Abstract
Background
In the current era of targeted therapies, the benefits of resection of primary tumors in patients with unresectable stage IV colorectal cancer, specifically with regard to overall survival, are unknown.
Methods
Our study population comprised 208 consecutive patients with unresectable stage IV colorectal cancer who received chemotherapy containing at least one molecular target agent, bevacizumab, cetuximab, and panitumumab, at the National Cancer Center Hospital from 2006 to 2013. To lessen the effects of confounding factors between two treatment groups (resection versus non-resection) such as performance status, presence of severe symptoms, M subcategories (M1a versus M1b, M1c) according to the TNM classification, primary tumor site, and CEA value, we conducted three different propensity score analyses (regression adjustment, stratification, and matching).
Results
Of the 208 patients, 108 (52%) underwent resection of the primary tumor, while 100 (48%) did not. Regression adjustment revealed that resection was not associated with longer overall survival (hazard ratio of 0.70 (95% CI [0.49–1.00]; p = 0.051)). Stratification analysis of five strata revealed inconsistent results (hazard ratios ranged from 0.50 to 1.58); specifically, resection was associated with longer overall survival in four strata, but with shorter survival in one stratum. The propensity score-matched cohort (64 matched pairs) yielded a hazard ratio of 0.76 (95% CI [0.51–1.15]; p = 0.197).
Conclusions
All three analyses revealed that, in the current era of chemotherapy with target agents, primary tumor resection was only marginally influential and did not significantly improve overall survival over chemotherapy alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with unresectable stage IV colorectal cancer receive systemic chemotherapy immediately after diagnosis or subsequently after resection of the primary tumor.1 Theoretically, surgery delays the start of systemic chemotherapy, but may prevent the development of complications caused by the primary tumor, such as obstruction, perforation, or bleeding. These later complications may often require emergency interventions which are associated with increased perioperative mortality and less favorable long-term outcomes. Several retrospective studies, including one from our group,2 reported that primary tumor resection is associated with better outcomes in unresectable stage IV colorectal cancer using propensity analyses to lessen the effects of confounding factors between the two treatments (resection and non-resection).3,4,5 Until recently, it has been considered that the resection of the primary tumor can prevent the future complications and contribute to the prolongation of survival of patients with unresectable stage IV colorectal cancer.
The introduction of molecular target agents such as vascular endothelial growth factor (VEGF)-targeted antibody (bevacizumab) and epidermal growth factor receptor (EGFR)-targeted antibodies (cetuximab, panitumumab) has improved overall survival (OS) of stage IV colorectal cancer patients. Median OS of patients diagnosed with unresectable stage IV colorectal cancer has been improved, from approximately 1 year during the era of fluoropyrimidine monotherapy to > 30 months with the integration of multiple cytotoxic agents and molecular target agents.6 Combination of cytotoxic and molecular target agents (targeted therapy) is now standard treatments in the various lines setting of unresectable stage IV patients.
In the current era of targeted therapies, good disease control may delay the emergence of medical conditions requiring emergency interventions. Moreover, it also seems that good tumor shrinkage can keep primary lesions asymptomatic all through the treatment course around 30 months and reserve surgery for patients with symptoms derived from the primary tumor. Marked progress in recent chemotherapy with molecular target agents may thereby change treatment strategies for unresectable colorectal cancer. Accordingly, the role of resection of primary tumors in unresectable stage IV colorectal cancer patients in the current era of targeted therapies should be reexamined.
Here, we hypothesized that, in the current era of targeted therapy, its efficacy would reduce the prognostic impact of palliative surgery. The present study aimed to evaluate the prognostic impact of primary tumor resection in patients with unresectable stage IV colorectal cancer who received targeted therapies, specifically with regard to OS. Given the observed heterogeneity and potential selection bias between the two treatment strategies (resection versus non-resection), we conducted several propensity score analyses to minimize the selection bias inherent to retrospective observational studies.7, 8
Materials and Methods
Study Population
The sources of the subjects in this study were patients with unresectable stage IV colorectal cancer who were referred to the divisions of surgery or gastrointestinal medical oncology at the National Cancer Center Hospital between January 2006 and December 2013. The selection criteria of this study were age 18 years or older, initial diagnosis of unresectable stage IV colorectal cancer with histologically confirmation of adenocarcinoma, and treatment with targeted therapy containing molecular target agents, such as bevacizumab, cetuximab, and panitumumab, at least once throughout the treatment course. Patients those with a histologic diagnosis other than adenocarcinoma (e.g., carcinoid, neuroendocrine tumor, or gastrointestinal stromal tumor) were excluded. We also excluded those who (1) underwent resection of both primary tumor and metastatic sites with curative intent,9 (2) received best supportive care only, and (3) received cytotoxic chemotherapy without molecular target agents throughout the treatment course. The patients with asymptomatic unresectable colorectal cancer who participated the randomized controlled trial, JCOG1007 study (iPACS trial, UMIN000008147) (chemotherapy with versus without primary tumor resection), as well as the patients with symptomatic unresectable colorectal cancer who participated the randomized controlled trial, JCOG1107 study (Encore trial, UMIN000009715) (open resection versus laparoscopic resection followed by chemotherapy), were also included. Initial treatment decisions for the remaining patients were typically made by the multidisciplinary team conference including colorectal surgeons, medical oncologists, hepatobiliary surgeons, thoracic surgeons, and radiologists, taking into account disease severity and patient condition including comorbidities. No patient underwent palliative resection of other disease sites.
