Abstract
Background or Purpose
There is controversy regarding the efficacy of nonsteroidal anti-inflammatory drugs (NSAIDs) for prophylaxis against endoscopic retrograde cholangiopancreatography (ERCP) postoperative pancreatitis. Therefore, we conducted a systematic review and meta-analysis to evaluate the efficacy of NSAIDs for prophylaxis against post-ERCP pancreatitis (PEP).
Methods
PubMed, EMBASE, and Cochrane library databases were searched for relevant randomized controlled trials (RCTs). Selected RCTs were pooled under a fixed effects model to generate the relative risks (RRs) and their corresponding 95% confidence intervals (CIs).
Results
Nineteen RCTs involving a total of 5031 patients (2555 in the intervention group and 2476 in the control group) were selected. Overall, NSAIDs were associated with a significant reduction in risk of PEP (RR = 0.54, 95% CI 0.45 to 0.64, I2 = 40.4%) and moderate to severe PEP (RR = 0.45, 95% CI 0.30 to 0.67, I2 = 0%) compared with the control group. Subgroup analyses were performed according to route of administration (rectal or other), type of NSAIDs (diclofenac, indomethacin, or other), timing of administration (pre-ERCP, post-ERCP, or other), and patient population (high risk or general). Subgroup analyses showed difference in clinical efficacy of NSAID prophylaxis regardless of route, timing, or specific type of NSAID.
Conclusion
NSAIDs were associated with a significant reduction in risk of PEP and moderate to severe PEP compared to the control group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) is a common diagnostic and therapeutic procedure for disorders of the biliary tree and pancreas. Acute pancreatitis is the most frequent and severe complication of ERCP. The occurrence of post-ERCP pancreatitis (PEP) varies between 1 and 25% depending on the risk factors and the indication of ERCP.1,2,3,4 The vast majority of PEP has a mild or moderate course, but in 0.3–0.6% of cases, PEP is severe in nature with a need for intensive care and invasive interventions and, at worst, can even lead to death.5,6
Several approaches to reduce the risk of PEP have been investigated. Insertion of pancreatic duct (PD) stents has been shown to reduce the risk of PEP in high-risk patients and the risk of severe PEP.7,8,9 However, stent placement has drawbacks, which include failed placement, migration, and ductal perforation.10,11 Thus, the use of PD stents is limited to patients with an increased risk of moderate to severe pancreatitis. Additionally, a significant proportion of endoscopists decide not to place PD stents due to lack of experience.12 Pharmacological prophylaxis of pancreatitis after PEP has remained favorable in various trials in recent years. Nonsteroidal anti-inflammatory drugs (NSAIDs) have shown the most encouraging results in this respect by attenuating the inflammatory response seen in pancreatitis.13 NSAIDs like diclofenac inhibit phospholipase A214 and suppress neutrophil/endothelial cell attachment, thereby restricting the accumulation of neutrophils at the site of tissue injury. In addition, they inhibit the expression of nitric oxide synthase, which is linked to inflammation and cell damage.15 Furthermore, NSAIDs are easily administered, inexpensive, and relatively safe when given as a single dose, making them an attractive treatment option.
Thus far, a number of randomized controlled trials (RCTs)13,14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 have been conducted to investigate the efficacy of NSAIDs for prophylaxis against PEP. However, the results of these trials were conflicting, as several trials13,14,16,18,19,21,22,24,26,27,28,30,31,32 showed promising results, whereas others17,20,23,25,29 showed null results. Therefore, we performed a systematic review and meta-analysis of published studies to provide a comprehensive assessment of the efficacy of NSAIDs for prophylaxis against PEP.
Materials and Methods
The present meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.33
Search Strategy
We searched PubMed, Embase, and the Cochrane Library databases for relevant publications up to December 2017. The following search terms and their combinations were used to identify relevant publications: (endoscopic retrograde cholangiopancreatography ORERCP) AND pancreatitis AND (nonsteroidal anti-inflammatory drugs ORNSAIDs). We did not impose any language restrictions on database searches. In addition, the references cited in the retrieved manuscripts were also manually searched to identify additional relevant publications that were missed during the database searches.
