Abstract
Background
“Hilar en bloc resection” using a no-touch technique has been advocated as a standard procedure in right-sided hepatectomies for treatment of perihilar cholangiocarcinoma (PHC). In principle, it has never been reported for left-sided tumors. The aim is to describe the procedures of total hilar en bloc resection with left hemihepatectomy and caudate lobectomy (THER-LH) for advanced PHC and discuss feasibility and clinical significance of this novel technique.
Methods
A retrospective study using a prospectively maintained database was performed to identify eight patients who had received THER-LH for advanced PHC from January 2013 to December 2015. The clinicopathological features, surgical procedures, and outcomes of these patients form the basis this study.
Results
The operative time was 546 ± 158 (380–870) min, and estimated blood loss was 875 ± 690 (400–2500) ml. Time of vessel resection and reconstruction was 25.6 ± 12.3 min for the portal vein and 19.1 ± 4.9 min for the hepatic artery. Time of hilum clamping was 27.3 ± 11.9 (15–41) min. Two patients had Clavien-Dindo grade II and IVa complications of bile leakage with one developing intraabdominal abscess and bleeding. There was no perioperative mortality. Histopathologic examination revealed that all of eight patients had tubular adenocarcinoma with microscopic invasion to the resected hepatic arteries and portal veins in seven patients. Negative bile duct margins were achieved in all of them. Three patients developed recurrence and died at 11, 18, and 24 months postoperatively. The remaining patients were alive at the time of last follow-up. The median survival was 24 months with one patient achieving a disease-free survival of 50 months.
Conclusion
THER-LH is a technically demanding procedure that is safe and feasible that may have some beneficial effects on the prognosis of these patients with advanced PHC. Further studies are required to confirm the oncological superiority and survival benefits of this novel technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perihilar cholangiocarcinoma (PHC) is the most common malignant tumor affecting extrahepatic bile ducts, and surgical resection represents the only curative option. Given the close proximity of the bile duct bifurcation to vascular inflow, perihilar vasculature invasion is common and historically been the main cause for unresectability.1 While portal vein (PV) resection has become routine, hepatic artery (HA) involvement remains an obstacle.2,3,4,5,6,7,8,9 “Hilar en bloc resection” using a “no-touch technique” with hepatectomy has been recommended for treatment of advanced PHC.10,11,12 The survivals of patients undergoing hilar en bloc resection for right-sided PHC are reported to be significantly higher than those after standard resection.11 However, in principle, hilar en bloc resection has never been reported for left-sided tumors given technical difficulty and fear of associated morbidity and mortality.10,11,12,13,14 As the refinement of surgical technique developed in liver transplantation, we have advocated an aggressive surgical strategy to perform major hepatectomy with simultaneous resection of the PV and HA for the treatment of PHC from 2012. Here, we report a novel technique of performing total hilar en bloc resection with left hemihepatectomy and caudate lobectomy (THER-LH) for treatment of left-sided PHC.
Methods
Patients
From January 2005 to December 2015, a total of 128 consecutive patients with PHC underwent surgical exploration with the intention of performing curative resection at the Department of Hepatobiliary and Pancreatic Surgery, Lihuili Hospital of Ningbo University, Ningbo, China. A retrospective study using a prospectively maintained database was performed to identify patients who had received THER-LH. Between January 2013 to December 2015, forty-seven consecutive patients with PHC underwent curative resection, and eight patients had received THER-LH.
Patients diagnosed with PHC were evaluated using computed tomography and magnetic resonance cholangiopancreatography (MRCP) to determine Bismuth-Corlette classification, vascular invasion, and metastatic disease. Three dimensional visualization technologies were applied to help making surgical procedures individually from 2015 (Video 1). Left hemihepatectomy combined with caudate lobectomy was considered for type IIIb or IV PHC with tumor extension predominantly in the left side. The preliminary decision to perform a THER-LH was made based on preoperative imaging that the tumor abutted or encircled the PV and HA in hilum. When the findings were confirmed by palpation by the surgeons, that the HA and PV at the hilum were involved and could not be freed from tumor, THER-LH was performed, when there were no contraindications. Clinicopathological and follow-up data of the eight patients were collected to form the basis of this retrospective study.
Surgical Procedures
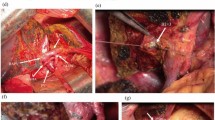
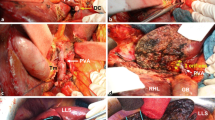
Surgical procedures of THER-LH are described sequentially and illustrated by Figs. 1, 2 and Video 2.