This retrospective study was approved by the Institutional Review Board (IRB) of the National Cancer Center Hospital (IRB code, 2015-320).
Statistical Analysis
Pearson’s chi-square test was used to compare categorical variables between the two treatment groups (resection versus non-resection). OS was defined as the interval between the date of diagnosis of stage IV colorectal cancer and the date of either death or the end of the observation period. Patients alive at the end of follow-up were censored. The Kaplan-Meier method was used to estimate OS. Differences in survival outcomes were assessed with the log-rank test. Multivariate Cox proportional hazards regression models were subsequently fitted to evaluate the relationship between resection of the primary tumor and OS adjusting potential confounding covariates. Results are presented as a hazard ratio (HR) and 95% confidence interval (CI).
In order to adjust for heterogeneity between the treatment groups (resection and non-resection), propensity score analyses were conducted as described previously.2, 7 Multivariable logistic regression was used to generate propensity score-predicting treatment (resection versus non-resection) based on confounding covariates, including ECOG performance status, presence of severe symptoms (severe anemia, bowel obstruction, abdominal pain), M subcategories (M1a versus M1b, M1c) according to the TNM classification (8th edition),10 primary tumor site (right-sided: cecum, hepatic flexure, and transverse colon; left-sided: splenic flexure, sigmoid, rectosigmoid junction, and rectum), and CEA value (≥ 30 ng/ml versus < 30 ng/ml). Each patient was then assigned an estimated propensity score, which is the probability that the patient receives resection given their measurable characteristics.
By applying propensity scores to adjust for group differences in the following three manners, Cox models were created.2, 7 First, propensity scores were used for regression adjustment, which include the score as a linear predictor in the model.7, 8 In a regression model, treatment effect is estimated by adjusting background covariates. Second, propensity scores were used for stratification, because stratification based on propensity score is known to produce strata in which the average treatment effect within strata is an unbiased estimate of the true treatment effect.7 Defined by quintiles of the estimated propensity score, the entire cohort was divided into five strata. Third, propensity scores were used for matching, which pairs resection patients and non-resection patients according to similarities in his/her observed baseline characteristics. Each patient who underwent resection of the primary tumor was matched 1:1 with a non-resection patient with the closest estimated propensity on the logit scale within a specified range (smaller than 0.05 of estimated logits as the caliper width) to reduce differences between treatment groups. All statistical analyses were performed using the JMP12 software program (SAS Institute Japan Ltd., Tokyo, Japan). p < 0.05 was considered statistically significant.
Results
Characteristics of the Study Cohort
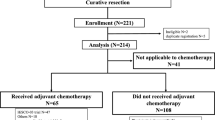
Details of our study cohort are shown in Fig. 1. Between January 2006 and December 2013, a total of 551 patients with stage IV colorectal cancer were referred to the National Cancer Center. Of these, 151 (27% of all stage IV patients) patients who underwent curative resection and 23 patients who received only best supportive care because of poor performance status were excluded. We also excluded 169 patients who were treated with cytotoxic agents without target agents. Thus, 208 patients met the aforementioned selection criteria for unresectable stage IV colorectal cancer who received targeted therapy. One hundred thirty and 78 patients were first referred to the surgery division and to the gastrointestinal oncology division, respectively. Of these, 117 patients received VEGF-targeted antibody (bevacizumab), 24 patients received EGFR-targeted antibody (cetuximab or panitumumab), and 67 patients received both bevacizumab and one of the EGFR-targeted antibodies at least once throughout the treatment course.
