Abstract
Background
Laparoscopic cholecystectomy is a high-volume surgery that is an end-stage treatment for gallstones. There is little understanding of the surgery’s effect on the gain in patients’ health relative to its cost. The objective of this study is to measure health gain, cost and cost utility of elective laparoscopic cholecystectomy.
Methods
Participants completed the EQ-5D(3L) pre-operatively and post-operatively. Quality adjusted life years attributable to cholecystectomy were calculated by comparing health state utility values between the pre- and post-operative time points. Laparoscopic cholecystectomy cost was calculated from a health system perspective and included hospital and specialists’ fees (in 2016 Canadian dollars). Cost per QALY was calculated for the entire sample and demographic sub-groups.
Results
The cohort consisted of 135 participants who completed surveys between February 2013 and June 2017. The response rate among eligible patients was 50%. Assuming that health gain accrued to the participant for 25 years after cholecystectomy, the mean gain in QALYs was 1.7430, corresponding to an average cost per QALY of $2102. Older patients, on average, had less gain in QALYs than younger patients.
Conclusion
Laparoscopic cholecystectomies are inexpensive relative to the gains in health they provide patients. The gains in health were not uniform across age categories. These results should provide health system planners confidence that incremental increases in surgical capacity for elective cholecystectomies is beneficial.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Cholelithiasis, or gallstones, affects 10–15% of adults in developed countries.1,2 There are a number of risk factors for gallstone formation including older age, being female, obesity and a sedentary lifestyle.1 Cholecystectomy, or gallbladder removal, is the end-stage treatment for cholelithiasis. Cholecystectomies are a high-volume procedure for hospitals; in fiscal year from April 1, 2014 to March 31, 2015, there were 28,315 cholecystectomies performed in Canada, making it the eighth most common surgical intervention. The estimated hospital costs to Canada’s public health insurance system for those surgeries were $135 million.3,4
There is a limited amount of literature evaluating the value of patients’ gain in health attributable to cholecystectomy relative to the surgery’s cost. A meta-analysis of two Norwegian randomized control trials found that cholecystectomy was cost-effective over conservative management for uncomplicated symptomatic gallstones at an incremental cost-effectiveness ratio of £13,205 or $24,672 (all currencies shown as 2016 Canadian dollars).5 A Canadian study compared early cholecystectomy (surgery within 1 week of presentation), delayed cholecystectomy (surgery within 8 to 12 weeks of presentation) and watchful waiting (ongoing symptom monitoring); early cholecystectomy was found to be the most cost-effective treatment pathway at almost $7000 per case versus $8500 for delayed cholecystectomy.6 However, the evidence is ambiguous; a US economic evaluation focused on older patients found otherwise—watchful waiting was more cost-effective than surgical options among older patients reporting tolerable symptoms due to increased risk of complications.7
The objective of this study is to use preference-based measures of health, to estimate the cost-utility of cholecystectomy among symptomatic patients. The analyses’ output is expressed as cost per quality-adjusted life year (QALY). The study takes advantage of a cohort of cholecystectomy patients who have completed patient-reported outcomes (PROs) pre-operatively and post-operatively.
Methods
Study Protocol
This study is based on a secondary analysis of a prospectively recruited longitudinal cohort of elective cholecystectomy patients of 14 general and colorectal surgeons in Vancouver Coastal Health authority (VCH) hospitals. All general and colorectal surgeons in three hospitals were approached to participate. Vancouver Coastal Health, in the province of British Columbia, is responsible for funding hospital services to over one million residents of the geographic region which includes greater Vancouver and coast Garibaldi regions, Canada.8
Prospective participants were contacted by phone pre-operatively by VCH. To be included, participants had to be not permanently residing in a nursing home or long-term care residence, 19 years of age or older, scheduled for surgery at least 2 weeks from being enrolled on the surgical queue in order to remove emergent cases, and able to respond with or without assistance to survey questions in English.9
Participants complete a pre-operative survey consisting of a battery of PRO instruments. Six months post-operatively, participants complete the same PROs. The PROs data are linked to hospital discharge summaries; participants’ comorbidities are identified from patients’ hospital discharge summaries.
