-410LR; Olympus Co. Ltd., Japan). Both white light and narrow-band imaging (NBI) were used to visualize lesions. Mucosal markings were made at 2-mm intervals around the lesion using the needle knife or dual knife (Fig. 1). Submucosal injection using a 10% glycerol or hydroxyethyl starch solution mixed with indigo carmine and dilute epinephrine was performed to elevate the lesion. A small mucosal incision was made using a standard needle knife. From this opening, the insulated tip or dual knife was introduced and used to complete the circumferential mucosal incision around the tumor, followed by submucosal dissection at the plane between the submucosa and muscularis propria. Most dissections were performed from distal to proximal, as this enhances visualization throughout the procedure. Specimens were extracted by the mouth and pinned on a specimen board before fixation. Patients were mostly admitted for observation overnight, discharged on a progressive liquid and soft diet the following day, and treated with both proton pump inhibitors and sucralfate orally for 8 weeks.
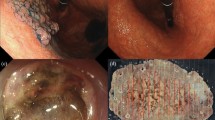
Endoscopic submucosal dissection of a cT1 adenocarcinoma of the gastric antrum. a Initial lesion seen straddling the incisura of the antrum. b Marking of the eventual resection margins with the needle knife and submucosal mucosal injection with 10% glycerol/methylene blue/epinephrine solution. c Mucosal incision completed with the IT2 knife prior to submucosa dissection. d Post-resection ESD ulcer with muscularis propria visualized. e Specimen pinned before fixation to allow accurate assessment of margins
Pre-ESD staging was initially performed by endoscopic ultrasound (EUS) for all patients; however, in our experience, the lack of accuracy of this technique led us to largely abandon routine EUS prior to ESD. Rather, the decision to proceed to ESD was based on the endoscopic appearance of the lesion, including pit pattern on NBI, and if the lesion was lifted with submucosal injection. ESD was thus used not infrequently as a diagnostic and staging modality with potential therapeutic intent. Pre-ESD CT scans were undertaken in all patients with prior biopsy-confirmed invasive cancer (67/79 = 85%) to assess for regional or distant disease. PET scans were not performed in any patient prior to ESD; however, PET scans were performed selectively post-ESD based on the cases’ curability status and lymph node involvement risk in those with non-curative ESD. Active surveillance with endoscopy, EUS, and CT was employed similarly for non-curative resections that were otherwise complete (R0) and which did not proceed to surgery.
Post-ESD Oncologic Management
In the case of curative resection, follow-up endoscopies with biopsy at the resection site were offered at post-procedure at 3-month intervals in the first year, 6-month intervals in the second year, and yearly subsequently. All patients with cancer or dysplastic lesions who did not undergo subsequent surgical resection completed endoscopic follow-up largely according to this schedule. Patients with non-curative resections were evaluated for either surveillance, repeat ESD or EMR, or organ resection based on the risk of nodal involvement, location of margin involvement, viability of the patient as a surgical candidate, and patient preference. These patients were also offered EUS and computed tomography (CT) scan in order to confirm or rule out regional or distant disease. Patients discovered to have local recurrence were treated with repeat ESD or EMR, or organ resection based on similar criteria. No adjuvant radiation or chemotherapy was used in this series.
Statistical Analysis
Descriptive statistics were calculated and presented as median and range or interquartile range (IQR) for continuous variables and percentages for categorical variables. The Kaplan-Meier estimator was used to generate the survival function and corresponding survival curves in Fig. 4.
Results
Table 1 shows the characteristics of the patients and lesions. Of the 103 patients who underwent ESD for gastric or esophageal lesions, 69% (71/103) were male, with a median age of 72 (38–90) years. The majority of these (59%: 61/103) were performed for esophageal or gastroesophageal junction (GEJ) lesions, while the rest were gastric. The median size of resected specimens was 35 (17–65) mm and on histological examination, the median lesion size was 19 (5–55) mm. There were 79 cases of either adenocarcinoma or squamous cell carcinoma; of these, the vast majority of lesions were confined to either the mucosa (44%: 35/79) or the submucosa (52%: 41/79). Of these 79 carcinoma cases, 51% (40/79), including all 4 cases of squamous cell carcinoma, were of the esophagus, and the rest gastric (49%). Lymphovascular invasion was found in 19% of cases.