Of the 208 patients, 108 (52%) underwent resection of the primary tumor, and 100 (48%) did not. Patients who underwent diverting stoma construction without primary tumor resection (n = 20), or probe laparotomy (n = 4), were included in the non-resection group. Patient characteristics of these two treatment groups are shown in Table 1. Significant differences were observed in ECOG performance status (p = 0.013), presence of severe symptoms (p = 0.039), and CEA values (p < 0.001); namely, patients with good performance status (PS0), with severe symptoms, and with smaller CEA values, tended to undergo resection. These results indicate that a clear bias existed for treatment selection from two treatment strategies. On the other hand, distribution of primary tumor sites, which is a prognostic factor of stage IV colorectal cancer which recently gathers a lot of attention,11,12,13,14 as well as distribution of M subcategories (M1a versus M1b and M1c), a well-known prognostic factor of stage IV colorectal cancer,15 did not differ significantly between the two groups (p = 0.093 and p = 0.294, respectively).
Effects of Resection of the Primary Tumor on OS
Without adjusting background variables, patients in this study (n = 208) who underwent resection of the primary tumor had a significantly longer OS than patients who did not undergo resection (Fig. 2). Median OS was 32.9 and 23.5 months for resection and non-resection patients, respectively, and the HR for OS for resection compared with non-resection was 0.61 (95% CI [0.44–0.84]; p < 0.01) (Table 2).
(Upper left) overall survival with and without palliative resection of the primary tumor in the entire cohort (n = 208). Of these, 108 (52%) underwent palliative resection of the primary tumor, and 100 (48%) did not. (Lower left) overall survival with and without palliative resection of the primary tumor in the propensity score-matched population (64 matched pairs). (Right) estimated propensity scores of each patient represented their predicted probability of receiving palliative resection on the basis of the observed baseline characteristics. Distribution of propensity scores in the entire cohort (upper right) and in the propensity score-matched groups (lower right)
Propensity scores (probability of receiving primary tumor resection) of the entire cohort, generated by using multivariable logistic regression with confounding covariates including performance status, presence of severe symptoms, M subcategories, primary tumor site, and CEA value, showed unbalance between the two groups (Fig. 2, upper right).
Regression Adjustment Including the Propensity Score
By way of regression adjustment (i.e., include propensity score as a linear predictor in the model), Cox models created by applying propensity scores were used to adjust group differences. In our entire cohort (n = 208), the HR for OS for resection compared with non-resection was 0.70 (95% CI [0.50–0.71]; p = 0.051) (Table 2).
Stratification Based on Propensity Score
As defined by quintiles of estimated propensity scores, the entire cohort (n = 208) was divided into five strata. Stratification analysis of the five strata revealed inconsistencies (HRs ranged from 0.50 to 1.58) (Table 2). The confidence intervals for the hazard ratios in Table 2 for the propensity score-adjusted model all cross 1, so none of these hazard ratios are significant.
Propensity Score Matching
After propensity score matching, 64 matched pairs of patients were selected. The demographics of the propensity score-matched patients was described in Table 3, showing patient distributions were balanced between the resection and non-resection groups. In the cohort of matched patients (n = 128), those who underwent primary tumor resection did not show significant better outcomes than those who did not (Fig. 2), shown as median OS values of 31.0 versus 28.1 months for resection and non-resection patients, respectively, and HR of 0.76 (95% CI [0.51–1.15]; p = 0.197) in the propensity score-matched model (Table 2). Distributions of propensity scores in the matched groups are shown in Fig. 2 (lower right).
Discussion
Of our study population, 52% were patients in the resection group and 48% were in the non-resection group. In some previous studies of unresectable colorectal cancer investigating the impact of primary tumor resection, the numbers of patients in each treatment group have been markedly unbalanced (only 10–28% of patients subject to analysis were non-resection patients).4, 5, 16 This issue of imbalance is critical when comparing prognoses of the two treatments (resection versus non-resection). According to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results CRC registry in the USA, the annual rate of resection of the primary tumor has been decreasing, down to 57.4% in 2010.17 Thus, the composition of our study population (from 2006 to 2013) seems to be a relatively accurate representation of the patient population with unresectable stage IV colorectal cancer.
We found that resection of the primary tumor in unresectable stage IV colorectal cancer patients who received targeted therapy containing at least one molecular target agent such as bevacizumab, cetuximab, and panitumumab was not associated with significant longer OS. This finding was supported by three different propensity score analyses, namely, regression adjustment, stratification, and matching. Each of these analyses represents a way to adjust covariates prior to calculation of the treatment effect (matching and stratification) or during calculation of the treatment effect (regression adjustment and stratification).7 HR comparisons of OS for resection and non-resection were analyzed in three different ways. The resulting HRs ranging from 0.50–1.58 indicate that outcomes for resection over non-resection were neither significantly better nor worse (Table 2).