This analysis is based on participants who completed their post-operative surveys between February 2013 and July 2017. Vancouver Coastal Health made an anonymized dataset available to the research team for this study’s analysis. The University of British Columbia’s Behavioural Research Ethics Board (BREB) approved the study.
Measures
All study participants complete EuroQoL’s EQ-5D(3L)10 pre-operatively and post-operatively. This instrument measures an individual’s general health in the domains of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The individual ranks each domain based on the severity of problems they experience.
Participants’ responses to the EQ-5D(3L) instrument are used to determine participants’ health state10 at pre-operative and post-operative survey points. Each participants’ health state is linked to previously derived utility values.11,12 These utility values represent a preference-based measure of health, as measured by the EQ-5D(3L). Utility values for all health states generated for the EQ-5D(3L) have been derived from a representative sample of Canadians independent of this study;13 the values range from − 0.34 to 1. Values below 0 are considered worse than death, while a utility value of 1 represents perfect health. Considered over time, utility values provide a means to calculate QALYs.14
Demographic characteristics of the participants are summarized with frequencies and percentages. Health state utility values are summarized using mean, standard deviation, and range statistics for the pre-operative and post-operative time points. The significance of the difference in pre-operative and post-operative utility values is determined through paired t tests.
Calculating Quality Adjusted Life Year
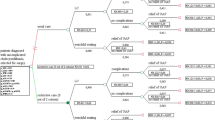
This study adopts an approach to measuring cost-utility similar to that of the United Kingdom’s National Health Service.15,16 Figure 1 provides a visual depiction of an example QALY calculation, where the shaded area is the gain in health attributable to cholecystectomy. Pre-operative and post-operative utility values are discounted at 3.5% per year to devalue health gains over time17 for both the non-surgical and surgical health states, respectively. The discount of 3.5% annually is consistent with the United Kingdom’s National Institute for Clinical Excellence recommendations.17
Pre-operative and post-operative utility values and surgery dates shown. The post-surgery value is deducted at 3.5% per annum. The gain in health, or quality-adjusted life year, attributable to cholecystectomy, is represented by the shaded area. The duration of health benefits is truncated at 15 years in the figure. Adapted from Appleby et al.15
For this study, two time periods are used to attribute the health benefits of cholecystectomy. The first scenario assumes that benefits accumulate for 25 years, reflecting the long-lasting effects of cholecystectomy. The second scenario assumes that gain in health accumulates until age 82, the life expectancy of the average Canadian.18 While the two scenarios extend longer than analyses assuming that benefits accrue for 5 years,6 the two scenarios are very plausible time horizons for realizing health benefits due to cholecystectomy selected in consultation with surgeons. QALYs are calculated as the difference of utility values between post-operative health and pre-operative health.19
Calculating Cost per QALY
This study adopts the perspective of the funder—the cost of the cholecystectomy to the health system. Other approaches may consider the economic loss attributable to reduction in employment income, or privately-paid expenditures on related medical expenses. While the perspective of the funder is less sophisticated than considering patients’ costs, this analysis provides a ‘floor’ for examining the value of cholecystectomy.
Cost per QALY is calculated as participants’ cholecystectomy cost divided by participants’ QALYs attributable to the gain in health. Patients’ cholecystectomy cost is based on two pieces of patient-level data. First, the hospital cost is based on the product of the participants’ case mix adjusted cost weight, generated by the Canadian Institute for Health Information for the population of hospitalizations, independent of this study,20 and the hospital’s cost per weighted case. If they exist, the costs of patients’ related readmissions within 30 days are included. Second, fee-for-service billing codes associated with the cholecystectomy (surgeon and anesthesiologist) are used to determine specialist (consultant) cost. Fee-for-service billings include the pre-operative consultation, the surgery, and hospital-based post-operative follow-up. The health system’s cost is the sum of the hospital’s cost and the specialists’ fees.