Selection Criteria
Randomized controlled trials evaluating the efficacy of NSAIDs for prophylaxis against PEP were selected, if they met the following criteria: (1) Reported either the effect estimates, such as RRs with 95% CIs, or sufficient information to calculate these values; (2) reported the following outcomes: the severity of PEP (any, mild, or moderate to severe).
Data Extraction and Quality Assessment
All available data was extracted from each study by two investigators independently based on the inclusion criteria listed above. Any disagreement was resolved through discussion with a third investigator. The following information was extracted from all included studies: first author, year of publication, country, gender, mean age, study design, intervention group, and outcomes assessed. The quality of the RCTs was evaluated using the Cochrane Collaboration’s tool for assessing the risk of bias.34 The assessment included the following components: random sequence generation, allocation concealment, blinding of patients and study personnel, blinding of outcome assessment, completeness of outcome data, selective reporting of outcomes, and other biases to validity.
Statistical Analysis
Dichotomous outcomes included rates or proportions from which pooled relative risk (RR) and 95% confidence intervals (CI) were estimated. When P > 0.1 or I2 < 50%, indicating a lack of heterogeneity, for these analyses, the fixed effects model was used; otherwise, the random effects model was applied. Sensitivity analysis by omitting a single study in each turn was performed to assess the relative influence of each study on the pooled estimate. Publication bias was evaluated by visual inspection of symmetry of Begg’s funnel plot and assessment of Begg’s and Egger’s test. Trim and fill analysis was applied if publication bias was detected. The Q and I2 statistics were used to assess statistical heterogeneity across studies. For the Q statistic, P < 0.1 was considered statistically significant; for the I2 statistic, the following cutoff points were used: < 25% (low heterogeneity), 25–50% (moderate heterogeneity), > 50–75% (high heterogeneity), and > 75% (severe heterogeneity)35. One study32 investigated the prevention of PEP using rectal and intramuscular administration, and the data was analyzed separately for each group; hence, we analyzed them as two separate studies. We performed subgroup analyses according to route of administration (rectal or other), type of NSAIDs (diclofenac, indomethacin, or other), timing of administration (pre-ERCP, post-ERCP, or other), and population (high risk or general). All statistical analyses were performed using STATA Software (version 12.0, StataCorp, College Station, TX). All P values were two-sided, and the level of significance was set at < 0.05.
Results
Study Selection
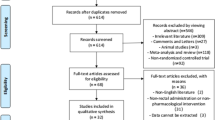
From the initial electronic database searches, we identified 293 relevant studies. We found four additional studies by searching the reference lists of review articles.36 Based on the inclusion criteria, 31 articles qualified for full-text evaluation. After evaluation, 12 articles were deemed unsuitable, of which eight were not focused on NSAIDs and four did not present usable data, and hence were excluded. Finally, 19 studies13,14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 with 5031 patients were included into the present meta-analysis. The flowchart for the selection of studies and reasons for exclusion are presented in Fig. 1.
Characteristics of the Studies
The main characteristics of the selected studies are shown in Table 1. The included studies were published between 2003 and 2016. The studies were performed in various countries, and the study size ranged from 80 to 665 patients (the intervention group from 40 to 347 and the control group from 40 to 318). The mean patient age ranged from 42.93 to 75. Nineteen RCTs were included in our meta-analysis, and a total of four different types of NSAIDs were used; diclofenac,13,14,16,17,22,25,31,32 indomethacin,18,19,20,21,23,24,26,28,29 flurbiprofen,27 and naproxen.30 The summary of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases identified for each individual RCT is shown in Fig. 2. All of the included RCTs showed moderate or high quality with acceptable and moderate risk of bias.