We recently reported the positive prognostic impact of resection of the primary tumor in unresectable stage IV colorectal cancer.2 Several systematic reviews in the literature have also evaluated the positive prognostic effects of resection of the primary tumor in unresectable stage IV colorectal cancer.18,19,20 These studies differ from the present study in the points that they included patients not only in the old era of fluoropyrimidine monotherapy, but also in the modern era when combination of cytotoxic agents was popular and in the current era of targeted therapies. The present study focused on patients who received targeted therapies, and found that primary tumor resection has only marginal impacts; this is in clear contrast to our previous report.2 These opposing conclusions may be explained by a greater efficacy of therapeutic combinations of multiple cytotoxic agents and targeted therapies, which might reduce the impact of primary tumor resection. Even in the era of multiple cytotoxic agents without target agents, one study showed a lower incidence of major intestinal complications such as obstruction, peritonitis, and gastrointestinal hemorrhage requiring hospital admission, even in the non-resection group, and concluded that chemotherapy may be successfully used as initial treatment for unresectable colorectal cancer.21 Thus, the role of resection of the primary tumor in unresectable stage IV colorectal cancer in the current era of targeted therapies should be reexamined anew. To this end, well-designed randomized clinical trials are currently ongoing, and include the CAIRO4 study22 and the JCOG1007 study (iPACS trial, UMIN000008147), both of which define systemic therapy as fluoropyrimidine-based chemotherapy in combination with bevacizumab.
There are some potential limitations to this study. First, since the present study was retrospective in design, biases may exist. However, this was one of the main reasons for conducting three different propensity score analyses. Second, the sample size was relatively small. Third, the chemotherapy regimen differed by patient, with some being administered bevacizumab and others administered cetuximab/panitumumab. This heterogeneity might affect the efficacy of chemotherapy. Fourth, a significant proportion of the cohort was enrolled in one of two randomized controlled trials. Thus, the inclusion of data from study patients in randomized controlled trials introduces some potential inconsistencies in treatment decision-making processes throughout the study period. Nonetheless, our observations warrant further consideration and validation in a larger patient population with unresectable colorectal cancer.
Conclusion
By minimizing selection bias through the use of propensity score analyses, the present study revealed that, in the current era of targeted therapies, primary tumor resection was not associated with improved OS. That is, the efficacy of palliative surgery might be losing their impact, which might be surpassed by that of targeted therapies. Our study indicated that systemic chemotherapy without resection of the primary tumor might be standard therapy for unresectable stage IV colorectal cancer in the current era of targeted therapies. Even for symptomatic patients, standard therapy might become not resection of the primary tumor but just palliative surgery such as stoma construction without primary tumor resection in order to minimize the surgical risk and not to delay the start of systemic chemotherapy. Further investigations such as randomized trials are needed to confirm this result.
Abbreviations
- CEA:
-
Carcinoembryonic antigen
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- HR:
-
Hazard ratio
- OS:
-
Overall survival
- PS:
-
Performance status
References
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 2018;23(1):1–34. https://doi.org/10.1007/s10147-017-1101-6.
Shida D, Hamaguchi T, Ochiai H, Tsukamoto S, Takashima A, Boku N et al. Prognostic Impact of Palliative Primary Tumor Resection for Unresectable Stage 4 Colorectal Cancer: Using a Propensity Score Analysis. Ann Surg Oncol. 2016;23(11):3602–8. https://doi.org/10.1245/s10434-016-5299-8.
Tarantino I, Warschkow R, Worni M, Cerny T, Ulrich A, Schmied BM et al. Prognostic Relevance of Palliative Primary Tumor Removal in 37,793 Metastatic Colorectal Cancer Patients: A Population-Based, Propensity Score-Adjusted Trend Analysis. Ann Surg. 2015;262(1):112–20. https://doi.org/10.1097/SLA.0000000000000860.
Ishihara S, Hayama T, Yamada H, Nozawa K, Matsuda K, Miyata H et al. Prognostic impact of primary tumor resection and lymph node dissection in stage IV colorectal cancer with unresectable metastasis: a propensity score analysis in a multicenter retrospective study. Ann Surg Oncol. 2014;21(9):2949–55. https://doi.org/10.1245/s10434-014-3719-1.