-
Step 1.
After laparotomy, the whole abdominal cavity is thoroughly examined to confirm the absence of liver metastasis and disseminated disease. The extension of tumor invasion and vascular involvement are confirmed. Kocher’s maneuver is used to facilitate all subsequent procedures and to confirm the absence of para-aortic lymph node metastasis by obtaining frozen sections. Clearance of the para-aortic lymph node is not performed routinely unless microscopic metastasis of these nodes is observed (Figs. 1a, b and 2a).
-
Step 2.
The dissection of hepatoduodenal ligament (HDL) begins at level of the pancreas and duodenum with transection of the right gastric artery (RGA) followed by dissection along gastroduodenal artery (GDA), which is divided as close to the pancreas as possible. The proper hepatic artery (PHA) and the main portal vein (MPV) are identified and isolated using vessel loops. A complete lymphadenectomy of HDL is performed, including the clearance of para-aortic lymph nodes and soft tissues in the case where metastasis to these nodes has been confirmed, proceeding proximally to the hilum with skeletonization of the PHA and the MPV. The distal common bile duct (CBD) is then divided at its entrance into the pancreas. Frozen sections of the margin of the CBD stump are performed to ensure that a Whipple procedure is not necessary. The dissection toward the hilum is stopped in the vicinity of the tumor without attempts to dissect the HA and the PV at the level of the bile duct bifurcation. If possible, the right posterior sectional branches of the HA (RPSA) and the PV (RPSPV) are isolated with vessel loops inside the Rouviere’s sulcus (Figs. 1b, c and 2b).
-
Step 3.
The left liver and caudate lobe are mobilized from the left to right by dividing the Arantius ligament at its attachment to the root of left hepatic vein. Belgiti’s hanging maneuver15 is used to transect parenchyma along the left side of middle hepatic vein (MHV) or along the right side of MHV in case it has been involved by tumor, to expose the right pedicle without mobilization of the right liver. The anterior and posterior sectional branches of the right hepatic artery (RHA), the right portal vein (RPV), and the right hepatic duct (RHD) are identified and isolated, respectively (Figs. 1d–f and 2c).
-
Step 4.
The anterior and posterior sectional branches of RHD are then divided first by scissors with great care being taken to avoid an injury to the corresponding arterial branches. At this point, the specimen including the left liver with the caudate lobe and total hilum with tumor and dissected tissues is connected to the right liver only by the HA and PV. After evaluating the lengths of involved vessels that need to be resected, the HA and PV are clamped both proximal and distal to the tumor using vascular clamps and divided. The distal division of the HA and the PV is at the points where the anterior and posterior sectional branches of RHA and RPV or the RHA and the RPV per se can safely be controlled via vascular clamps. Frozen sections of each of the bile duct margins, if possible, including the cut margins of the PV and the HA, were performed to ensure a radical resection. The dissected specimen is removed immediately (Figs. 1e–h and 2c).
-
Step 5.
Subsequent reconstruction begins with PV by suturing the anterior and posterior sectional branches of PV: RASPV and RPSPV together followed by an end-to-end anastomosis to the MPV or direct end-to-end anastomosis between the remaining RPV and MPV in case there is no invasion of the second-order branches of RPV. After the portal flow to the right liver is restored, an end-to-end anastomosis of right anterior sectional artery (RASA) to PHA and RPSA to GDA is performed under a magnifying scope using a magnification of 2500. If first order of RHA ramification is clear, it may also serve as an alternative for arterial anastomosis to the PHA. Time for hilum clamping (THC) defined as time between PV clamping to restoration of PV flow, as well as time for HA and PV anastomosis, is recorded. Arterio-portal shunting can serve as an alternative in the case of arterial reconstruction failure (Figs. 1i–m and 2d, e).
-
Step 6.