Pre-operative endoscopic ultrasound (EUS) staging of lesions was performed in 57 patients in total. Of these, 4 cases did not yield a staging result due to the absence of a visualized lesion or to the procedure being aborted. One benign lipoma was correctly identified. Of the remaining 52 cases, the same depth staging was obtained post-operatively through pathological examination as through EUS in 58% (30/52) of cases, indicating a low rate of accuracy. EUS overestimated or underestimated the depth in 23% (12/52) and 19% (10/52) of cases respectively.
Approximately half (52%: 54/103) of the procedures were performed in the operating room under general anesthesia, though Fig. 2 shows that the use of the endoscopy suite under conscious sedation increased with the number of cases performed. Most of the resections were successfully performed en bloc (87%: 90/103) and discovered on pathological examination to be histologically complete R0 (72%: 74/103). Of those resections with positive margins, the majority were at the deep margin (90%: 26/29). Regarding the 79 cases of carcinoma specifically, 91% (72/79) were performed en bloc and the R0 resection rate was 66% (52/79). Of those found to be R1, involvement was principally at the deep margin (93%: 25/27). Of the 9 cases (7 esophageal, 2 gastric) in which a perforation occurred, 6 were successfully managed endoscopically, with either clips or stents. None of the endoscopically repaired perforations required additional drains. Of these 9 cases, the median length of stay (LOS) was 2 (1–15) days. Three cases, all in the early experience (initial 30 cases), required operative repair including the following: laparoscopic wedge gastrectomy (LOS = 1 day), laparoscopic primary repair (LOS = 3 days), and left thoracotomy with primary repair (LOS = 13 days). On pathological exam, depth was principally submucosal (T1b) or deeper (67%: 6/9). Although intra-procedural bleeding was common, most were managed successfully with either the coag-grasper or clips. Post-ESD bleeding occurred in 2 cases, both of whom were successfully managed endoscopically (clips and/or coag-grasper). No patient died post-ESD (i.e., no in-hospital mortality). The median length of stay was 1 day; however, over the past 2 years, more patients have been discharged on the same day of the ESD (8%: 8/103). Only one patient required dilatation of an ESD stricture, an esophageal case with a 75% circumferential resection.
After the final pathological assessment, 51 procedures were determined to be curative under either absolute or expanded guidelines. These patients underwent endoscopic follow-up with a median time of most recent follow-up of 23 (2–119) months. Of the 43 absolute curative resections, there were 2 cases of recurrent high-grade dysplasia, treated with repeat ESD and EMR respectively. Of the 8 expanded criteria cases, there was one case of recurrent adenocarcinoma, for which the patient underwent subtotal gastrectomy. No patient with curative ESDs has died from malignancy.
Forty-five patients had non-curative ESD based on either histopathologic features (e.g., T1b(SM2) or lymphovascular invasion) associated with a relatively high risk of occult lymph node metastasis (16 patients) or incomplete (R1) resections (29 patients) (Fig. 3a and b). Overall, 21 elected to undergo active surveillance despite the non-curative nature of the ESD, most of which (16) were due to poor performance status or comorbidities and the demographics reflect this. The median age of this group was 79 years (60–90), and almost half (43%: 9/21) were of the American Society of Anesthesiologists (ASA) classification level 3. The remaining 24 had a resection and the median age in this group was 69 (38–79), and only 13% (3/21) were of ASA class 3, representing a significantly healthier population than those who underwent active surveillance.
For the 28 esophageal cancer patients who had “non-curative” resection (Fig. 3a), half proceeded to esophagectomy and the other half elected to undergo active surveillance. All 14 patients underwent minimally invasive esophagectomy (with 2 field lymphadenectomy) and lymph node yield following these resections was adequate for appropriate pathological staging: 28 (19–48). Pathologic outcomes from the surgical resections are detailed in Table 1. No residual malignancy was identified in 7 patients and in the 7 patients with remnant cancer post-ESD, 4 had positive lymph nodes. These resected patients have undergone follow-up for 21 (4–53) months and all remain alive and disease free except for 1 patient who has recurrent liver metastases at 6 months and died of disease at 21 months and another who succumbed to a stroke at 22 months. The patients who have undergone active surveillance have been followed for 35 (9–69) months. Two patients have recurred, one locally at 8 months who underwent repeat ESD and died without disease at 36 months, and another who died of metastatic disease at 16 months at the age of 90 years. Four other patients undergoing active surveillance have died without disease, not entirely unexpected given the median age of this cohort.
