Abstract
Insufficient number of examined lymph nodes (eLNs) was considered to increase significantly the risk of stage migration in gastric cancer patients. The aim of our study is to establish a nomogram predicting the overall survival (OS) for patients with an insufficient number of eLNs. A total of 872 gastric cancer patients with extended lymphadenectomies were assigned randomly (2:1) to the development cohort and the validation cohort. The nomogram was established based on the Cox regression model using the development cohort. The concordance index (C-index) was used to evaluate the discriminative ability. We also compared our model with two other staging systems. Using multivariate analysis, age, sex, tumor location, depth of invasion, macroscopic type, lymphovascular invasion, the number of eLNs, and metastatic lymph nodes were selected and incorporated into the nomogram. The C-index of the nomogram was 0.742 and 0.743 in development and validation cohorts, respectively, which were significantly superior to the C-indices (range 0.705–0.712, all P < 0.001) of American Joint Committee on Cancer (AJCC) seventh edition and lymph node ratio staging systems in both cohorts. We established a nomogram which could predict accurately OS for gastric cancer patients with insufficient number of eLNs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The American Joint Committee on Cancer (AJCC) TNM staging system is the most commonly used staging system in gastric cancer, which stratifies patients into several risk groups according to the depth of tumor invasion, number of metastatic lymph nodes (mLNs), and distant metastasis. The seventh edition published in 2010 suggested that at least 16 lymph nodes should be examined for optimal staging.1
Several studies have demonstrated that an adequate number of examined lymph nodes (eLNs) were associated with improved overall survival (OS) in gastric cancer.2–4 Insufficient number (<16) of eLNs may cause “stage migration,” which was observed in 10–15% cases of gastric cancer,5–7 and further affects the treatment and prognosis of this group of patients.8
Lymph node ratio (LNR) is defined as the ratio between mLNs and eLNs. Several studies proposed the ratio-based staging systems, which have been approved to reduce stage migration.7, 9–11 However, the revised ratio-based classification still has some limitations.12, 13 In addition, several proposed ratio-based staging systems have different cutoff values.9, 11, 14 Thus, the extensive implication of ratio-based systems could also be limited by the different cutoff values.
Nomogram, a statistics-based tool that provides the overall probability of a specific outcome, has been widely used in many kinds of malignant tumors.15 Several nomograms have been established to predict the OS of gastric cancer patients.16–20 However, they were mainly based on the data of patients with sufficient numbers of eLNs, and no current nomogram has been validated in the patients with insufficient number of eLNs. Therefore, the predictive values of these nomograms may be limited in those with insufficient number of eLNs.
In the current study, we attempt to develop and validate a nomogram which may predict accurately OS of patients with insufficient number of eLNs, compared with AJCC TNM staging systems and the revised ratio-based staging systems.
Methods
Patients
Since D2 lymphadenectomy was associated with lower loco-regional recurrence and cancer-related death and recommended as standard surgery in both Asian and Western,21, 22 patients with gastric cancer were recruited who underwent extended lymphadenectomy (≥D2) and achieved radical (R0) resection at the Department of Surgical Oncology, The First Affiliated Hospital of China Medical University between January 1980 and December 2006. The extent of lymphadenectomy was performed according to the guidelines of the Japanese Gastric Cancer Association (JGCA).23 R0 resection was defined as complete removal of all tumors without microscopic or macroscopic tumor residual.
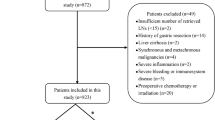
Patients with insufficient number of eLNs (<16) and without other combined cancer were included. Patients were excluded if they received neoadjuvant chemotherapy or if any information had been missed. Patients who died in the postoperative period were also excluded. Finally, 872 patients with insufficient numbers of eLNs and complete information were included in our primary cohort. This study was approved by the Institutional Ethics Committee of China Medical University.
