Abstract
Introduction
The use of intensity-modulated radiation therapy (IMRT) in rectal cancer has steadily increased over traditional 3D conformal radiotherapy (3D-CRT) due to perceived benefit of delivering higher treatment doses while minimizing exposure to surrounding tissues. However, IMRT is technically challenging and costly, and its effects on rectal cancer outcomes remain unclear.
Material and Methods
Adults with clinical stage II and III rectal adenocarcinoma who underwent neoadjuvant chemoradiotherapy with 45–54 Gy of radiation and surgery were included from the 2006–2013 National Cancer Data Base. Patients were grouped based the modality of radiation received: IMRT or 3D-CRT. Multivariable regression modeling adjusting for demographic, clinical, and treatment characteristics was used to examine the impact of IMRT vs. 3D-CRT on pathologic downstaging, resection margin positivity, sphincter loss surgery, 30-day unplanned readmission and mortality after surgery, and overall survival.
Results
Among 7386 patients included, 3330 (45 %) received IMRT and 4056 (55 %) received 3D-CRT. While the mean radiation dose delivered was higher with IMRT (4735 vs. 4608 cGy, p < 0.001), it was associated with higher risks of positive margins (adjusted odds ratio (OR) 1.57; p < 0.001) and sphincter loss surgery (OR 1.32; p < 0.001). There were no differences between IMRT and 3D-CRT in the likelihood of pathologic downstaging (OR 0.89, p = 0.051), unplanned readmission (OR 0.79; p = 0.07), or 30-day mortality (OR 0.61; p = 0.31) after surgery. Additionally, there were no differences in overall survival at 8 years (IMRT vs. 3D-CRT: 64 vs. 64 %; adjusted hazard ratio 1.06, p = 0.47).
Conclusion
IMRT is associated with worse local tumor control without any long-term survival benefit for patients with locally advanced rectal cancer. Given the lack of significant advantage and the higher cost of IMRT, caution should be exercised when using IMRT instead of traditional 3D-CRT for rectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the treatment of locally advanced rectal cancer, fluorouracil-based neoadjuvant chemoradiation is associated with improved tumor downstaging, increased R0 resection rates, and reduced local recurrence [1, 2]. To achieve the target radiation dose, three-dimensional conformal radiotherapy (3D-CRT), which involves triangulating multiple radiation beams, has traditionally been utilized to treat rectal tumors. While this modality is well established, clinically significant gastrointestinal toxicity is common due to radiation-induced injury to normal tissues adjacent to the target volumes, exacerbated by concurrent administration of chemotherapy [1–3].
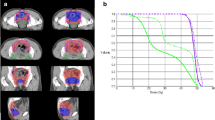
Recently, intensity-modulated radiation therapy (IMRT) has been increasingly adopted as an alternative to 3D-CRT. By leveraging computer-based optimization, IMRT can deliver radiation of variable intensity within a single beam, with the goal of maximizing radiation dose to the tumor while minimizing exposure to healthy tissues [4]. Single institution reports suggest that IMRT can be used with good patient compliance, low rates of acute bowel toxicity, and high tumor pathologic response rates [5, 6]. However, IMRT also requires greater technical proficiency, more time, and higher cost. It also can be associated with a higher risk of missing the target volume [7].
Furthermore, despite the rising implementation of IMRT, direct comparisons between IMRT and 3D-CRT use in rectal cancer treatment remain limited. Moreover, there is little data evaluating either short- or long-term oncologic outcomes associated with IMRT-treated rectal tumors. Therefore, our objective was to examine the perioperative and survival outcomes of 3D-CRT and IMRT in a large cohort of patients with locally advanced rectal cancer.
Material and Methods
Study Design
We queried the National Cancer Data Base (NCDB), which collects information on approximately 70 % of all newly diagnosed cancer cases in the USA and Puerto Rico from more than 1500 cancer centers. The NCDB 2006–2013 Participant Use File for rectal cancer was used for the analysis, representing a contemporary cohort of rectal cancer patients.
Adults with clinical stage II and III rectal adenocarcinomas who received neoadjuvant chemoradiotherapy to a dose of 45–54 Gy of radiation and surgery were selected based on International Classification of Diseases for Oncology, Third Edition histology codes (8140, 8141, 8143, 8144, 8145, 8147, 8150, 8210, 8211, 8260, 8261, 8262, 8263, 8310, 8320, 8323, 8380, 8401, 8410, 8440, 8460, 8470, 8490, 8500, 8503, 8510). Patients with more than one primary tumor or received incomplete doses (<45 Gy) of radiation were excluded. Patients with unspecified radiation modality were also excluded.