Uncertainty in the estimated cost per QALY is quantified by using non-parametric bootstrap sampling methods.21 For each of the two scenarios, the cost per QALY is recalculated for resampled patient data, and the empirical distribution of cost per QALY was used to derive 95% confidence intervals. This approach is used for the overall sample of participants and among demographic subgroups. Two hundred bootstrap samples are calculated for each cost per QALY statistic. The cost per QALY statistics are compared to the often-cited benchmark of cost per QALY of $50,000.22
Results
The demographics of participants are summarized in Table 1. The study cohort consisted of 135 participants, representing a response rate of 50.5% of eligible patients. Participants were, on average, 4 years older than non-participants (not shown), though no other differences between participants and non-participants were observed.
Almost three-quarters of the participants were female. The numbers of participants in each age category were fairly evenly distributed. The participants had an average of one comorbidity. The average pre-operative utility value was 0.8394 and the average post-operative value was 0.9066; the difference in mean utility values was highly statistically significant (p < 0.001), representing a significant gain in post-operative health.
Table 2 summarizes participant’s health system cost, gain in QALYs, and the cost per QALY based on gains in health from cholecystectomy accumulating to participants for a duration of 25 years. The average health system cost for participant’s cholecystectomy was $3676, an amount lower than cited in previous literature.5,6,7
Under this scenario, the mean gain in QALYs was 1.7430 and the average cost per QALY was $2102. For the youngest age categories, participant’s cost per QALY was less than $1700. Gains in QALYs were observed to be smaller among participants older than 70 years, contributing to a higher cost per QALY among older participants. Literature examining cholecystectomies in older adults have found higher odds of mortality, complications, and non-routine discharge, as well as longer length of stay;23,24 complex post-operative courses may decrease the gains in health among older patients. The large confidence intervals among the oldest participants show that their gains in health may be more variable relative to age groups of younger participants.
The results of Table 3 summarize participants’ cost per QALY, based on the second scenario, where participants accumulate health benefits from cholecystectomy until age 82. The cost per QALY was higher for males than for females, reflecting that male participants in our sample tended to be older than females. The results also show that the cost per QALY increased to almost $10,000 for the oldest participants.
Discussion
Patients’ perspectives of the outcomes of their surgery are becoming increasingly important; the results of this study fill a gap in understanding the cost-utility of cholecystectomies—findings that are particularly relevant to countries whose publicly funded hospitals balance access to elective surgery with government restrictions on hospital sector spending growth and wait times for elective (planned) surgery.25,26 The findings will increase the knowledge base that policy-makers can draw upon to understand the effectiveness of spending on elective surgery.
This study found that cholecystectomy has a very low cost per QALY for patients with symptomatic cholelithiasis. Compared to the commonly cited cost-effectiveness benchmark of $50,000 per QALY, even the most conservative assumptions, and among the oldest participants, the gains in health relative to the surgery’s cost were very beneficial when compared with benchmarks used to evaluate and approve new drugs and devices.
This study reports that the average gain in utility value among participants was 0.066. This value can be contrasted against the minimally important difference (MID), a value which represents the minimum change that a patient would find to be important or meaningful.27,28 Consistent with threshold MID values of this instrument reported elsewhere,29,30 the mean gain in utility among this study’s participants exceeded the MID.
The subgroup analyses revealed interesting findings; patients older than 70 years experienced much more variability in their gains in health. There are a number of plausible clinical scenarios supporting the finding—older patients may have not experienced significant gains in health owing to comorbid conditions or other post-surgical treatments which impacted health status. Further, their surgical care may have been delayed due to their comorbidities or their age, which may have resulted in an unrecoverable deterioration in their overall health. Alternatively, older patients may have experienced non-routine discharges or in-hospital complications that affected their post-operative health status which, in turn, limited their gain in QALYs.
These results are broadly concordant with previous literature, which have reported that cholecystectomies were more cost-effective than non-operative interventions like watchful waiting.5,6 The results are also partially in accordance with Parmar et al. who reported that cholecystectomies may not be as cost-effective in older patients (greater than 65 years old) due to increased risk of complications.7
Determining that cholecystectomy provides a significant gain in health relative to its cost, among all subgroups, should be reassuring to decision- and policy-makers. In a public health care system, such as the setting of this study, consideration of the health benefits derived from surgery relative to its costs is an important consideration; the findings from this study found that surgical management of cholelithiasis is a good investment of public funding. As the government provides increased funding to hospitals to increase surgical capacity, the finding of this study may serve as an empirical basis for evaluating incremental investments in increasing surgical capacity. Additional research might clarify strategies for optimizing gains in health among the oldest patients; factors associated with smaller gains in health should be identified and used in improving surgical pathways in this subgroup.