Quantitative Synthesis
The Incidence of PEP
This outcome was reported in 19 trials that compared NSAIDs to placebo. Of the 5031 patients, 2555 were in the intervention group and 2476 were in the control group. Heterogeneity between these studies was low (I2 = 40.4%), which was derived from the fixed effects model. Administration of NSAIDs was associated with a significant reduction in risk of PEP compared to the control group (RR = 0.54, 95% CI 0.45 to 0.64, I2 = 40.4%) (Fig. 3a). All subgroup analyses (Fig. 3b–e) were generally consistent with the overall results and are summarized in Table 2.
The Incidence of Moderate to Severe PEP
This outcome was reported in 13 trials16,17,19,21,22,23,24,25,26,27,29,30,32 and compared NSAIDs to placebo. Of the 4071 patients, 2034 were in the intervention group, and 2037 were in the control group. No significant heterogeneity between these studies was found (I2 = 0%) using the fixed effects model. Administration of NSAIDs was associated with a significant reduction in the risk of moderate to severe PEP compared to the control group (RR = 0.45, 95% CI 0.30 to 0.67, I2 = 0%) (Fig. 4a). All subgroup results (Fig. 4b–e) were generally consistent with the overall results and are summarized in Table 2.
Sensitivity Analysis
Sensitivity analysis revealed that the overall results were free from the influence of a single study (Fig. 5).
Publication Bias
Begg’s and Egger’s regression test demonstrated no evidence of asymmetrical distribution in the funnel plot for the incidence of moderate to severe PEP (Begg’s test P = 0.381; Egger’s test P = 0.337) (Fig. 6a). However, Begg’s test showed the presence of publication bias for incidence of PEP (Begg’s test P = 0.019; Egger’s test P = 0.053) (Fig. 6b). The results remained statistically significant after trim and fill method was performed, suggesting that there were no studies to be filled (Fig. S1).
Discussion
The meta-analysis included 19 RCTs with 5031 patients (2555 in the intervention group and 2476 in the control group) demonstrated that NSAIDs were associated with a significant reduction in risk of post-ERCP pancreatitis and moderate to severe PEP compared to the control group.
The efficacy of NSAIDs for prophylaxis against PEP has been investigated in several previous meta-analyses.37,38,39 To our knowledge, the current meta-analysis is the largest and most comprehensive in investigating the efficacy of NSAIDs for prophylaxis against PEP and consisted of 5031 patients from 19 RCTs. Recently, Yang et al39 conducted a comprehensive meta-analysis on the efficacy of NSAIDs for prophylaxis against PEP. Compared to the meta-analysis conducted by Yang et al., we included seven additional studies.14,17,25,27,28,31,32 In addition, we performed more comprehensive subgroup analyses compared to the work performed by Hou et al.37 The study by Luo et al.38 from a meta-analysis by Hou et al. was not included in our meta-analysis because of the treatment and control group receiving NSAID administration.
Rectal NSAID administration has shown potential benefit on PEP prevention despite conflicting findings in multiple single-center RCTs. Elmunzer et al. performed a multicenter RCT comparing a single dose of 100 mg of rectal indomethacin to placebo following ERCP in selected high-risk patients and found that 9.2% of patients in the indomethacin group developed PEP compared to 16.9% in the placebo group, demonstrating a statistically significant difference (P = 0.005).21 The incidence of moderate to severe pancreatitis was also significantly decreased in the indomethacin group compared to that in the placebo. However, the majority of patients in this study had possible sphincter of Oddi dysfunction, thus limiting the generalizability of the findings. For such patients, the benefit of ERCP is unclear and there may be an elevated risk of PEP.40 Additionally, the majority of patients also had a PD stent attempted or placed, and as a result, it was unclear whether indomethacin was the sole contributor for the improved outcomes. Finally, the authors specifically excluded patients with malignant biliary obstruction and patients with other common low-risk indications for ERCP. In a subsequent meta-analysis of 7 RCTs with a total of 2133 patients, rectal indomethacin demonstrated a similar reduction in PEP.41 However, the majority of patients were at high risk and all studies included patients with suspected SOD.41 A recent RCT involving mainly average-risk patients failed to find a benefit with rectal indomethacin administration when compared to placebo.29 Therefore, the benefit of rectal NSAIDs has not been definitively demonstrated in low-risk patients and patients with malignant obstruction, who, together, comprise the majority of patients undergoing ERCP in real-world practice.1
Several limitations of our meta-analysis should be addressed. Firstly, the characteristics of the included patients, diagnostic criteria of pancreatitis as well as the criteria of pancreatitis severity, definition of the risk stratification of the patients, administration dose, and intervention regimen varied across studies, which may influence the results, hence limiting comparability to some extent. Secondly, there may have been potential publication bias in this meta-analysis since we did not include several unpublished papers because the data was not available to us. Thirdly, our results were based on unadjusted assessment of RRs, which may influence our results. Based on these limitations mentioned above, the results should be regarded with caution.