The following demographic and pathologic characteristics were collected: age, sex, tumor location, tumor size, macroscopic type, histological type, lymphovascular invasion, extent of lymphadenectomy, depth of invasion, the number of eLNs and mLNs, the follow-up period, and survival status. The tumor location was classified into three categories according to the location of the main lesion: upper third, middle third, and lower third. The macroscopic type was classified into five categories: early gastric cancer and advanced gastric cancer Borrmann types I to IV. The histological type was divided into two categories: differentiated type, including papillary adenocarcinoma and well- and moderately differentiated tubular adenocarcinoma, and undifferentiated type, including poorly differentiated tubular adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma. The depth of invasion was classified according to the seventh edition of the AJCC TNM classification.1 In our present study, 464 (53.2%) patients received adjuvant therapy during our study period and the treatment pattern was highly variable. Therefore, adjuvant therapy may not be an ideal model covariate in our present study. In addition, Balachandran et al. suggested that treatment should be avoided as a covariate in the prediction model unless there are validated data from a randomized clinical trial.24 In the light of these considerations, we did not include adjuvant therapy in our prediction model and only used clinicopathological characteristic variables to predict survival.
After being discharged from the hospital, the patients received telephone interview or outpatient follow-up every 3 months in the first 3 years, 6 months in the fourth and fifth years, and once a year thereafter. The follow-up period was measured from the time of operation to the date of last follow-up (March 31, 2015) or death date.
Development and Validation of the Nomogram
Nomogram construction and validation were performed on the basis of the guidelines proposed by Iasonos.15 We assigned randomly two thirds of patients to the development cohort (n = 582) and one third to the validation cohort (n = 290).
To identify the independent survival predictors, multivariable analysis was performed using a Cox proportional hazards regression model and the data of development cohort. Continuous variables (tumor size, the number of eLNs and mLNs) were fit using restricted cubic splines to relax the linearity assumption.25 The nomogram was constructed to predict the 5- and 10-year OS based on the results of the multivariable Cox regression model. And the final factors included in the model were identified by a backward stepwise selection process with the Akaike information criterion.25
The performance of the nomogram was evaluated with respect to discrimination and calibration. Discrimination was measured by the concordance index (C-index),26 which refers to models’ ability to distinguish the outcomes correctly. The larger the C-index, the more accurate the model predicted.27 Calibration curves were performed by comparing the means of the nomogram-predicted OS with the observed Kaplan–Meier estimate of actual OS. We used the bootstrapping (1000 repetitions) method to reduce the estimate bias. Additionally, the model validations were also performed using the validation cohort. For patients in validation cohort, we first calculated total points according to the established nomogram. Next, we performed the Cox regression analysis using the total points as a factor. Finally, the regression analysis was used to generate the C-index and calibration curves.
Categorization of Patients in Different Staging Systems
Patients were categorized according to the seventh edition of AJCC TNM staging systems.1 A novel LNR-based staging system, named TNrM, was established according to the same T and M definitions of the seventh AJCC staging system and the Nr classification (Nr0 0%, Nr1 1∼20%, Nr2 21∼50%, Nr3 >50%). The cutoff values of LNR were determined by using the log-rank test and were consistent with the results of previous studies which came from the same patient database.9, 13
Statistical Analysis
The clinicopathological characteristics were compared using the χ 2 test for categorical variables and two-tailed Student’s t test for continuous variables. OS was estimated using the Kaplan–Meier method. The log-rank test was used to examine the survival difference between subgroups. A two-tailed P value <0.05 was considered as statistically significant. All statistical analyses were performed using STATA version 12.0 for Windows (StataCorp, College Station, TX) and R software version 3.2.0 with the rms, Hmisc, and survival package (http://www.r-project.org/).
Results
Clinicopathological Characteristics and Survival
The clinicopathological characteristics of 872 patients are presented in Table 1. In the primary cohort, three quarters of patients were male and the median age was 60 years old (range 26–90). Most of tumors were located in the lower third (n = 533, 61.1%). More than 70% patients were classified as pT3 or pT4 stage according to the seventh AJCC TNM staging system. A majority of patients underwent D2 lymphadenectomy (n = 645, 74.0%). The mean numbers of eLNs and mLNs were 9.45 ± 4.02 (range 1–15) and 2.14 ± 2.92 (range 0–14), respectively. In the development and validation cohorts, the mean numbers of eLNs were 9.36 ± 4.03 and 9.62 ± 4.01, and the mean numbers of mLNs were 2.15 ± 2.94 and 2.14 ± 2.88. No significantly different variables were observed between development and validation cohorts (all P > 0.05).