There were a number of limitations to this study. The participants may not have been representative of the population of cholecystectomy patients even though every effort was made to ensure population-based recruitment and retention. Participants scheduled for elective cholecystectomy tended to be in good health pre-operatively which may undermine the study’s generalizability, also criterion which excluded non-English speakers may limit the generalizability of the findings. Additionally, while this study assumed that health devalued at 3.5% per year, when left untreated, the condition may cause emergent health problems that are not reflected in the rate of 3.5%. As a result, the cost utility of gall bladder surgery may even be better than the results shown in these analyses. Finally, this analysis presumed the perspective of the health system as payer; in more holistic cost-utility studies, broader measures of patient-borne and societal costs, such as missed work or impact on family/care-givers, might further improve the cost per QALY, leaving the estimates of this study as the upper bound.
Conclusion
Cholecystectomy for symptomatic cholelithiasis provides significant health gains relative to its cost as measured by cost per QALY, particularly among patients under 60 years of age.
References
Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. Editorial Office of Gut and Liver; 2012 Apr;6(2):172–87.
Aerts R, Penninckx F. The burden of gallstone disease in Europe. Aliment Pharmacol Ther. Blackwell Publishing Ltd; 2003 Nov;18(s3):49–53.
Canadian Institute for Health Information. Patient Cost Estimator. 2014.
Canadian Institute for Health Information. Inpatient Hospitalizations, Surgeries and Newborn Indicators, 2014-2015. 2016.
Brazzelli M, Cruickshank M, Kilonzo M, Ahmed I, Stewart F, McNamee P, et al. Systematic review of the clinical and cost effectiveness of cholecystectomy versus observation/conservative management for uncomplicated symptomatic gallstones or cholecystitis. Surg Endosc. 2015 Mar;29(3):637–47.
de Mestral C, Hoch JS, Laupacis A, Wijeysundera HC, Rotstein OD, Alali AS, et al. Early Cholecystectomy for Acute Cholecystitis Offers the Best Outcomes at the Least Cost: A Model-Based Cost-Utility Analysis. J Am Coll Surg. 2016 Feb;222(2):185–94.
Parmar AD, Coutin MD, Vargas GM, Tamirisa NP, Sheffield KM, Riall TS. Cost-effectiveness of elective laparoscopic cholecystectomy versus observation in older patients presenting with mild biliary disease. J Gastrointest Surg. NIH Public Access; 2014 Sep;18(9):1616–22.
Vancouver Coastal Health. Vancouver Coastal Health [Internet]. 2017 [cited 2019 Mar 26]. Available from: http://www.vch.ca/
Sutherland JM, Crump RT, Chan A, Liu G, Yue E, Bair M. Health of Patients on the Waiting List: Opportunity to Improve Health in Canada? Health Policy (New York). Elsevier Ireland Ltd; 2016;120(7):749–57.
EuroQoL Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy (New York). 1990;16(3):199–208.
Aygören-Pürsün E, Bygum A, Beusterien K, Hautamaki E, Sisic Z, Boysen HB, et al. Estimation of EuroQol 5-Dimensions health status utility values in hereditary angioedema. Patient Prefer Adherence. Dove Press; 2016;10:1699–707.
Hao Y, Wolfram V, Cook J. A structured review of health utility measures and elicitation in advanced/metastatic breast cancer. Clinicoecon Outcomes Res. Dove Press; 2016;8:293–303.
Bansback N, Tsuchiya A, Brazier J, Anis A. Canadian valuation of EQ-5D health states: preliminary value set and considerations for future valuation studies. PLoS One. 2012;7(2):e3111.
Brazier J. Valuing health states for use in cost-effectiveness analysis. Pharmacoeconomics. 2008;26(9):769–79.
Appleby J, Poteliakhoff E, Shah K, Devlin N. Using patient-reported outcome measures to estimate cost-effectiveness of hip replacements in English hospitals. J R Soc Med. 2013;106(8):323–331.
