Abstract
Introduction
Safety of synchronous hepatectomy and colorectal resection (CRR) for metastatic colorectal cancer remains controversial. We hypothesized that both the extent of hepatectomy and CRR influences postoperative outcomes.
Methods
Prospective 2005–2013 ACS-NSQIP data were retrospectively reviewed for mortality and major morbidity (MM) after (1) isolated hepatectomy, (2) isolated CRR, and (3) synchronous resection for colorectal cancer. Hepatectomy and CRR risk categories were created based on mortality and MM of respective isolated resections. The synchronous cohort was then stratified based on risk categories. Cumulative asynchronous mortality and MM were estimated compared to that observed in the synchronous cohort via unadjusted relative risk and risk difference.
Results
There were 43,408 patients identified. Among isolated hepatectomy patients (N = 6,661), trisectionectomy and right hepatectomy experienced the greatest mortality and were defined as “major” hepatectomy. Among isolated CRR patients (N = 35,825), diverted left colectomy, abdominoperineal resection, total abdominal colectomy, and total abdominal proctocolectomy experienced the greatest MM and were defined as “high risk” CRR. Synchronous patients (N = 922) were stratified by hepatectomy and CRR risk categories; mortality and MM varied from 0.9 to 5.0 % and 25.5 to 55.0 %, respectively. Mortality and MM were greatest for patients undergoing “high risk” CRR and “major” hepatectomy and lowest for synchronous CRR and “minor” hepatectomy. As both CRR and hepatectomy risk categories increased, there was a significant trend in increasing mortality and MM in synchronous patients. Additionally, comparison of the synchronous resections versus the estimated cumulative asynchronous outcomes showed that (1) mortality was significantly less after synchronous minor hepatectomy and either low or high risk CRR, and (2) neither mortality nor major morbidity differed significantly after major hepatectomy with either high or low risk CRR.
Conclusion
Major morbidity after synchronous hepatic and colorectal resections vary incrementally and are related to both the risk of hepatectomy and CRR. Stratification of outcomes by the hepatectomy and CRR components may reflect a more accurate description of risks. Comparison of synchronous and combined outcomes of individual operations supports a potential benefit for synchronous resections with minor hepatectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colon and rectal cancer is the third most common cancer diagnosis and the third leading cause of cancer death among both men and women in the USA.1 , 2 Recent Surveillance, Epidemiology, and End Results Program (SEER) statistics reveal that 20 % of colorectal cancer patients have distant disease at diagnosis.3 The liver is the most common site of distant metastases, and among patients with stage IV colorectal cancer, the liver is the only site of distant metastases in as many as 77 % of patients.4 Modern chemotherapy has resulted in improved response and survival rates for patients with hepatic metastases and also has increased the rate of resectability for hepatic metastases in selected patients.5 – 8 To date, resection remains the best opportunity for long-term survival for patients with hepatic metastases from colorectal cancer.9 – 13
The synchronous management of both the primary and metastatic colorectal cancer continues to evolve. Whether sequential resection of the primary and metastatic colorectal cancer or synchronous resection of stage IV colorectal cancer is in the best interest of the patient remains controversial.14 – 16 The overall safety of hepatic resection for both primary hepatobiliary and metastatic disease continues to improve due to advances in preoperative imaging, improved understanding of liver anatomy, improved surgical techniques, improved perioperative management, and patient selection.17 – 19 Moreover, centralization of these procedures to high volume and academic centers where they are performed by surgeons with advanced specialty training and supporting institutional infrastructure has contributed to the improved safety of major hepatic resection.20 Consequently, synchronous resection of stage IV colorectal cancer has been undertaken more frequently. Although low operative mortality has been reported,8 , 14 , 21 – 23 selection of patients often has been limited to patients with lower T-stage primary colorectal cancers and limited hepatic metastases compared to patients undergoing sequential resection of stage IV colorectal cancer.24 , 25
Currently, data regarding the surgical outcomes of synchronous colorectal and hepatic resection for stage IV colorectal cancer are limited largely to single-institution case series.9 , 15 , 21 , 26 – 28 Whether such reports are representative broadly of other US hospitals is unknown. Many single-institution studies lack sufficient power to adequately address the differences in perioperative outcomes for different combinations of synchronous colorectal and hepatic resections. Moreover, most studies addressing mortality and morbidity after synchronous hepatic and colorectal resections for stage IV colorectal cancer have not stratified the outcomes by both extent of hepatic resections and the location or type of concurrent colorectal resections. Most studies report morbidity and mortality rates stratified only by the extent of hepatic resection as major or minor hepatectomy but consolidate all colorectal resections into a singular entity.14 , 23 , 29 However, current evidence suggests that morbidity for colorectal resections vary based on the type and location of resection as well as the use of intestinal diversion.30 , 31 Therefore, it is reasonable to expect that type of colorectal resections may influence the morbidity of synchronous resections as well.