At the last follow-up, a total of 694 patients had died and the median and mean follow-up time was 48 and 86 months (range 3–410 months), respectively. The 5-year OS of the primary cohort was 45.2%.
Nomogram Development and Validation
In the univariate analysis, sex, age at operation, tumor location, tumor size, macroscopic type, lymphovascular invasion, depth of invasion, and the number of mLNs were associated with OS, whereas the histological type, the extent of lymphadenectomy, and the number of eLNs were not (Table 2). We also incorporated the number of eLNs in multivariate analysis to improve the model performance of our nomogram.
Multivariate analysis showed that sex, tumor location, macroscopic type, lymphovascular invasion, depth of invasion, and the number of mLNs and eLNs were significantly associated with OS (all P < 0.05) (Table 2).
On the basis of the results of multivariate analysis, we built up a nomogram which could predict the 5- and 10-year OS (Fig. 1a). For each prognostic factor in the nomogram, the point can be determined by drawing a line straight upward from each predictor to the point axis. The total points are estimated by summing each point and located in the total point axis. The probability of 5- and 10-year OS can be determined by drawing a line straight down from the total point axis. The C-index for the developed nomogram to predict the OS was 0.742 [95% confidence interval (CI) 0.719∼0.764]. The calibration curves showed good agreement between the nomogram-predicted probability and the actual survival probability (Fig. 1b).
a Nomogram predicting 5- and 10-year overall survival of patients who underwent extended lymph node dissection with insufficient examined lymph nodes. Calibration curves of the nomogram in the development cohort (b) and validation cohort (c). The nomogram-predicted probability of overall survival is plotted on the x-axis; the actual overall survival estimated by the Kaplan–Meier method is plotted on the y-axis (EGC early gastric cancer, LNs lymph nodes)
In the validation cohort, the C-index for predicting OS was 0.743 (95% CI 0.710∼0.776). The calibration curves showed that the nomogram performed well in predicting the 5- and 10-year OS (Fig. 1c).
We also evaluated the discrimination ability of the nomogram by dividing the patients into quartiles according to the points and plotted the Kaplan–Meier curves of both development and validation cohorts (Fig. 2). The survival curves showed that our nomogram had good discriminatory ability for patients.
Comparison Between the Developed Nomogram and Other Staging Systems
The TNrM staging system was established according to the method that we have mentioned earlier. The novel staging system has the same T and M classification with the seventh AJCC staging system, and the N classification was just replaced by the Nr classification. The novel revised staging system is presented in Table 3.
The C-index of our nomogram in the development cohort was 0.742 (95% CI 0.719∼0.764), which was significantly higher than the seventh AJCC TNM staging system (C-index 0.707; 95% CI 0.683∼0.732) and the TNrM staging system (C-index 0.712; 95% CI 0.688∼0.735) (all P < 0.001). The same results were also observed in the validation cohort; the C-index of the nomogram (C-index 0.743; 95% CI 0.710∼0.776) was higher than the C-indices of the seventh AJCC staging system (C-index 0.705; 95% CI 0.670∼0.740) and the TNrM staging system (C-index 0.707; 95% CI 0.673∼0.741) (all P < 0.001). In addition, Fig. 3 shows the distribution of nomogram-predicted 5-year survival according to the seventh AJCC TNM staging system and TNrM staging system. A wide range of nomogram-predicted survival was identified in both TNM staging system and TNrM staging system. The heterogeneity of OS in each stage of TNM staging systems and TNrM staging systems is significant, especially in stages II and III.
These results revealed that our nomogram might be superior to the current staging systems for prediction of OS in gastric cancer patients with insufficient numbers of eLNs.