Coronini-Cronberg S, Appleby J, Thompson J. Application of patient-reported outcome measures (PROMs) data to estimate cost-effectiveness of hernia surgery in England. J R Soc Med. 2013;106(7):278–87.
National Institute for Health and Clinical Excellence (NICE). Guide to the methods of technology appraisal 2013. 2013.
The World Bank. Life expectancy at birth, total (years) - Canada. 2017.
Whitehead SJ, Ali S. Health outcomes in economic evaluation: The QALY and utilities. Br Med Bull. 2010;96(1):5–21.
Lavergne MR, Barer M, Law MR, Wong ST, Peterson S, McGrail K. Examining regional variation in health care spending in British Columbia, Canada. Health Policy. Elsevier Ireland Ltd; 2016;9–14.
Efron B, Tibshirani RJ. An Introduction to the Bootstrap. First. Chapman and Hall; 1993.
Neumann PJ, Cohen JT, Weinstein MC. Updating Cost-Effectiveness — The Curious Resilience of the $50,000-per-QALY Threshold. N Engl J Med. 2014;371(9):796–7.
Kuy S, Sosa JA, Roman SA, Desai R, Rosenthal RA. Age matters: a study of clinical and economic outcomes following cholecystectomy in elderly Americans. Am J Surg. 2011 Jun;201(6):789–96.
Nielsen LBJ, Harboe KM, Bardram L. Cholecystectomy for the elderly: no hesitation for otherwise healthy patients. Surg Endosc. Springer US; 2014 Jan;28(1):171–7.
Schut FT, Varkevisser M. Tackling hospital waiting times: the impact of past and current policies in the Netherlands. Health Policy. 2013 Nov;113(1–2):127–33.
Siciliani L, Hurst J. Tackling excessive waiting times for elective surgery: a comparative analysis of policies in 12 OECD countries. Health Policy (New York). OECD Publishing; 2005 May;72(2):201–15.
Jaeschke R, Singer J, Guyatt G. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–15.
Copay AG, Subach BR, Glassman SD, Polly DW, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–6.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–108.
Clemens S, Begum N, Harper C, Whitty JA, Scuffman PA. A comparison of EQ-5D-3L population norms in Queensland, Australia, estimated using utility value sets from Australia, the UK and USA. Qual Life Res. 23(8):2375–2381.
Funding
This study was funded by the Canadian Institutes for Health Research (CIHR) and in-kind support of Vancouver Coastal Health (VCH) Authority. The first author is a Scholar of the Michael Smith Foundation for Health Research (MSFHR). CIHR, VCH, and MSFHR had no role in developing the methods, data analyses, interpreting the results or manuscript preparation.
Author information
Authors and Affiliations
Contributions
JS contributed to the study in the conceptualization of the study, acquisition of the data, analysis of the data, interpretation of the results, writing of the manuscript, final approval of the text and is responsible for the accuracy and integrity of the study.
JM contributed to the study in the interpretation of the results, writing of the manuscript, final approval of the text and is responsible for the accuracy and integrity of the study.
GL contributed to the study in the acquisition of the data, analysis of the data, interpretation of the results, writing of the manuscript, final approval of the text and is responsible for the accuracy and integrity of the study.
AK contributed to the study in the conceptualization of the study, acquisition of the data, interpretation of the results, final approval of the text and is responsible for the accuracy and integrity of the study.
TC contributed to the study in the conceptualization of the study, acquisition of the data, interpretation of the results, writing of the manuscript, final approval of the text and is responsible for the accuracy and integrity of the study.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Jason M. Sutherland has no conflicts of interest or financial ties to disclose.
Ms. Janice Mok has no conflicts of interest or financial ties to disclose.
Dr. Guiping Liu has no conflicts of interest or financial ties to disclose.
Dr. Ahmer Karimuddin has no conflicts of interest or financial ties to disclose.
Dr. Trafford Crump has no conflicts of interest or financial ties to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sutherland, J.M., Mok, J., Liu, G. et al. A Cost-Utility Study of Laparoscopic Cholecystectomy for the Treatment of Symptomatic Gallstones. J Gastrointest Surg 24, 1314–1319 (2020). https://doi.org/10.1007/s11605-019-04268-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04268-z