Finally, biliary reconstruction is performed using a Roux-en-Y loop after connecting multiple orifices of bile ducts into one by biliary plasty (Figs. 1n, o and 2f).
a A right sub-coastal incision like contra-L type. b Isolated HA and divided CBD at its entrance into the pancreas. The PV remained to be skeletonized. c Isolated MPV. The dissection toward the hilum is stopped in the vicinity of the tumor without attempts to dissect the HA and the PV at the hilum. d The left liver and caudate lobe are mobilized from left side to right. e Transection line between right and left liver and a rubber catheter passed from retrohepatic tunnel. f Isolated RPHD, RASA, RPSA, RASPV, and RPSPV with transected RAHD. g Transection of MPV and PHA with scissors under vascular clamping. h En bloc resected specimen including left liver, caudate lobe, involved PV and HA, and bile duct tumor (BD T). i Vein plasty of RASPVwith RPSPV. j An end-to-end anastomosis of RPV to MPV. k Accomplished anastomosis of RASPV and RPSPV to MPV and restoring of the portal flow. l An end-to-end anastomosis of GDA to RPSV. m An arterial-portal shunting of the PHA to RASPV. n Biliary plasty of RAHD with RPHD. o Hepatojejunostomy by Roux-en-Y anastomosis
a Incision (diagrammatical representation of Fig. 1a). b Tumor location in bile duct and involvement regions of PV and HA (diagrammatical representation of Fig. 1b, c). c Transected vessels and bile ducts with en block resected specimen going to be removed (diagrammatical representation of Fig. 1e f, g). d Anatomical representation of the PV resection and reconstruction (diagrammatical representation of Fig. 1i, j, k). e Diagrammatical representation of accomplished anastomosis of PV, HA, and arterial-portal shunting of the PHA to RASPV (Fig. 1k–m). f Plastic RHD and ready for hepatojejunostomy (diagrammatical representation of Fig. 1n, o)
Occasionally, tumor invasion is limited to the left-side bifurcation of the MPV and the ramification of PHA, i.e., both the RPV and RHA can be divided extrahepatically with negative margins. The resection and reconstruction of the PV and HA for the right liver can be accomplished prior to hemihepatectomy.
Histology and Statistical Analysis
Histopathologic examinations were performed using AJCC classification system.16 Microscopic tumor invasion to the PV and HA was classified as grade 0 (no involvement), grade I (cancer invasion limited to the tunica adventitia or media), or grade II (cancer invasion reaching the tunica intima) according to the Nagoya’s criteria.9 Postoperative complications were classified using the Clavien-Dindo classification.17 Overall survival (OS) was defined as the time between surgery and death or most recent follow-up. Postoperative mortality was defined as death occurring within hospital stay or within 90 postoperative days. Continuous variables were reported as means with standard deviation or medians with interquartile range (IQR) as deemed appropriate. Categorical variables were reported as whole numbers and percentages. Statistical analyses were carried out using SPSS (SPSS, Inc., Chicago, 16.0).
Results
Eight patients underwent THER-LH. Clinical features and operative data are presented in Table 1. The median age was 64 (IQR 53.25–75.50) years, and four patients were females. The tumors were classified as Bismuth-Corlette type IIIb in three patients and type IV with left-sided predominance in five patients. None of the eight patients received neoadjuvant chemotherapy. Two patients developed PHC with underlying intrahepatic choledocholithiasis. One of them (patient 2) had undergone gastrectomy for gastric carcinoma and left lateral sectionectomy for early stage intrahepatic cholangiocarcinoma 2 and 5 years prior to THER-LH, respectively. Another patient (patient 7) had undergone a left lateral sectionectomy due to intrahepatic choledocholithiasis 5 years prior to THER-LH. Two patients with total bilirubin more than 200 μmol/l had undergone percutaneous transhepatic biliary drainage (PTBD). Given macroscopic involvement, concomitant organ resections were performed in three patients. One patient each required a pancreatoduodenectomy, gastric antrum resection, and combined gastric antrum and duodenal bulb resection. The median operative time and estimated blood loss were 520 (IQR 415–630) min and 700 (IQR 425–950) ml, respectively. Because of past history of abdominal surgery and combined pancreatoduodenectomy with clearance of para-aortic lymph nodes and soft tissues, patient 2 has the longest operation time of 870 min. The median length of resected vessel was 3.35 (IQR 1.6–3.5) cm for HA and 3.10 (IQR 1.5–3.5) cm for PV, respectively. Reconstructions were accomplished by an end-to-end anastomosis of RPV stump to MPV in five patients, and by combining anterior with posterior branches of RPV together, and subsequent end-to-end anastomosis to the MPV in three patients (Fig. 1). Reconstruction of the HA was performed after portal flow was restored. In six patients, one artery was reconstructed