Discussion
For malignant tumor, staging is a critical component of therapy. The accurate staging of patients could convey the extent of disease, help oncologists make appropriate clinical therapy decisions, and evaluate the prognosis. The status of mLNs was considered one of the most important prognostic indicators of gastric cancer,28–30 and the AJCC TNM staging systems defined N classification according to the absolute number of mLNs since 1997.1, 31, 32 However, some authors reported that the accurate classification of the mLNs is influenced by the number of eLNs significantly.33, 34 Insufficient number of eLNs can lead to an underestimation of stage, which is also called “Will Rogers” phenomenon.35
The number of eLNs is often determined by two independent parts: one is the extent of lymphadenectomy, and the other is lymph nodes harvested from specimen after surgery, and furthermore, the difference of lymph nodes number was mainly associated with lymph nodes harvested from the specimen after surgery, rather than the extent of lymphadenectomy.36 Surgeons may also be influenced by the suggested minimum number of LNs required for examination and carry out retrieval carelessly or not strictly.3 In addition, some clinicopathological factors and the immunologic response of patients may be associated with insufficient number of eLNs.4, 8, 37, 38 Actually, many previous studies also found that about 10%–20% of patients had insufficient number of lymph nodes examined despite the extended lymphadenectomy was performed, especially in western population.6, 39, 40
In the current study, we developed and validated a nomogram predicting the OS of patients who underwent extended lymphadenectomy with insufficient number of eLNs. The C-indices and calibration curves revealed that our nomogram performed well in predicting OS. Comparing with the conventional TNM staging systems, nomograms attempt to combine all proven prognostic factors and quantify risk as precisely as possible, rather than producing different risk groups.20 Thus, for many cancers, nomograms are proved to be more accurate in predicting individualized survival than TNM staging systems.16, 41–43 In our present study, our nomogram showed better predictive accuracy of OS than the seventh edition AJCC TNM staging systems.
Several nomograms categorized the mLNs according to the seventh AJCC TNM staging system or using statistical methods and incorporated into the nomogram.16–18 Categorical variable is convenient for clinical implication, but insufficient number of eLNs may limit accuracy of classification. For instance, it is impossible to categorize patients with insufficient numbers of eLNs as N3b group. Besides that, N1 group can be upstaged to N2 or N3 group when more LNs were examined.33 Thus, categorization of mLNs may be not able to reflect truly the extent of disease and assign patients inaccurate points.
One most recent nomogram based on a western database incorporated LNR but without the number of eLNs included.44 On the one hand, previous studies have showed that patients with different lymph nodes examined had different survival rates, especially for patients with insufficient number of eLNs and rN0 group.9, 12 Therefore, the impact of eLNs should not be ignored. On the other hand, patients with the same LNR may have the different survival12 and incorporating LNR into our model may introduce bias. In our nomogram, we incorporated both the number of eLNs and mLNs into the nomogram and using continuous variables to predict the OS, which may help to reflect accurately the status of lymph node involvement in some extent. In addition, we noted that including both the number of eLNs and mLNs had a larger goodness-of-fit statistic (R 2) than we included LNR or mLNs alone and showed better model performance. However, including more covariates may lead to overfitting of the model.24 In our present study, we used the bootstrapping method (1000 repetitions) to quantify the overfitting of our model. Our calibration curves showed good agreement between nomogram-predicted and the actual survival (Fig. 1b, c) These results revealed minimal evidence of overfitting.
There are some limitations in the current study. First, our nomogram was established based on clinicopathological data from a single institution. Thus, our results might be biased by institutional practice patterns.24 And whether this nomogram can be applied to other patient groups with insufficient number of eLNs remained to be evaluated. Second, our study cohort lasted for a long time period (1980–2006), which may introduce external variations in long-time outcomes.45 Third, adjuvant chemotherapy has been confirmed to improve OS in gastric cancer patients after extended lymphadenectomy in recent years.46, 47 Nonetheless, we did not include adjuvant chemotherapy in our analysis, because adjuvant therapy was not a common treatment during the long period of our study and many patients did not receive the standard adjuvant chemotherapy. Thus, our current study could not evaluate precisely the effect of adjuvant therapy, which may affect the accuracy of our conclusions. In addition, adjuvant therapy was not controlled in our predictive model; however, it may be a confounder for OS and the validity of our results may be limited. Thus, the treatment effect of adjuvant chemotherapy will be considered in our further studies.
Conclusions
In summary, the nomogram established in current study predicted accurately the OS of gastric cancer patients with insufficient number of eLNs after extended lymphadenectomies. We provided a new method to assess the prognosis for gastric cancer patients who had high risk of stage migration. The value of this nomogram for gastric cancer patients from other institutions would be determined in the future studies.
References
Author names in bold designate shared co-first authorship
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2010.
Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 2005; 23: 7114–7124.
Coburn NG, Swallow CJ, Kiss A, Law C. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer 2006; 107: 2143–2151.