Conclusion
In conclusion, despite the limitations of our meta-analysis, our study confirmed that NSAIDs were associated with a significant reduction in risk of post-ERCP pancreatitis and moderate to severe PEP compared to the control group, especially via rectal administration. Further studies with larger cohorts and well-designed protocols are required to validate our findings.
References
Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007;102:1781–1788.
Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS, Shaw MJ, Snady HW, Erickson RV, Moore JP, Roel JP. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001;54:425–434.
Rabenstein T, Hahn EG. Post-ERCP pancreatitis: new momentum. Endoscopy 2002;34:325–329.
Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP, Jr., Montes H, Roston AD, Slivka A, Lichtenstein DR, Ruymann FW, Van Dam J, Hughes M, Carr-Locke DL. Risk factors for complications after performance of ERCP. Gastrointest Endosc 2002;56:652–656.
Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, Lande JD, Pheley AM. Complications of endoscopic biliary sphincterotomy. N Engl J Med 1996;335:909–918.
Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, Minoli G, Crosta C, Comin U, Fertitta A, Prada A, Passoni GR, Testoni PA. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol 2001;96:417–423.
Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc 1991;37:383–393.
Singh P, Das A, Isenberg G, Wong RC, Sivak MV, Jr., Agrawal D, Chak A. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc 2004;60:544–550.
Tarnasky PR, Palesch YY, Cunningham JT, Mauldin PD, Cotton PB, Hawes RH. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology 1998;115:1518–1524.
Aizawa T, Ueno N. Stent placement in the pancreatic duct prevents pancreatitis after endoscopic sphincter dilation for removal of bile duct stones. Gastrointest Endosc 2001;54:209–213.
Cha SW, Leung WD, Lehman GA, Watkins JL, McHenry L, Fogel EL, Sherman S. Does leaving a main pancreatic duct stent in place reduce the incidence of precut biliary sphincterotomy-associated pancreatitis? A randomized, prospective study. Gastrointest Endosc 2013;77:209–216.
Dumonceau JM, Rigaux J, Kahaleh M, Gomez CM, Vandermeeren A, Deviere J. Prophylaxis of post-ERCP pancreatitis: a practice survey. Gastrointest Endosc 2010;71:934–939, 939.e931-932.
Khoshbaten M, Khorram H, Madad L, Ehsani Ardakani MJ, Farzin H, Zali MR. Role of diclofenac in reducing post-endoscopic retrograde cholangiopancreatography pancreatitis. J Gastroenterol Hepatol 2008;23:e11–16.
Senol A, Saritas U, Demirkan H. Efficacy of intramuscular diclofenac and fluid replacement in prevention of post-ERCP pancreatitis. World J Gastroenterol 2009;15:3999–4004.
Dai HF, Wang XW, Zhao K. Role of nonsteroidal anti-inflammatory drugs in the prevention of post-ERCP pancreatitis: a meta-analysis. Hepatobiliary Pancreat Dis Int 2009;8:11–16.
Murray B, Carter R, Imrie C, Evans S, O'Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology 2003;124:1786–1791.
Cheon YK, Cho KB, Watkins JL, McHenry L, Fogel EL, Sherman S, Schmidt S, Lazzell-Pannell L, Lehman GA. Efficacy of diclofenac in the prevention of post-ERCP pancreatitis in predominantly high-risk patients: a randomized double-blind prospective trial. Gastrointest Endosc 2007;66:1126–1132.