with an end-to-end anastomosis of RHA to PHA for each. In one patient who had two arterial stumps of RPSA and RASA, an end-to-end anastomosis of RPSA to GDA and an end-to-side anastomosis of PHA to anterior branch of RPV (arterio-portal shunting) with the ligation of RASA were performed. In another patient with RHA coming from the superior mesenteric artery, the aberrant RHA stump was reconstructed using an end-to-end anastomosis to the PHA. The time taken for vessel resection and reconstruction was 25.6 ± 12.3 min for PV and 19.1 ± 4.9 min for HA. Therefore, the mean THC was 27.3 ± 11.9 min (15–41). Postoperatively, serum ALT, AST, and total bilirubin levels were elevated on day 1, but returned to a normal value within 10 days after surgery (Fig. 3). Patients 3 and 7 experienced a Clavien-Dindo grade IVa and II complications, respectively. Patient 7 who had bile leakage classified as grade II18 was treated with extended drainage, while patient 3 developed intraperitoneal abscess with bleeding required re-laparotomy. There was no perioperative mortality. Histopathologic examination demonstrated that all eight patients had tubular adenocarcinoma with well, moderate, and poor differentiations presented in one, four, and three patients, respectively (Table 2). Perineural involvement was observed in all patients. Microscopic examination of vessels revealed no invasion to both the PV and HA for patient 5 (grade 0) who had marked fibrosis around the resected HA and PV. The extent of PV invasion for other patients was grade I in six and grade II in one. And the extent of HA invasion was grade I in the other seven patients (Fig. 4). Therefore, approximately all of the patients with PV and HA resections appeared to have microscopic invasion except one. Negative bile duct margins were achieved in all patients. The number of harvested nodes was 15.5 (IQR 13.25–17.75), and positive nodes were identified in six patients with periaortic node metastasis of N2 in two patients. As per the AJCC staging system,16 one patient had stage IIIB, two patients had stage IVB diseases due to N2 metastasis, and the other five patients had stage IVA diseases (Table 2). The median length of hospital stay was 16.5 (IQR 14.25–20.00) days. On most recent follow-up in March, 2017, three patients developed recurrence and died at 11, 18, and 24 months after surgery, respectively. The remaining patients were alive. The median survival was 24 months with one patient having a disease free survival of 50 months (Table 2).
Discussion
It has been reported that operative macroscopic PV infiltration is a significant prognostic factor.19 Advancements in surgical techniques has made resection of tumors with PV involvement possible with acceptable morbidity and mortality.1,2,3 However, a systematic review revealed that there were no proven survival advantage with portal vein resection, and arterial resection results in higher morbidity and mortality with no proven benefit.1 Dissection of bile ducts and tumor off of the PV bifurcation and RHA risks spillage of tumor cells locally, which may contribute to the high rate of local recurrence. Neuhaus et al. advocated en bloc hilar resection using the no-touch technique for treatment of right-side PHC.10 More recent data have reported longer survival as compared to that after standard resection for same stage disease.11 However, in principle, hilar en bloc resection for left-sided tumors has never been reported because of the associated morbidity and the mortality associated with routine RHA reconstruction.12 Anatomically, the RPV is short and bifurcates early on into anterior and posterior branches. Also, the anterior and posterior branches of the RHA become smaller and lack elasticity. The technique of hilar en bloc resection for left-sided tumors is much more difficult than that for right-sided tumors because of the reconstruction of RPV and RHA. Therefore, Neuhaus and Tamoto concluded that hilar en bloc resection is only indicated for right-sided hepatectomies but not feasible for left-sided hepatectomies.10,14 Besides, blood flow of both the HA and PV has to be interrupted during hilar en bloc resection with left hepatectomy which can induce ischemic reperfusion liver damage. In order to avoid long-term ischemic injury, Miyazaki et al.20 reported a technique of keeping arterial blood supply intact by initially resecting and reconstructing the PV with subsequent HA resection and reconstruction. To resect HA and PV separately, the dissection of hilar vessels might be necessary in a manner that does not follow principles of an en bloc resection. Meanwhile, the reconstruction of RPV might be difficult for most of advanced tumors using this approach due to limited space occupied by the being resected specimen. Miyazaki et al. reported 33% mortality and no survivors at 3 years.5,20 These poor outcomes could be related to technical difficulty of the anastomosis as well as tumor recurrence. In the last decade, only Nagino et al. have reported a large series on patients undergoing combined HA and PV resection for PHC.9 The time taken for vessel resection and reconstruction was 25 ± 19 min for the PV and 119 ± 56 min for the HA. The technique of simultaneous resection of PV and HA was not described in detail. The time of vascular flow interruption of both the HA and PV has not been reported by the same team.9,21 We inferred from the long time for HA resection and reconstruction and lack of comment on liver ischemic time that they may employ a technique similar to that described by Miyazaki et al.20