We sought to assess perioperative major morbidity and mortality after synchronous colorectal and hepatic resection for stage IV colorectal cancer from a large, multi-institutional database. Our primary aim was to identify whether operative risk does in fact vary by both the risk due to the extent of hepatectomy as well as the risk due to the type and location of the colorectal resection. We further hypothesized that preoperative risk stratification of synchronous resections by both hepatic and colorectal resection risk type would be useful clinically and potentially allow more accurate identification of patient outcomes and assist in preoperative planning and counseling of this patient population. A secondary aim was to compare the synchronous cohort’s morbidity and mortality to those of similar isolated resections.
Methods
Data Source
The American College of Surgeons–National Surgical Quality Improvement Program (ACS-NSQIP) database is a prospectively maintained outcome database of 135 variables including 30-day morbidity and mortality for patients undergoing surgical procedures. Specific details regarding data collection, outcome variable definitions, quality control, and personnel training are available on the ACS-NSQIP website.14
Design
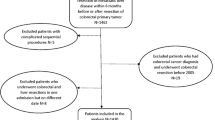
The 2005–2013 ACS-NSQIP database Participant-User File (PUF) contains 2.97 million records and was reviewed for three distinct cohorts: (1) all isolated open colorectal resections for primary colorectal cancer, (2) all isolated open hepatic resections for metastatic colorectal cancer, and (3) all synchronous colorectal and hepatic resections for primary and metastatic colorectal cancer. Patients in the isolated colorectal and isolated hepatic resection cohorts were identified by primary procedure using Current Procedural Terminology (CPT) codes.15 For colorectal resections, 44,160 represented right colectomy; 44,140 represented left colectomy without diversion; 44,145, 44,146, 44,147, 45,111, 45,112, 45,114, and 44,119 represented partial proctectomy with low pelvic anastomosis/low anterior resection (LAR); 44,141, 44,143 and 44,144 represented left colectomy with anastomosis and diversion; 44,150 and 44,151 represented total abdominal colectomy; 44,155, 44,156, 44,157, and 44,158 represented total abdominal proctocolectomy, and 45,110 represented abdominal perineal resection (APR). For hepatic resections, 47,120, 47,125, 47,130, and 47,122 represented partial hepatectomy (PH), left hepatectomy (LH), right hepatectomy (RH), and trisectionectomy (TS), respectively. The same colorectal and hepatic resection codes were used to identify the synchronous resection cohort; patients were included in the synchronous cohort if they had both a colorectal and hepatic resection during the same operation with one of these was coded as their primary procedure. Patients with procedure codes for more than one type of hepatectomy were categorized based on the larger of resections performed. For example, if a right hepatectomy and a partial hepatectomy were coded for a patient, right hepatectomy was used. If a patient had two or more partial hepatectomies, partial hepatectomy was used. Patients having procedure codes for more than one type of colorectal resection were excluded from all cohorts (n = 686).