Montano Loza A, Rodriguez Lomeli X, Garcia Correa JE, Davalos Cobian C, Cervantes Guevara G, Medrano Munoz F, Fuentes Orozco C, Gonzalez Ojeda A. [Effect of the administration of rectal indomethacin on amylase serum levels after endoscopic retrograde cholangiopancreatography, and its impact on the development of secondary pancreatitis episodes]. Rev Esp Enferm Dig 2007;99:330–336.
Sotoudehmanesh R, Khatibian M, Kolahdoozan S, Ainechi S, Malboosbaf R, Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol 2007;102:978–983.
Dobronte Z, Toldy E, Mark L, Sarang K, Lakner L. [Effects of rectal indomethacin in the prevention of post-ERCP acute pancreatitis]. Orv Hetil 2012;153:990–996.
Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, Hayward RA, Romagnuolo J, Elta GH, Sherman S, Waljee AK, Repaka A, Atkinson MR, Cote GA, Kwon RS, McHenry L, Piraka CR, Wamsteker EJ, Watkins JL, Korsnes SJ, Schmidt SE, Turner SM, Nicholson S, Fogel EL. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med 2012;366:1414–1422.
Otsuka T, Kawazoe S, Nakashita S, Kamachi S, Oeda S, Sumida C, Akiyama T, Ario K, Fujimoto M, Tabuchi M, Noda T. Low-dose rectal diclofenac for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled trial. J Gastroenterol 2012;47:912–917.
Dobronte Z, Szepes Z, Izbeki F, Gervain J, Lakatos L, Pecsi G, Ihasz M, Lakner L, Toldy E, Czako L. Is rectal indomethacin effective in preventing of post-endoscopic retrograde cholangiopancreatography pancreatitis? World J Gastroenterol 2014;20:10151–10157.
Andrade-Davila VF, Chavez-Tostado M, Davalos-Cobian C, Garcia-Correa J, Montano-Loza A, Fuentes-Orozco C, Macias-Amezcua MD, Garcia-Renteria J, Rendon-Felix J, Cortes-Lares JA, Ambriz-Gonzalez G, Cortes-Flores AO, Alvarez-Villasenor Adel S, Gonzalez-Ojeda A. Rectal indomethacin versus placebo to reduce the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography: results of a controlled clinical trial. BMC Gastroenterol 2015;15:85.
Lua GW, Muthukaruppan R, Menon J. Can Rectal Diclofenac Prevent Post Endoscopic Retrograde Cholangiopancreatography Pancreatitis? Dig Dis Sci 2015;60:3118–3123.
Patai A, Solymosi N, Patai AV. Effect of rectal indomethacin for preventing post-ERCP pancreatitis depends on difficulties of cannulation: results from a randomized study with sequential biliary intubation. J Clin Gastroenterol 2015;49:429–437.
Fujita Y, Hasegawa S, Kato Y, Ishii K, Iwasaki A, Sato T, Sekino Y, Hosono K, Nakajima A, Kubota K. Intravenous injection of low-dose flurbiprofen axetil for preventing post-ERCP pancreatitis in high-risk patients: An interim analysis of the trial. Endosc Int Open 2016;4:E1078-e1082.
Hosseini M, Shalchiantabrizi P, Yektaroudy K, Dadgarmoghaddam M, Salari M. Prophylactic Effect of Rectal Indomethacin Administration, with and without Intravenous Hydration, on Development of Endoscopic Retrograde Cholangiopancreatography Pancreatitis Episodes: A Randomized Clinical Trial. Arch Iran Med 2016;19:538–543.
Levenick JM, Gordon SR, Fadden LL, Levy LC, Rockacy MJ, Hyder SM, Lacy BE, Bensen SP, Parr DD, Gardner TB. Rectal Indomethacin Does Not Prevent Post-ERCP Pancreatitis in Consecutive Patients. Gastroenterology 2016;150:911–917; quiz e919.