By the refinement of surgical technique developed in liver transplantation, we have advocated an aggressive surgical strategy to perform major hepatectomy with simultaneous resection of the PV and HA for the treatment of PHA starting in 2012 and have been able to perform THER-LH for the treatment of eight patients with left-sided PHC from 2013 to 2015. To our knowledge, this is one of the first reports on hilar en bloc resection with left-sided hepatectomy. Our technique of THER-LH is quite different from hilar en block resection proposed by Neuhaus.10 There are some technical and oncological superiorities of THER-LH over other techniques: (1) preserved volume of right liver is larger than that of the left liver; therefore, preoperative portal vein embolization (PVE) is rarely required; (2) perineural invasion and lymphangial carcinomatosis can frequently be observed up to 2 cm from the tumor into the liver and HDL, and the resection of hilar structures including HA and PV by THER-LH provides three-dimensional resected safety margins with maximized oncological dissection; (3) given that RPV is short or bifurcates early, its dissection is easier after exposure of right pedicle, making an end-to-end anastomosis of PV more feasible; (4) with the help of Belgiti’s maneuver, liver parenchyma transection becomes easier without mobilization of the right liver which benefits protection of collateral circulation. When performing this technique, we observed a rich retrograde blood flow from the distant ends of the transected vessels.
The interruption of the blood flow during THER-LH can induce ischemic liver damage. Clinical studies have established 60 min as the safe duration under normthermic conditions.22,23,24 To reduce the ischemic time, we performed the PV resection lastly and reconstruction immediately after removing the specimen. This procedure was accomplished within 40 min in our study. The duration of hilum clamping in our patients was 27.3 ± 11.9 min (15–41). The serum enzymes and bilirubin levels returned to near normal range within 10 postoperative days suggesting that the ischemic effect on liver was minimal.
Jarnagin et al.25 had proposed a criterion of non-resectability in patients with PHC that included local factors such as encasement or occlusion of the main PV proximal to its bifurcation and atrophy of one lobe with encasement of contralateral PV branch. The extent of disease of the reported eight patients is within these criteria; however, an R0 resection was achieved safely, resulting in a median survival of 24 months with one patient being disease free at 50 months after surgery. Therefore in our opinion, the technique of THER-LH can have a significant impact on the revision of guidelines for the treatment of PHC.
In conclusion, we believe that the THER-LH is safe and feasible and can have some benefit in terms of the prognosis of these patients. However, due to the limited number of patients and short follow-up, further studies are required to determine the true oncological superiority of this novel approach for the treatment of advanced PHC.
B-C Bismuth-Corlette classification, TB total bilirubin, OR operation, N no, Y yes, THC time of hilum clamping, rHA resected hepatic artery, rPV resected portal vein, Pre-OR BD preoperative biliary drainage, PD pancreatoduodenectomy, GA gastric antrum resection, DB duodenal bulb resection
Grade 0: no involvement; grade I: invasion limited to the tunica adventitia or media; grade II: invasion reaching to the tunica intima
rHA resected hepatic artery, rPV resected portal vein, PLN positive lymph nodes, TLN number of harvested lymph nodes, a alive, d dead
aN2 metastasis16
bGrand II or more according to Clavien-Dindo Classification of Surgical Complications17
References
Abbas S, Sandroussi C. Systematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2013;15(7):492–503.
Gerhards MF, van Gulik TM, de Wit LT, Obertop H, Gouma DJ. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma—a single center experience. Surgery. 2000;127(4):395–404.
Hemming AW, Mekeel K, Khanna A, Baquerizo A, Kim RD. Portal vein resection in management of hilar cholangiocarcinoma. Journal of the American College of Surgeons. 2011;212(4):604–613; discussion 613-606.
Ota T, Araida T, Yamamoto M, Takasaki K. Operative outcome and problems of right hepatic lobectomy with pancreatoduodenectomy for advanced carcinoma of the biliary tract. Journal of hepato-biliary-pancreatic surgery. 2007;14(2):155–158.
