Abstract
A 3-year-old girl presented with multiple episodes of vomiting, fever, and hematemesis for the past 2 months. Except for hemoglobin, her rests of the laboratory tests were unremarkable. Her barium X-ray showed absence of the duodenal bulb and the C-loop. Her endoscopy showed deformed stomach with multiple ulcers and diverticuli. The gastric outlet was not visualized. Distal gastrectomy with gastro-duodenal anastomosis was performed. Histopathological findings revealed transmural dense infiltrates of eosinophils, consistent with eosinophilic gastritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
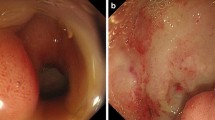
A 3-year-old girl was brought by her parents with history of multiple episodes of vomiting, fever with chills and rigors, and hematemesis for past 2 months. Her barium skiagram showed deformed stomach with absence of the duodenal bulb and the C-loop (Fig. 1a). Her endoscopy revealed narrow, deformed, shortened, and cicatrized stomach with multiple ulcers and diverticuli (Fig. 1b). The gastric outlet could not be seen. Her endoscopic gastric biopsy was diagnosed as erosive gastritis. Her hemoglobin was 10 g/dl; rests of the laboratory tests including eosinophil counts were unremarkable. She underwent distal gastrectomy with gastro-duodenal anastomosis. A per-operative finding was suspicious of malignancy due to thickened appearance of the distal stomach. Histopathology revealed transmural dense infiltrates of eosinophils (≈80 eosinophils /high power field; 182 eosinophils/mm2) (Fig. 2) with erosion of the gastric foveolar lining, inflammatory granulation tissue, and occasional multinucleate giant cells. Histological evidence of parasite, granuloma, vasculitis, or malignancy was not seen. Special stains for Helicobacter pylori, fungi, and acid-fast organisms were negative. There was no history of food allergy or connective tissue disorders. Based on history and histopathological findings, final diagnosis of primary eosinophilic gastritis was made.
Eosinophilic gastritis (EG) is an uncommon disorder that is characterized by dense infiltrates of eosinophils and tissue damage involving antral or pyloric region of the stomach. Primary EG is demonstrated by gastric eosinophilia without any known specific etiology like Strongyloides stercoralis, Anisakis spp., and Helicobacter pylori infections, drugs, connective tissue disorders, hematopoietic disorders, and food allergy.1 We could not identify a known etiologic agent for EG in our case. The Klein classification divides patients of EG into predominantly mucosal, muscular, or subserosal types based on the layer involved.2 The mucosal type clinically presents with bleeding manifestations, the muscular type with obstructive features, and the subserosal type presents with peripheral eosinophilia and ascites; this latter type has a good response to corticosteroid treatment. Our patient had severe type of EG involving the mucosal and muscular layers of the stomach and clinically presented with both bleeding and obstructive manifestations of the upper gastrointestinal tract. Clinically, patients of EG show upper gastrointestinal tract symptoms and signs along with poor growth, gastrointestinal bleeding, and diarrhea.3 Elevated levels of serum IgE and peripheral blood eosinophilia are noted in most but not all patients. Endoscopic findings in EG includes erythema, erosions, swollen mucosal folds, scattered mucosal blebs, or nodular lesions.3 Histopathologically, the mean eosinophil count in normal gastric mucosa was found to be 15.5 ± 16.8 eosinophils/mm2 with a peak density of ≈50 eosinophils/mm2.1 The histological diagnosis of EG can be offered to the patients where gastric biopsy shows ≥127 eosinophils/mm2 without any known cause of eosinophilia.1 Excessive recruitment of eosinophils in the gastric wall leads to destruction of the epithelium.2
References
Lwin T, Melton SD, Genta RM. Eosinophilic gastritis: histopathological characterization and quantification of the normal gastric eosinophil content. Mod Pathol 2011;24:556–63.
Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut 1990;31:54–8.
Dohil R, Hassall E, Jevon G, Dimmick J. Gastritis and gastropathy of childhood. J Pediatr Gastroenterol Nutr 1999;29:378–94.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katiyar, R., Patne, S.C.U., Dixit, V.K. et al. Primary Eosinophilic Gastritis in a Child with Gastric Outlet Obstruction. J Gastrointest Surg 20, 1270–1271 (2016). https://doi.org/10.1007/s11605-016-3074-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3074-6