Diagnoses of primary and metastatic colorectal cancer were identified using ICD9 codes 153, 153.0-4, 153.6-9, 154, 154.0-3, 154.8, 197.5, and 197.7.16
In an attempt to achieve mutually exclusive groups and minimize ambiguity of diagnosis, patients were only included if identified by the aforementioned CPT and ICD-9 codes. Patients were also excluded for any of the following preoperative conditions: emergent operation, pregnant, American Society of Anesthesiologists (ASA) class 5, ventilator dependent, sepsis, septic shock, systemic inflammatory response syndrome (SIRS), pneumonia, open wound, wound infection, acute renal failure, coma, transfusion of >4 units RBCs in prior 72 h, and dialysis(n = 43,772). In an attempt to reduce confounding of operations and to create sample populations that were limited to the inherent risks of each of the three cohorts, patients with concurrent operations not typical of an isolated colectomy, isolated hepatectomy, and/or synchronous colorectal and hepatic resection also were excluded (n = 3,147) (e.g., hysterectomy, splenectomy, hernia repair, nephrectomy, pneumonectomy, thyroidectomy, etc.). Cases were not excluded for common concomitant operations such as feeding tube, central line placement, and laparoscopy.
Patient demographic and outcome variables were reviewed. Primary end points for this study were major morbidity and mortality within 30 days postoperatively. Complications defined as major morbidity included cardiac arrest requiring cardiopulmonary resuscitation (CPR), myocardial infarction, stroke/CVA with neurological deficit, wound disruption, deep incisional surgical site infection (SSI), organ space SSI, sepsis or septic shock, unplanned intubation, ventilator >48 h, pneumonia, acute renal failure, progressive renal insufficiency, DVT/thrombophlebitis, pulmonary embolism, and return to the operating room.
Statistical Methodology and Analysis
As previously described, mortality among open hepatic resections can accurately and sufficiently differentiate hepatic resections into major and minor resection categories.32 , 33 Therefore, as previously described, mortality for each isolated hepatic resection was reviewed. The two resections associated with the greatest mortality were compared as were the two resections with the lowest mortality32 , 33 and consolidated into major or minor hepatic resection categories. Chi-square tests were then performed to assess differences in major morbidity and mortality between the major and minor resection categories.
Regardless of the type of colorectal operation, postoperative mortality after elective resections remains relatively low and without great variation31 , 34 , 35 and therefore, a priori, was considered an unlikely differentiating factor among colorectal operations. Therefore, major morbidity, instead of mortality as with hepatic resections, was reviewed for each isolated colorectal resection. The rate of major morbidity for each isolated colorectal procedure was calculated and ranked, least to greatest. The isolated colorectal operations where then divided into two groups based on the major morbidity rate per operation. The operations within each of these two groups were then compared via chi-square test to determine whether they were statistically not different and therefore could sufficiently be consolidated into a singular risk category. These two risk categories were labeled “high risk” and “low risk” with respect to their relative major morbidity rates. After consolidation of the isolated colorectal resections into two risk categories, chi-square test was performed to compare major morbidity and mortality between the two risk categories.
After defining the risk categories for both CRR (high and low risk) and hepatectomy (major and minor), the synchronous cohort was then stratified according to the combination of the new CRR and hepatectomy risk categories. Major morbidity and mortality rates of the stratified synchronous cohort were determined and compared. A one-sided Cochran-Armitage trend test was utilized to assess whether major morbidity and mortality rates increased significantly as the as the risk of CRR and risk of hepatectomy increases.
Currently, the ACS-NSQIP PUF data are reported without patient identifiers, any longitudinal data, or ability to link patient occurrences and therefore all entries are assumed to be independent patient encounters. As such, each encounter represents an occurrence and a contribution to the probability of an outcome for their respective operation specific cohort. For further inferential and supplemental analysis, we estimated the cumulative asynchronous probabilities of major morbidity and mortality of the two independent events isolated hepatectomy (major or minor) and an isolated colectomy (high risk or low risk). The cumulative asynchronous estimates of major morbidity and mortality were performed via the addition law of probability (or the sum rule) method of adding mutually inclusive probabilities which accounts for the statistical “overlap” that is not accounted for by simply adding the two outcomes of interest.36 , 37 The method estimates the probability of an outcome of interest occurring in either or both operations. The probability of experiencing morbidity or mortality after the two isolated procedures was derived as one minus the probabilities of being morbidity or mortality free from the two independent procedures. Standard errors of the combined estimate were derived using the delta method, as previously described.38 , 39 The estimated cumulative asynchronous major morbidity and mortality then were compared to actual observed outcomes of the synchronous cohort via relative risk (RR) and risk difference (RD). RR was calculated with the synchronous cohort’s outcomes in the numerator, whereby RR >1 confers a greater estimated risk within the synchronous cohort. RD was calculated by subtracting the estimated cumulative asynchronous outcomes from the outcomes of the synchronous cohort, whereby a positive RD confers greater risk in the synchronous cohort.