Miyazaki M, Kato A, Ito H, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007;141(5):581–588.
Hidalgo E, Asthana S, Nishio H, et al. Surgery for hilar cholangiocarcinoma: the Leeds experience. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2008;34(7):787–794.
Konstadoulakis MM, Roayaie S, Gomatos IP, et al. Aggressive surgical resection for hilar cholangiocarcinoma: is it justified? Audit of a single center’s experience. American journal of surgery. 2008;196(2):160–169.
Sakamoto Y, Sano T, Shimada K, et al. Clinical significance of reconstruction of the right hepatic artery for biliary malignancy. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2006;391(3):203–208.
Nagino M, Nimura Y, Nishio H, et al. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Annals of surgery. 2010;252(1):115–123.
Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Annals of surgery. 1999;230(6):808–818; discussion 819.
Neuhaus P, Thelen A, Jonas S, et al. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Annals of surgical oncology. 2012;19(5):1602–1608.
Govil S, Reddy MS, Rela M. Surgical resection techniques for locally advanced hilar cholangiocarcinoma. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2014;399(6):707–716.
Hirano S, Kondo S, Tanaka E, Shichinohe T, Tsuchikawa T, Kato K. No-touch resection of hilar malignancies with right hepatectomy and routine portal reconstruction. Journal of hepato-biliary-pancreatic surgery. 2009;16(4):502–507.
Tamoto E, Hirano S, Tsuchikawa T, et al. Portal vein resection using the no-touch technique with a hepatectomy for hilar cholangiocarcinoma. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2014;16(1):56–61.
Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. Journal of the American College of Surgeons. 2001;193(1):109–111.
Edge SB BD, Compton CC, et al. Perihilar bile ducts. In: AJCC Cancer Staging Handbook. 7th ed. Chicago, IL: Springer; 2010:718.
Dindo D, Demartines D, Clavien PA. Classification of Surgical Complications A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg. 2004;240(2): 205–213.
Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: A definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149(5):680–688.
Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Annals of surgery. 2003;238(5):720–727.
Miyazaki M, Kimura F, Shimizu H, et al. Recent advance in the treatment of hilar cholangiocarcinoma: hepatectomy with vascular resection. Journal of hepato-biliary-pancreatic surgery. 2007;14(5):463–468.
Ebata T, Ito T, Yokoyama Y et al. Surgical technique of hepatectomy combined with simultaneous resection of hepatic artery and portal vein for perihilar cholangiocarcinoma (with video) J Hepatobiliary Pancreat Sci June 2014; 21:E57–E61.
Smyrniotis V, Farantos C, Kostopanagiotou G, Arkadopoulos N. Vascular control during hepatectomy: review of methods and results. World journal of surgery. 2005;29(11):1384–1396.
Midorikawa Y, Kubota K, Takayama T, et al. A comparative study of postoperative complications after hepatectomy in patients with and without chronic liver disease. Surgery. 1999;126(3):484–491.
Smyrniotis VE, Kostopanagiotou GG, Contis JC, et al. Selective hepatic vascular exclusion versus Pringle maneuver in major liver resections: prospective study. World journal of surgery. 2003;27(7):765–769.
House MG, D’Angelica MI, Jarnagin R. Cancer of the bile ducts: extrahepatic biliary tumors. In: Blumgart’s Surgery of the Liver, Biliary Tract, and Pancreas. 5th ed. Philadelphia, PA:Elsevier; 2012:783.
Acknowledgements
The authors are grateful to Yu Xi for her assistance in preparing the manuscript.
Funding
The Scientific Innovation Team Project of Ningbo (Fund Number 2013B82010)
Author information
Authors and Affiliations
Corresponding author
Additional information
Synopsis
We described our technique of total hilar en bloc resection with left hemihepatectomy and caudate lobectomy for eight patients with advanced PHC and discussed its feasibility. It is a technically demanding procedure that is safe and feasible and may have some beneficial effects on the prognosis of these patients.
Rights and permissions
About this article
Cite this article
De Lu, C., Huang, J., Wu, S.D. et al. Total Hilar En Bloc Resection with Left Hemihepatectomy and Caudate Lobectomy: a Novel Approach for Treatment of Left-Sided Perihilar Cholangiocarcinoma (with Video). J Gastrointest Surg 21, 1906–1914 (2017). https://doi.org/10.1007/s11605-017-3561-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-017-3561-4