Study Outcomes
The primary outcome of our analysis was overall survival, defined from date of surgery. Secondary outcomes included pathologic downstaging (either by T- or N-stage), sphincter loss surgery, resection margin positivity (either distal or circumferential margin), unplanned 30-day postoperative readmission (as a surrogate for postoperative complications), and 30-day postoperative mortality.
Statistical Analysis
The initial patient cohort was stratified based on the modality of radiation received: IMRT or 3D-CRT. Unadjusted outcomes were evaluated using Kruskal-Wallis or chi-squared test for continuous and categorical variables, respectively. Survival was plotted using the Kaplan-Meier method. Multivariable logistic regression modeling was utilized to compare perioperative outcomes while accounting for patient age, sex, race, insurance status (private vs. government vs. no insurance), Charlson-Deyo comorbidity score, tumor size, hospital type (community, comprehensive community, or academic), clinical tumor stage, and extent of surgery (sphincter-preserving surgery including low anterior resection or coloanal pull-through vs. non-sphincter preserving surgery including abdominoperineal resection or pelvic exenteration). For survival, a Cox proportional hazards model was employed while adjusting for postoperative chemotherapy in addition to all variables defined in the perioperative outcome models.
Multivariable logistic regression models were created to analyze patient and hospital factors independently associated with using IMRT; a backward variable elimination method was used to produce the most parsimonious and fit model based on the lowest Akaike information criterion.
A p value of less than 0.05 was considered statistically significant. Statistical analysis was performed using R 3.2.1 (R Foundation, Vienna, Austria). The Duke Institutional Review Board exempted this retrospective study.
Results
Baseline Demographics and Trends of Use
Among 7386 rectal cancer patients included, 3330 (45 %) received IMRT and 4056 (55 %) received 3D-CRT. Utilization of IMRT increased over the study period from 24 % in 2006 to 50 % in 2013 (Fig. 1). Patients who received IMRT were more likely to be female (38.8 vs. 36 %, p = 0.01) and non-White (12.7 vs. 10.7 %, p = 0.03), possess government-based insurance (42.5 vs. 39.6 %, p = 0.03), and treated at an academic institution (38.6 vs. 35.6 %, p = 0.02) (Table 1). There were no differences in presenting disease stage, tumor size, and tumor grade (all p > 0.05). Mean radiation dose delivered (4735 vs. 4608 cGy, p < 0.001) as well as extent of surgery (sphincter loss 34.7 vs. 28.3 %, p < 0.001) was greater with IMRT.
After adjustment, female gender was independently associated with a higher likelihood of using IMRT (adjusted odds ratio (OR) 1.15, 95 % confidence interval (CI) 1.04–1.28, p = 0.01) (Fig. 2). Patient age, race, insurance status, Charlson-Deyo co-morbidity score, hospital type, income and education status, and clinical stage of disease were not predictive of IMRT or 3D-CRT use (all p > 0.05).
Forest plot of factors associated with intensity-modulated radiation therapy (IMRT) use among patients with stage II/III rectal cancers. Black circles represent odds ratios for the independent association of each factor with likelihood of using IMRT; 95 % confidence interval bounds are represented by the corresponding horizontal lines
Short-Term Outcomes and Overall Survival
Before adjustment, IMRT was associated with a higher rate of sphincter loss surgery (34.7 vs. 28.3 %, p < 0.001) and positive resection margin (8 vs. 5.6 %, p < 0.001), but a lower rate of postoperative readmission (6.4 vs. 7.9 %, p = 0.02). Rates of pathologic downstaging (55 vs. 57 %, p = 0.09) and 30-day postoperative mortality (0.6 vs. 0.8 %, p = 0.36) were not different.
After adjustment for patient, clinical, and tumor characteristics, patients receiving IMRT had higher odds of sphincter loss surgery (OR 1.32, CI 1.14–1.52, p < 0.001) and positive resection margin (OR 1.57, CI 1.21–2.03, p < 0.001) (Table 2). Patients treated with IMRT trended towards a lower likelihood of pathologic downstaging (OR 0.89, CI 0.79–1.01, p = 0.051) and a lower likelihood of postoperative readmissions (OR 0.79, CI 0.61–1.02, p = 0.07). There were no adjusted differences in 30-day mortality (OR 0.61, CI 0.24–1.57, p = 0.31).