A non-curative ESD was performed in 17 gastric adenocarcinoma patients (Fig. 3b). Active surveillance was performed in 7 patients, one of whom had a recurrence (regional) and underwent delayed gastrectomy at 23 months (pT0N1-1/47 lymph node positive). A total of 11 patients had a post-ESD gastrectomy (9 laparoscopic subtotal and 2 total, 1 each laparoscopic and open) and D2 lymphadenectomy yielding 25 (15–47) lymph nodes. Final surgical pathology (Table 1) revealed no residual cancer in 5 patients and lymph node–positive disease in 2. Of these 17 non-curative ESDs, 4 patients have died, but only one from gastric cancer, at 24 months (Table 2).
Overall survival of the patients with invasive cancer is depicted in Fig. 4 and the 1-, 3-, and 5-year cumulative overall survival is shown in Table 3, demonstrating no difference in survival between tumor types (esophagus vs gastric, p = 0.520) but a trend towards worse survival in the non-curative resections based on the histologic features of the resected specimen (absolute/expanded curative vs non-curative, p = 0.196). Including the entire cohort of 79 patients with invasive carcinoma, three patients have died of recurrent malignancy.
Discussion
Since its origination in Japan, ESD has proven to be a safe and effective treatment for lesions of the esophagus and stomach, with widespread prevalence in Asia and also increasingly in Europe.8,15,16 In comparison with EMR, ESD is able to resect larger and deeper lesions in an en bloc fashion, allowing for not only lower local recurrence rates as a therapeutic modality but also to function as a more accurate diagnostic and pathological staging assessment tool.6 However, despite these numerous advantages, the adoption of this technique in North America has been slow, although interest in recent years has been increasing.17,18
Hesitancy can likely be attributed to the current lack of data supporting its use in the West, as well as the higher difficulty of this technique, with some reports indicating a requirement of at least 30 cases to reach proficiency.19 This in conjunction with the need for specialized tools and intubation means that ESD is generally performed in the operating theater.20,21 However, in our experience, we have shown that ESD can also be effectively performed in an endoscopy suite under conscious sedation and indeed this is currently the location of preference in over 80% of cases. Our results corroborate reports that ESD as a procedure requires a certain number of cases to achieve proficiency; all the perforations requiring surgical repair occurred in the first 30 cases.
Currently, direct visualization and endoscopic ultrasound are the two main modalities for pre-operative staging of superficial esophageal and gastric lesions.22,23 However, direct visualization is very poor at depth staging for all but the most superficial lesions, and EUS is also prone to over or underestimation in deeper lesions. Indeed, some reports indicate a 56% rate of either over or underestimation of T2 lesions in esophageal cancers and a 53% accuracy in T staging in the blinded evaluation of gastric lesions.24,25 In our study, correct T staging was obtained by EUS in only 58% of cases. ESD may also therefore serve as a more accurate diagnostic alternative to EUS for pre-operative T staging of these superficial lesions and, in a significant proportion of patients, provide a definitive therapeutic modality. Based on the inaccuracy of EUS, we employ this investigative procedure only selectively prior to ESD in patients with larger or more bulky lesions. Rather, an attempt at submucosal injection is performed to see if the lesion lifts off the muscularis propria, and if this is successful, then an ESD is attempted as both a staging and potentially therapeutic modality.
The majority of the cases in the present series are esophageal adenocarcinomas, representing one of the largest single institution series on this histology. With ESD, we were able to achieve an acceptable rate of complete resection (74%) in non-benign cases, and similarly in strict cases of carcinoma (66%: 52/79). More importantly, when dissecting directly off of the muscularis propria with ESD, our R1 deep rate is very low (3%), despite having a significant number of T1b lesions (over 50%). Although we do not have an EMR comparison group, this ability to achieve a complete resection in T1b lesions is precisely the proposed benefit of ESD over EMR. This is particularly important as there is increasing data to support endoscopic resection as a curative intent treatment for T1b(SM1) cancers26 of the esophagus and the modality that offers the best chance for complete resection should be embraced. Although our rate of R0 resection could be considered low compared with certain literature,9 we record also a significant number of submucosal or deeper cases (56%: 44/79) likely due to the fact that we employ ESD as not only a therapeutic tool but also for accurate diagnosis and T staging. With regard to our choice of management technique for these cases, we note that an endoscopic resection does not preclude more aggressive treatment, such as a subsequent definitive resection or adjuvant therapy. Indeed, endoscopic resection is also an investigative technique that not only provides accurate T staging but also can frequently result in definitive therapy.27 It is our opinion that it is preferable to favor an endoscopic approach for a potentially under-staged lesion than to begin directly with more aggressive treatment. Should ESD prove itself to be non-curative in a given case based on a high risk of lymph node metastasis, the option still exists to proceed to surgical resection and/or adjuvant and neoadjuvant therapy.