All p values were considered significant at p <0.05. All statistical analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Isolated Hepatectomy for Colorectal Cancer Cohort
Six thousand six hundred sixty-one (6661) patients were identified in NSQIP 2005–2013 PUF undergoing an isolated hepatectomy and met inclusion criteria. There were 4009 partial hepatectomies, 583 left hepatectomies, 1466 right hepatectomies, and 603 trisectionectomies (Fig. 1).
Definition of Hepatic Resection Risk Category Using the Isolated Hepatectomy Cohort
Overall 30-day major morbidity rate for all isolated hepatic resections was 14.0 % (n = 934). Overall 30-day mortality was 1.11 % (n = 74). Hepatic resections associated with the greatest mortality were right hepatectomy (2.3 %) and trisectionectomy (2.0 %). Mortality rates did not differ between these hepatic resections (p = 0.71); therefore, they were combined and labeled as “Major” hepatectomies. Hepatic resections associated with the lowest mortality were partial hepatectomy (0.6 %) and left hepatectomy (0.5 %). Mortality rates after these hepatic resections were not different (p = 1.00) and therefore were combined and labeled as “Minor” hepatectomies.
Mortality of minor hepatectomies (0.6 %) was significantly lower than major hepatectomies (2.2 %) (p < 0.001) (Fig. 2). Major morbidity was 11.0 % in the minor hepatectomy category compared to 20.8 % in the major hepatectomy category (p < 0.001).
Isolated Colectomy for Colorectal Cancer Cohort
Thirty-five thousand eight hundred twenty-five (35,825) patients were identified in ACS-NSQIP 2005–2013 PUF undergoing an isolated colectomy for colorectal cancer and met inclusion criteria. There were 5644 right colectomies, 11,751 left colectomies without diversion, 11,025 LAR, 2287 left colectomies with diversion, 787 total abdominal colectomies, 812 total abdominal proctocolectomies, and 3519 APRs (Fig. 3).
Definition of Colorectal Resection Risk Categories Using the Isolated CRR Cohort
Overall 30-day major morbidity rate for all isolated colorectal resections was 17.1 % (n = 6141). Overall 30-day mortality for all isolated colorectal resections was 2.1 % (n = 759). Colorectal resections associated with the greatest major morbidity were left colectomy with diversion (22.2 %), total abdominal colectomy (24.5 %), total abdominal proctocolectomy (24.0 %), and APR (21.8 %). Major morbidity rates did not differ among these colorectal resections (p = 0.26); therefore, they were combined and labeled as “High Risk”. Colorectal resections associated with the least major morbidity were right colectomy (15.8 %), left colectomy without diversion (15.4 %), and LAR (16.2 %). Major morbidity rates did not differ among these colorectal resections (p = 0.27) and therefore were combined and labeled as “Low Risk”.
Major morbidity after colorectal resections categorized as low risk (15.8 %) was significantly lower than after colorectal resections categorized as high risk (22.4 %) (p < 0.001) (Fig. 4). The mortality rate was significantly greater for the high risk category (2.5 %) than for the low risk category (2.0 %) (p = 0.014).
Synchronous Resection of Colorectal Primary and Liver Metastasis Cohort
Nine hundred twenty-two (922) patients were identified in ACS-NSQIP 2005–2013 PUF undergoing synchronous colorectal and liver resection for primary and metastatic colorectal cancer and met inclusion criteria. The synchronous cohort was stratified by the previously defined “high” or “low” risk colorectal resections and by “major” or “minor” hepatectomy. Thus, there were four possible synchronous resection categories: (1) high risk CRR and major hepatectomy (n = 20), (2) low risk CRR and major hepatectomy (n = 148), (3) high risk CRR and minor hepatectomy (n = 112), and (4) low risk CRR and minor hepatectomy (n = 642) (Fig. 5).