At 5 years, unadjusted overall survival (follow up range: 1–102 months) was not different between patients who received IMRT vs. 3D-CRT (73 vs. 75 %, p = 0.131) (Fig. 3). After adjustment, IMRT was not associated with a difference in the rate of mortality (adjusted hazards ratio 1.06, CI 0.89–1.28, p = 0.47).
Discussion
In this nationwide analysis of neoadjuvant radiotherapy modalities for locally advanced rectal cancer, we found that IMRT is not associated with benefits in perioperative outcomes or long-term survival, despite its rising use. Female gender appears to be independently associated with IMRT use, possibly related to a greater desire to avoid radiation-induced toxicity. Notably, other factors such as socioeconomic indicators or hospital type did not influence the likelihood of receiving IMRT, suggesting that there may be unreported provider factors influencing the choice of radiation modality.
While the primary motivation for using IMRT has been for its favorable gastrointestinal morbidity profile in the treatment of other cancers, data supporting IMRT for reduction of gastrointestinal toxicity are conflicting in the treatment of rectal tumors. In dosimetric studies, IMRT demonstrated better tumor target conformity and decreased irradiation of organs at risk compared to 3D-CRT [7]. However, these improvements did not necessarily translate to consistent results in reduced toxicity. While a retrospective review from the Mayo Clinic Arizona described 32 % grade ≥2 toxicity with IMRT compared to 62 % with 3D-CRT [5], RTOG 0822 reported a 52 % grade ≥2 gastrointestinal toxicity, including 15 % grade ≥3 diarrhea [8]. Moreover, the RTOG 0822 study utilizing IMRT was initiated as a response to the high rate of adverse events observed in RTOG 0247 that used 3D-CRT in conjunction with oxaliplatin, concluding that IMRT did not improve gastrointestinal toxicity [9]. Taken together, it is unclear that IMRT reduces acute toxicity of neoadjuvant therapy.
Similarly, data regarding the effects of IMRT on perioperative outcomes and long-term survival are limited. For example, Parekh et al. conducted a single institution retrospective analysis of 92 rectal cancer patients including 31 treated with IMRT [6]. The authors reported lower overall acute gastrointestinal toxicity rate with IMRT, as well as a 10 % decrease in pelvis-specific complications. However, patients undergoing IMRT did not experience improvements in overall perioperative complications (47 % postoperative morbidity requiring intervention). The Radiation Therapy Oncology Group (RTOG) 0822 study was a prospective, multicenter, single arm study that evaluated 68 patients who received IMRT with concurrent capecitabine and oxalipatin for rectal cancer [8]. Among these patients, sphincter loss surgery was performed in 21 % of patients, and overall survival at 4 years was 83 %. However, no 3D-CRT comparison group was included as part of RTOG 0822 for patients who received conventional 3D-CRT. A Chinese prospective study including 63 locally advanced rectal cancer patients who received IMRT reported a 34 % sphincter loss surgery rate with 96 % 2-year overall survival [10]. Similar to RTOG 0822, the Chinese study also did not report any outcomes in patients undergoing 3D-CRT, limiting the interpretation of its results. In contrast, our analysis provides meaningful comparisons for a contemporary cohort of rectal cancer patients receiving 3D-CRT or IMRT and shows that IMRT is associated worse R0 resection rates and sphincter preservation without differences in other perioperative endpoints and overall survival.
Our analysis has several limitations. Firstly, our comparison was retrospective and thus subject to selection bias. Nevertheless, our expectation was that IMRT would be used more often in high-volume academic centers associated with well-integrated treatment teams, which may optimize clinical outcomes and therefore bias our results to favor IMRT. Instead, patients who underwent IMRT were associated with worse perioperative outcomes without survival benefits, which persisted even after adjustment for confounding factors. Secondly, our dataset could not capture data on acute or late toxicities related to radiotherapy treatment; however, even if IMRT was associated with improvements in toxicity and recurrence, these benefits did not translate into statistically detectable differences in perioperative readmission rates (as a surrogate for surgical complications) or overall survival. Furthermore, our analysis could not adjust for tumor locations relative to the anal verge. Therefore, it is possible that IMRT may have been used for low-lying lesions in our cohort, accounting for the higher rate of margin positivity and sphincter loss surgery. Thus, IMRT may prove beneficial in these difficult cases where radiation fields involve targeting at risk areas in proximity to the genitalia [11]. Lastly, our analysis could only evaluate overall survival and thus cannot comment on cancer-specific survival.