Gresham G, Renouf DJ, Chan M, Kennecke HF, Lim HJ, Brown C et al. Association between palliative resection of the primary tumor and overall survival in a population-based cohort of metastatic colorectal cancer patients. Ann Surg Oncol. 2014;21(12):3917–23. https://doi.org/10.1245/s10434-014-3797-0.
Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015;33(16):1809–24. https://doi.org/10.1200/JCO.2014.59.7633.
D’Agostino RB Jr Propensity scores in cardiovascular research. Circulation. 2007;115(17):2340–3. https://doi.org/10.1161/CIRCULATIONAHA.105.594952.
Zhu J, Sharma DB, Gray SW, Chen AB, Weeks JC, Schrag D. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA. 2012;307(15):1593–601. https://doi.org/10.1001/jama.2012.454.
Shida D, Tsukamoto S, Ochiai H, Kanemitsu Y. Long-Term Outcomes After R0 Resection of Synchronous Peritoneal Metastasis from Colorectal Cancer Without Cytoreductive Surgery or Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol. 2018;25(1):173–8. https://doi.org/10.1245/s10434-017-6133-7.
UICC. TNM classification of malignant tumors 8th edition. New York: John Wiley & Sons, Ltd; 2017.
Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713–29. https://doi.org/10.1093/annonc/mdx175.
Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F et al. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2016. https://doi.org/10.1001/jamaoncol.2016.3797.
Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(3). https://doi.org/10.1093/jnci/dju427.
Shida D, Yoshida T, Tanabe T, Tsukamoto S, Ochiai H, Kanemitsu Y. Prognostic Impact of R0 Resection and Targeted Therapy for Colorectal Cancer with Synchronous Peritoneal Metastasis. Ann Surg Oncol. 2018;25(6):1646–53. https://doi.org/10.1245/s10434-018-6436-3.
Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–19. https://doi.org/10.1016/S1470-2045(16)30500-9.
Ferrand F, Malka D, Bourredjem A, Allonier C, Bouche O, Louafi S et al. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: results from the multicenter, randomised trial Federation Francophone de Cancerologie Digestive 9601. Eur J Cancer. 2013;49(1):90–7. https://doi.org/10.1016/j.ejca.2012.07.006.
Hu CY, Bailey CE, You YN, Skibber JM, Rodriguez-Bigas MA, Feig BW et al. Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival. JAMA Surg. 2015;150(3):245–51. https://doi.org/10.1001/jamasurg.2014.2253.
Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg. 2010;34(4):797–807. https://doi.org/10.1007/s00268-009-0366-y.
Anwar S, Peter MB, Dent J, Scott NA. Palliative excisional surgery for primary colorectal cancer in patients with incurable metastatic disease. Is there a survival benefit? A systematic review. Colorectal Dis. 2012;14(8):920–30. https://doi.org/10.1111/j.1463-1318.2011.02817.x.
Eisenberger A, Whelan RL, Neugut AI. Survival and symptomatic benefit from palliative primary tumor resection in patients with metastatic colorectal cancer: a review. Int J Colorectal Dis. 2008;23(6):559–68. https://doi.org/10.1007/s00384-008-0456-6.
Tebbutt NC, Norman AR, Cunningham D, Hill ME, Tait D, Oates J et al. Intestinal complications after chemotherapy for patients with unresected primary colorectal cancer and synchronous metastases. Gut. 2003;52(4):568–73.
t Lam-Boer J, Mol L, Verhoef C, de Haan AF, Yilmaz M, Punt CJ et al. The CAIRO4 study: the role of surgery of the primary tumour with few or absent symptoms in patients with synchronous unresectable metastases of colorectal cancer--a randomized phase III study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer. 2014;14:741. https://doi.org/10.1186/1471-2407-14-741.
Acknowledgements
The authors thank Moriya M., Shimada Y., Akasu T., Fujita S., Yamamoto S., Hamaguchi T., and Ochiai H., who were former staff members of our divisions and nurses who took care of the patients.
Author information
Authors and Affiliations
Contributions
NB and YK designed the study. NB, TT, TY, ST, and AT collected the data, performed the treatments, and wrote the paper. DS convinced of the study, and participated in its design and coordination. DS was responsible for writing the paper and for its supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This retrospective study was approved by the Institutional Review Board (IRB) of the National Cancer Center Hospital (IRB code, 2015-320).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Shida, D., Boku, N., Tanabe, T. et al. Primary Tumor Resection for Stage IV Colorectal Cancer in the Era of Targeted Chemotherapy. J Gastrointest Surg 23, 2144–2150 (2019). https://doi.org/10.1007/s11605-018-4044-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-4044-y