Mansour-Ghanaei F, Joukar F, Taherzadeh Z, Sokhanvar H, Hasandokht T. Suppository naproxen reduces incidence and severity of post-endoscopic retrograde cholangiopancreatography pancreatitis: Randomized controlled trial. World J Gastroenterol 2016;22:5114–5121.
Shafique MS, Khan JS, Fayyaz MU, Zafar S, Nasrullah M, Ahmad R. Prophylactic rectal NSAIDs in the prevention of post-ERCP pancreatitis. Journal of Postgraduate Medical Institute (Peshawar-Pakistan) 2016;30.
Ucar R, Biyik M, Ucar E, Polat I, Cifci S, ATASEVEN H, Demir A. Rectal or intramuscular diclofenac reduces the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography. Turkish Journal of medical sciences 2016;46:1059–1063.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269, W264.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 2011;343:d5928.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj 2003;327:557–560.
Sheikh I, Fontenot E, Waghray N, Ismail MK, Tombazzi C, Smith JL. The role of nonsteroidal anti-inflammatory drugs in the prevention of post endoscopic retrograde cholangiopancreatography pancreatitis. Jop Journal of the Pancreas 2014;15:219.
Hou YC, Hu Q, Huang J, Fang JY, Xiong H. Efficacy and safety of rectal nonsteroidal anti-inflammatory drugs for prophylaxis against post-ERCP pancreatitis: a systematic review and meta-analysis. Sci Rep 2017;7:46650.
Luo H, Zhao L, Leung J, Zhang R, Liu Z, Wang X, Wang B, Nie Z, Lei T, Li X, Zhou W, Zhang L, Wang Q, Li M, Zhou Y, Liu Q, Sun H, Wang Z, Liang S, Guo X, Tao Q, Wu K, Pan Y, Guo X, Fan D. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet 2016;387:2293–2301.
Yang C, Zhao Y, Li W, Zhu S, Yang H, Zhang Y, Liu X, Peng N, Fan P, Jin X. Rectal nonsteroidal anti-inflammatory drugs administration is effective for the prevention of post-ERCP pancreatitis: An updated meta-analysis of randomized controlled trials. Pancreatology 2017;17:681–688.
Cotton PB, Durkalski V, Romagnuolo J, Pauls Q, Fogel E, Tarnasky P, Aliperti G, Freeman M, Kozarek R, Jamidar P, Wilcox M, Serrano J, Brawman-Mintzer O, Elta G, Mauldin P, Thornhill A, Hawes R, Wood-Williams A, Orrell K, Drossman D, Robuck P. Effect of endoscopic sphincterotomy for suspected sphincter of Oddi dysfunction on pain-related disability following cholecystectomy: the EPISOD randomized clinical trial. Jama 2014;311:2101–2109.
Sethi S, Sethi N, Wadhwa V, Garud S, Brown A. A meta-analysis on the role of rectal diclofenac and indomethacin in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas 2014;43:190–197.
Funding
This study received a financial support from the National Natural Science Foundation of China (81560400) and Natural Science Research Foundation of Jilin Province for Sciences and Technology (20160101181JC).
Author information
Authors and Affiliations
Contributions
Lan Liu and Haiyan Jin conceived and supervised the study; Lan Liu, Chenghao Li, and Yuan Huang performed the research; Lan Liu and Chenghao Li analyzed and interpreted the data; Lan Liu and Haiyan Jin wrote the manuscript; Yuan Huang and Haiyan Jin made manuscript revisions. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Figure S1
Filled funnel plot for PEP using Trim and Fill method. (PNG 45 kb)
Rights and permissions
About this article
Cite this article
Liu, L., Li, C., Huang, Y. et al. Nonsteroidal Anti-inflammatory Drugs for Endoscopic Retrograde Cholangiopancreatography Postoperative Pancreatitis Prevention: a Systematic Review and Meta-analysis. J Gastrointest Surg 23, 1991–2001 (2019). https://doi.org/10.1007/s11605-018-3967-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-3967-7