Synchronous Resection Cohort: Preoperative Characteristics
Among patients undergoing synchronous resection, 33.9 % of patients were 65+ years, 9.3 % were 75+, 29.4 % were obese (BMI ≥ 30), 13.0 % were diabetic requiring insulin or non-insulin oral medication, and 19.9 % had received chemotherapy within 30 days prior to their operation. These patient characteristics were not significantly different among the four synchronous resections (p > 0.05). Similarly preoperative laboratory findings including preoperative serum creatinine, serum albumin, total bilirubin, SGOT, INR, and platelet counts did not differ among the four synchronous resections (p > 0.05). There was a significant differences in preoperative alkaline phosphatase (p = 0.004) in patients who underwent synchronous resection and major hepatectomy compared to patients who underwent synchronous resection and minor hepatectomy (Table 1).
Synchronous Resection Cohort: Postoperative Outcomes
Major Morbidity Stratified by Colorectal and Hepatic Resection Risk Groups
The overall observed rate of major morbidity for patients after synchronous resection was 29.0 % (n = 267). Observed major morbidity among the four synchronous resection categories was 55.0 % (n = 11) for high risk CRR and major hepatectomy, 39.2 % (n = 58) for low risk CRR and major hepatectomy, 30.4 % (n = 34) for high risk CRR and minor hepatectomy, and 25.5 % (n = 164) for low risk CRR and minor hepatectomy (p < 0.001). Cochran-Armitage Trend Test confirmed these findings as a statistically significant trend in major morbidity across the four synchronous resection risk categories (p < 0.001) (Fig. 6).
Mortality Stratified by Colorectal and Hepatic Resection Risk Groups
The overall 30-day mortality rate for the synchronous cohort was 1.7 % (n = 16). Observed 30-day mortality rate among the four possible synchronous resection categories was 5.0 % (n = 1) for high risk CRR and major hepatectomy, 3.4 % (n = 5) for low risk CRR and major hepatectomy, 0.9 % (n = 1) for high risk CRR and minor hepatectomy, and 1.4 % (n = 9) for low risk CRR and minor hepatectomy (p = 0.15). Cochran-Armitage Trend Test again confirmed these findings as a statistically significant trend in mortality (p = 0.038) (Fig. 7).
Additional Analysis
Comparison of Estimated Cumulative Major Morbidity and Mortality of Isolated Colorectal and Hepatic Resections to that of the Synchronous Resection Cohort
Estimated cumulative asynchronous risk of major morbidity and mortality for isolated high risk colorectal resection and isolated major hepatectomy was 38.6 and 4.6 %, respectively. When compared to the synchronous high risk colorectal resection and major hepatectomy cohort, the relative risk for major morbidity and mortality was 1.43 and 1.09, respectively, and the risk difference was 16.4 and 0.4 %, respectively. The risk difference was not significantly different for either major morbidity or mortality (p > 0.05) (Tables 2 and 3).
Estimated cumulative asynchronous risk of major morbidity and mortality for isolated low risk colorectal resection and isolated major hepatectomy was 33.3 and 4.2 %, respectively. When compared to the synchronous low risk colorectal resection and major hepatectomy cohort, the relative risk for major morbidity and mortality were 1.18 and 0.81, respectively, and the risk difference was 5.9 and −0.78 %, respectively. The risk difference was not significantly different for either major morbidity or mortality (p > 0.05) (Tables 2 and 3).
Estimated cumulative asynchronous risk of major morbidity and mortality for isolated high risk colorectal resection and isolated minor hepatectomy was 31.0 and 3.1 %, respectively. When compared to the synchronous high risk colorectal resection and minor hepatectomy cohort, the relative risk for major morbidity and mortality was 0.98 and 0.29, respectively, and the risk difference was −0.60 and −2.2 %, respectively. The risk difference revealed a statistically significant decrease in the risk of mortality in the synchronous cohort compared to estimated cumulative asynchronous risk observed in the isolated resections (p = 0.016). The risk difference for major morbidity was not significant (p = 0.89) (Tables 2 and 3).