Baxter NN, Tuttle TM. Inadequacy of lymph node staging in gastric cancer patients: a population-based study. Ann Surg Oncol 2005; 12: 981–987.
Inoue K, Nakane Y, Iiyama H, Sato M, Kanbara T, Nakai K, Okumura S, Yamamichi K, Hioki K. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol 2002; 9: 27–34.
Kong SH, Lee HJ, Ahn HS, Kim JW, Kim WH, Lee KU, Yang HK. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: the reappraisal of positive lymph node ratio as a proper N-staging. Ann Surg 2012; 255: 50–58.
Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol 2002; 9: 775–784.
Son T, Hyung WJ, Lee JH, Kim YM, Kim HI, An JY, Cheong JH, Noh SH. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer 2012; 118: 4687–4693.
Sun Z, Zhu GL, Lu C, Guo PT, Huang BJ, Li K, Xu Y, Li DM, Wang ZN, Xu HM. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Ann Oncol 2009; 20: 897–905.
Maduekwe UN, Lauwers GY, Fernandez-Del-Castillo C, Berger DL, Ferguson CM, Rattner DW, Yoon SS. New metastatic lymph node ratio system reduces stage migration in patients undergoing D1 lymphadenectomy for gastric adenocarcinoma. Ann Surg Oncol 2010; 17: 1267–1277.
Wang J, Dang P, Raut CP, Pandalai PK, Maduekwe UN, Rattner DW, Lauwers GY, Yoon SS. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg 2012; 255: 478–485.
Wang J, Hassett JM, Dayton MT, Kulaylat MN. The prognostic superiority of log odds of positive lymph nodes in stage III colon cancer. J Gastrointest Surg 2008; 12: 1790–1796.
Sun Z, Xu Y, Li de M, Wang ZN, Zhu GL, Huang BJ, Li K, Xu HM. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer 2010; 116: 2571–2580.
Deng J, Zhang R, Wu L, Zhang L, Wang X, Liu Y, Hao X, Liang H. Superiority of the ratio between negative and positive lymph nodes for predicting the prognosis for patients with gastric cancer. Ann Surg Oncol 2015; 22: 1258–1266.
Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008; 26: 1364–1370.
Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, Sano T, Park BJ, Kim WH, Yang HK. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 2012; 30: 3834–3840.
Song KY, Park YG, Jeon HM, Park CH. A nomogram for predicting individual survival of patients with gastric cancer who underwent radical surgery with extended lymph node dissection. Gastric Cancer 2014; 17: 287–293.
Eom BW, Ryu KW, Nam BH, Park Y, Lee HJ, Kim MC, Cho GS, Kim CY, Ryu SW, Shin DW, Hyung WJ, Lee JH. Survival nomogram for curatively resected Korean gastric cancer patients: multicenter retrospective analysis with external validation. PLoS One 2015; 10: e0119671.
Hirabayashi S, Kosugi S, Isobe Y, Nashimoto A, Oda I, Hayashi K, Miyashiro I, Tsujitani S, Kodera Y, Seto Y, Furukawa H, Ono H, Tanabe S, Kaminishi M, Nunobe S, Fukagawa T, Matsuo R, Nagai T, Katai H, Wakai T, Akazawa K. Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann Oncol 2014; 25: 1179–1184.
Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol 2003; 21: 3647–3650.
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010; 11: 439–449.
Okines A, Verheij M, Allum W, Cunningham D, Cervantes A, Group EGW. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21 Suppl 5: v50–54.
Memon MA, Subramanya MS, Khan S, Hossain MB, Osland E, Memon B. Meta-analysis of D1 versus D2 gastrectomy for gastric adenocarcinoma. Ann Surg 2011; 253: 900–911.
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. The Lancet Oncology 2015; 16: e173-e180.
Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–387.
Harrell FE, Jr., Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982; 247: 2543–2546.
Huitzil-Melendez FD, Capanu M, O’Reilly EM, Duffy A, Gansukh B, Saltz LL, Abou-Alfa GK. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol 2010; 28: 2889–2895.
Siewert JR, Bottcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998; 228: 449–461.
Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 2005; 241: 27–39.
Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer 1998; 1: 125–133.
Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC cancer staging manual. 6th ed. New York: Springer-Verlag; 2002.