In a recent cost analysis of IMRT vs. 3D-CRT, the overall treatment cost of IMRT was $5405 greater than 3D-CRT in soft tissue sarcoma patients [12]. Although IMRT use other malignancies such as soft tissue sarcomas may prove cost effective by consistently reducing toxicity and tumor recurrence, these benefits may not persist in the treatment of rectal cancer. Therefore, based on an estimated 12,000 rectal cancer patients receiving IMRT each year, the additional cost of IMRT could translate to more than $64 million dollars annually. As such, caution should be exercised when using IMRT in lieu of traditional 3D-CRT for rectal cancer.
Conclusion
In conclusion, our study demonstrates that IMRT use in rectal cancer is associated with worse R0 resection rates and sphincter preservation, without differences in pathologic downstaging, postoperative readmission, postoperative mortality, or long-term overall survival. Given the lack of consistent benefit in using IMRT for rectal cancer and its higher cost, utilization of IMRT should be further scrutinized.
References
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23.
Gérard J-P, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne P-L, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. Journal of Clinical Oncology. 2010;28(10):1638–44.
Verhey LJ. Comparison of three-dimensional conformal radiation therapy and intensity-modulated radiation therapy systems. Semin Radiat Oncol. 1999;9(1):78–98.
Samuelian JM, Callister MD, Ashman JB, Young-Fadok TM, Borad MJ, Gunderson LL. Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. International Journal of Radiation Oncology* Biology* Physics. 2012;82(5):1981–7.
Parekh A, Truong MT, Pashtan I, Qureshi MM, Martin NE, Nawaz O, et al. Acute gastrointestinal toxicity and tumor response with preoperative intensity modulated radiation therapy for rectal cancer. Gastrointest Cancer Res. 2013;6(5–6):137–43.
Arbea L, Ramos LI, Martinez-Monge R, Moreno M, Aristu J. Intensity-modulated radiation therapy (IMRT) vs. 3D conformal radiotherapy (3DCRT) in locally advanced rectal cancer (LARC): dosimetric comparison and clinical implications. Radiat Oncol. 2010;5:17.
Hong TS, Moughan J, Garofalo MC, Bendell J, Berger AC, Oldenburg NB, et al. NRG Oncology Radiation Therapy Oncology Group 0822: a phase 2 study of preoperative chemoradiation therapy using intensity modulated radiation therapy in combination with capecitabine and oxaliplatin for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2015;93(1):29–36.
Wong SJ, Winter K, Meropol NJ, Anne PR, Kachnic L, Rashid A, et al. RTOG 0247: a randomized phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiation therapy for patients with locally advanced rectal cancer. International Journal of Radiation Oncology, Biology, Physics. 2012;82(4):1367–75.
Li J-l, Ji J-f, Cai Y, Li X-f, Li Y-h, Wu H, et al. Preoperative concomitant boost intensity-modulated radiotherapy with oral capecitabine in locally advanced mid-low rectal cancer: a phase II trial. Radiotherapy and Oncology. 2012;102(1):4–9.
Kachnic LA, Winter K, Myerson RJ, Goodyear MD, Willins J, Esthappan J, et al. RTOG 0529: a phase II evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. International journal of radiation oncology, biology, physics. 2013;86(1):27–33.
Richard P, Phillips M, Smith W, Davidson D, Kim E, Kane G. Cost-effectiveness analysis of intensity modulated radiation therapy versus 3-dimensional conformal radiation therapy for preoperative treatment of extremity soft tissue sarcomas. International Journal of Radiation Oncology* Biology* Physics. 2016.
Grant Support
None
Statement of Authors’ Contributions
All authors contributed to the concept and design. Data acquisition, analysis, and interpretation of the data involved. Draft and revision of the work include all authors. Final approval of the work for submission includes all authors. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Z., Adam, M.A., Kim, J. et al. Intensity-Modulated Radiation Therapy Is Not Associated with Perioperative or Survival Benefit over 3D-Conformal Radiotherapy for Rectal Cancer. J Gastrointest Surg 21, 106–111 (2017). https://doi.org/10.1007/s11605-016-3242-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3242-8