In our series, we had a moderately high rate of “non-curative” endoscopic resections (43%). Slightly more than 50% of these patients underwent surgical resection by esophagectomy or gastrectomy; the rest had active surveillance with a small minority reporting disease recurrence over a 3-year follow-up. This indicates that our liberal employment of ESD is justified, as it spared a significant proportion of patients a surgical resection, particularly important given the advanced age and performance status of this group. For the 53% of “non-curative” ESDs who underwent surgical resection, we were able to identify residual malignancy in only approximately 50% of the esophageal and gastric cancer cases. Indicating that almost half of the surgical patients could have been managed with organ-sparing approaches, highlighting the urgent clinical need to develop an effective risk stratification tool to determine with more accuracy the presence of occult lymph nodes must be done. Overall, in our cohort, only 30% of “non-curative” ESD patients had a therapeutically valuable surgical resection, suggesting that in some of these patients, organ-sparing approaches may be feasible. However, this must be tempered with the fact that 6 patients undergoing surgical resection post “non-curative” ESD had regional disease, and two patients with comorbidities precluding esophagectomy died of disease. This implies that although organ-sparing approaches may be feasible for a subset of patients, at present, it is difficult to identify these patients and therefore referral for surgical consideration remains the standard of care for “non-curative” ESD resections. Reliable methods to differentiate those patients with borderline “non-curative” ESD into those that can continue with an organ-sparing approach and those that truly require surgical resection are lacking; however, some interesting options are on the horizon. Sentinel lymph node mapping (SLNM) and biopsy, common in breast and melanoma, has entered the upper GI arena. The theoretical rationale for this approach is that patients with high-risk lesions (LVI or deep T1b) resected by ESD could undergo SLNM and if negative, the stomach and esophagus could be spared. Indeed, our group and others have demonstrated that SLNM is feasible for gastric cancer,28,29 but concrete evidence in support of routine use is lacking. Additionally, there is emerging data out of Japan supporting the concept of chemoradiation in the adjuvant setting after “non-curative” ESD for esophageal squamous cell carcinoma.30
The main limitations of this study are its retrospective nature and the lack of a control group consisting of patients undergoing either EMR or organ resection. There may also have been a selection bias towards older and more comorbid patients, who would not have made good candidates for organ resection. However, this limits only the generalizability of our results and not its internal validity. As a relatively new technique, long-term follow-up and recurrence data are also limited but will become available as time elapses.
In conclusion, endoscopic submucosal dissection is a viable and effective therapeutic, diagnostic, and staging option for the management of superficial lesions of the stomach and esophagus. While challenging, it offers a safe and practical alternative to techniques such as endoscopic mucosal resection or organ resection. Efforts should be made to identify and address the barriers to the adoption of this technique in North America.
References
Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends - An update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16-27.
Bennett C, Wang Y, Pan T. Endoscopic mucosal resection for early gastric cancer. Cochrane Database Syst Rev. October 2009.
D’Journo XB, Thomas PA. Current management of esophageal cancer. J Thorac Dis. 2014;6(Suppl 2):S253.
Hiki Y. [Endoscopic mucosal resection (EMR) for early gastric cancer]. Nihon Geka Gakkai Zasshi. 1996;97(4):273-278.
Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146(3):652-660.e1.
Guo H-M, Zhang X-Q, Chen M, Huang S-L, Zou X-P. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol. 2014;20(18):5540-5547.
Hirao M, Masuda K, Asanuma T, et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 34(3):264-269.
Park HC, Kim DH, Gong EJ, et al. Ten-year experience of esophageal endoscopic submucosal dissection of superficial esophageal neoplasms in a single center. Korean J Intern Med. 2016;31(6):1064-1072.
Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: A large-scale feasibility study. Gut. 2009;58(3):331-336.
Tanabe S, Hirabayashi S, Oda I, et al. Gastric cancer treated by endoscopic submucosal dissection or endoscopic mucosal resection in Japan from 2004 through 2006: JGCA nationwide registry conducted in 2013. Gastric Cancer. 2017;20(5):834-842.
Wang J, Ge J, Zhang X-H, Liu J-Y, Yang C-M, Zhao S-L. Endoscopic submucosal dissection versus endoscopic mucosal resection for the treatment of early esophageal carcinoma: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(4):1803-1806.
Sgourakis G, Gockel I, Lang H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol. 2013;19(9):1424-1437.
Kodera Y, Sano T. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Japanese Gastric Cancer Association 1. Gastric Cancer. 2017;20:1-19.
Lee L, Ronellenfitsch U, Hofstetter WL, et al. Predicting lymph node metastases in early esophageal adenocarcinoma using a simple scoring system. J Am Coll Surg. 2013;217(2):191-199.
Yamamoto H. Endoscopic submucosal dissection-current success and future directions. Nat Rev Gastroenterol Hepatol. 2012;9(9):519-529.
Probst A, Aust D, Märkl B, Anthuber M, Messmann H. Early esophageal cancer in Europe: Endoscopic treatment by endoscopic submucosal dissection. Endoscopy. 2015;18(4).
Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol. 2015;110(6):784-791.
Cho KB, Jeon WJ, Kim JJ. Worldwide experiences of endoscopic submucosal dissection: not just Eastern acrobatics. World J Gastroenterol. 2011;17(21):2611-2617.
Tsou Y-K, Chuang W-Y, Liu C-Y, et al. Learning curve for endoscopic submucosal dissection of esophageal neoplasms. Dis esophagus Off J Int Soc Dis Esophagus. 2016;29(6):544-550.
Rong Q-H, Zhao G-L, Xie J-P, Wang L-X. Feasibility and safety of endoscopic submucosal dissection of esophageal or gastric carcinomas under general anesthesia. Med Princ Pract. 2013;22(3):280-284.
ASGE Technology Committee, Maple JT, Abu Dayyeh BK, et al. Endoscopic submucosal dissection. Gastrointest Endosc. 2015;81(6):1311-1325.
Papanikolaou IS, Triantafyllou M, Triantafyllou K, Rösch T. EUS in the management of gastric cancer. Ann Gastroenterol. 2011;24(1):9-15.
Quint LE, Bogot NR. Staging esophageal cancer. Cancer Imaging. 2008;8(SPEC. ISS. A).
Meining A, Dittler HJ, Wolf A, et al. You get what you expect? A critical appraisal of imaging methodology in endosonographic cancer staging. Gut. 2002;50(5):599-603.
Pech O, Günter E, Dusemund F, Origer J, Lorenz D, Ell C. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy. 2010;42(6):456-461.
Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11(6):630-635.
Hansen T, Nilsson M, Lindholm D, Sundström J, Hedberg J. Normal radiological lymph node appearance in the thorax. Dis esophagus Off J Int Soc Dis Esophagus. 2019;32(10):1-6.
Mueller CL, Lisbona R, Sorial R, Siblini A, Ferri LE. Sentinel Lymph Node Sampling for Early Gastric Cancer—Preliminary Results of A North American Prospective Study. J Gastrointest Surg. 2019;23(6):1113-1121.
Takeuchi H, Goto O, Yahagi N, Kitagawa Y. Function-preserving gastrectomy based on the sentinel node concept in early gastric cancer. Gastric Cancer. 2017;20:53-59.
Suzuki G, Yamazaki H, Aibe N, et al. Endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer: Choice of new approach. Radiat Oncol. 2018;13(1).
Author information
Authors and Affiliations
Contributions
AC and MC acquired and performed analysis on the data and drafted the manuscript. AS assisted with data acquisition and analysis. CM, JCL, JS, and LF were responsible for the design of the project, interpretation of the data, and final manuscript revisions.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, A., Chen, M., Trepanier, M. et al. Endoscopic Submucosal Dissection for Upper Gastrointestinal Neoplasia—a North American Perspective. J Gastrointest Surg 24, 2456–2465 (2020). https://doi.org/10.1007/s11605-020-04791-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04791-4