Fleming ID, Cooper JS, Henson DE, Hutter RVP, Kennedy BJ, Murphy GP, O’Sullivan B, Sobin LH, Yarbro JW. AJCC cancer staging manual. 5th ed. Philadelphia: Lippincott-Raven; 1997.
Bouvier AM, Haas O, Piard F, Roignot P, Bonithon-Kopp C, Faivre J. How many nodes must be examined to accurately stage gastric carcinomas? Results from a population based study. Cancer 2002; 94: 2862–2866.
Lee HK, Yang HK, Kim WH, Lee KU, Choe KJ, Kim JP. Influence of the number of lymph nodes examined on staging of gastric cancer. Br J Surg 2001; 88: 1408–1412.
Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 1985; 312: 1604–1608.
Bunt AM, Hermans J, van de Velde CJ, Sasako M, Hoefsloot FA, Fleuren G, Bruijn JA. Lymph node retrieval in a randomized trial on western-type versus Japanese-type surgery in gastric cancer. J Clin Oncol 1996; 14: 2289–2294.
Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Masunaga R, Kohno H, Nagasue N. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology 2000; 59: 18–23.
Dudeja V, Habermann EB, Zhong W, Tuttle TM, Vickers SM, Jensen EH, Al-Refaie WB. Guideline recommended gastric cancer care in the elderly: insights into the applicability of cancer trials to real world. Ann Surg Oncol 2011; 18: 26–33.
Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G, De Santis F, Baiocchi L, Coniglio A, Nitti D, Italian Research Group for Gastric C. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg 2007; 245: 543–552.
Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Belluco C, Lise M. Ratio Between Metastatic and Examined Lymph Nodes Is an Independent Prognostic Factor After D2 Resection for Gastric Cancer: Analysis of a Large European Monoinstitutional Experience. Annals of Surgical Oncology 2003; 10: 1077–1085.
Yang Y, Zhang YJ, Zhu Y, Cao JZ, Yuan ZY, Xu LM, Wu JX, Wang W, Wu T, Lu B, Zhu SY, Qian LT, Zhang FQ, Hou XR, Liu QF, Li YX. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T-cell lymphoma, nasal-type: a multicenter study. Leukemia 2015; 29: 1571–1577.
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, Lau W, Wu M, Shen F. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013; 31: 1188–1195.
Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol 2002; 20: 791–796.
Kim Y, Spolverato G, Ejaz A, Squires MH, Poultsides G, Fields RC, Bloomston M, Weber SM, Votanopoulos K, Acher AW, Jin LX, Hawkins WG, Schmidt C, Kooby D, Worhunsky D, Saunders N, Levine EA, Cho CS, Maithel SK, Pawlik TM. A nomogram to predict overall survival and disease-free survival after curative resection of gastric adenocarcinoma. Ann Surg Oncol 2015; 22: 1828–1835.
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011; 29: 4387–4393.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Kim YH, Ji J, Yeh TS, Button P, Sirzen F, Noh SH. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012; 379: 315–321.
Kim DH, Kim SM, Hyun JK, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. Changes in postoperative recurrence and prognostic risk factors for patients with gastric cancer who underwent curative gastric resection during different time periods. Ann Surg Oncol 2013; 20: 2317–2327.
Author information
Authors and Affiliations
Contributions
Research idea and study design: LFN, XHM, and WPL; data analysis/interpretation: WPL, XFT, and GBC; statistical analysis: WPL and LFN; supervision or mentorship: LFN and XHM. All the authors contributed important intellectual content for the overall work. LFN and XHM take responsibility for the honesty and accuracy of the present study.
Corresponding authors
Ethics declarations
This study was approved by the Institutional Ethics Committee of China Medical University.
Grant Support
This work was supported by the National Natural Science Foundation of China (No. 81001092) and the Natural Science Foundation of Liaoning Province of China (No. 2013021097).
Rights and permissions
About this article
Cite this article
Wang, Pl., Xiao, Ft., Gong, Bc. et al. A Nomogram for Predicting Overall Survival of Gastric Cancer Patients with Insufficient Lymph Nodes Examined. J Gastrointest Surg 21, 947–956 (2017). https://doi.org/10.1007/s11605-017-3401-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-017-3401-6