Estimated cumulative asynchronous risk of major morbidity and mortality for isolated low risk colorectal resection and isolated minor hepatectomy was 25.0 and 2.6 %, respectively. When compared to the synchronous low risk colorectal resection and minor hepatectomy cohort, the relative risk for major morbidity and mortality was 1.02 and 0.53, respectively, and the risk difference was 0.54 and −1.2 %, respectively. The risk difference revealed a statistically significant decrease in the risk of mortality in the synchronous cohort compared to the estimated cumulative asynchronous risk observed in the isolated resections (p = 0.011). The risk difference for major morbidity was not significant (p = 0.76) (Tables 1 and 2).
Discussion
To our knowledge, this study is the first large multi-institutional investigation assessing operative morbidity and mortality after synchronous surgical management of stage IV colorectal cancer by stratifying synchronous colorectal and hepatic resections by both the extent and risk of the hepatectomy as well as the type and risk of the colorectal resection. Our findings from this review of the ACS-NSQIP PUF reveal that both postoperative mortality and major morbidity can be effectively stratified by the type of isolated hepatectomy and isolated colorectal resection for stage IV colorectal cancer. Furthermore, we have shown that synchronous colorectal and hepatic resections for stage IV colorectal cancer can be stratified using these newly defined risk categories. Finally, our primary hypothesis was confirmed; outcomes after synchronous resections do in fact vary based upon both the extent of hepatic resection and the type of colorectal resection performed. In fact, when the synchronous resection cohort is stratified by both the risk of the hepatectomy and risk of the colectomy, there is a significant trend in major morbidity and mortality as risk of colectomy and risk of hepatectomy increase.
We also performed inferential statistical comparisons of the estimated cumulative mortality and morbidity of two isolated and independent operations (hepatectomy and colorectal resection) to that of a similarly stratified synchronous cohort. While sample size is certainly lacking from the synchronous cohort, several interesting observations were found. Synchronous colorectal and hepatic resections were associated with a reduced operative mortality risk for any colorectal resection (high or low risk) with minor hepatectomy compared to the estimated cumulative mortality from similar isolated resections. Conversely, we found no such association or benefit for synchronous resections involving major hepatectomy. Despite the overall patients’ sample size, the small number of patients undergoing synchronous major hepatic resection and CRR was insufficient to determine whether actual risk differed significantly.
Prior studies have demonstrated the overall safety of synchronous resections,40 though synchronous resections are often performed for less extensive hepatic disease. Clearly in patients who harbor metastases that require a major hepatectomy, careful patient selection has been advocated. In fact, even in selected patients, hepatectomy may be aborted intraoperatively for issues related to the CRR. Moreover, although extensive hepatic metastases may prompt major hepatectomy, staged hepatectomy may be required to address the extent of hepatic disease which precludes synchronous resection of the primary colorectal cancer and all hepatic metastases. However, because minor hepatic resections are not associated with increased risk of perioperative mortality or major morbidity, synchronous resection of the minor component of the staged hepatic resection and CRR are supported by these data. Regardless of specific patient selection criteria for complex hepatic resections, our results support the efficacy of any colorectal resection with minor hepatectomy. Specifically, these ACS-NSQIP data show that low risk colorectal resection and minor hepatectomy is the most frequent synchronous surgical approach for stage IV colorectal cancer, and these data support its efficacy. Further study of synchronous high risk colorectal resection with major hepatectomy will be required to establish clinical recommendations for such patients.
Although outcomes for synchronous resection have been published stratifying by major and minor hepatectomy, this report is first to (1) define major and minor hepatectomy as well as high and low risk colorectal resections for stage IV colorectal cancer based on the statistical differences in actual outcomes encountered, (2) stratify synchronous resection outcomes based on the individual risks of both the hepatic and colorectal resection components, and (3) estimate the potential benefit or harm posed by synchronous resection when compared to the estimated cumulative mortality and major morbidity experienced by the same resections performed in an isolated and independent fashion.
These findings have potential impact to patient care and quality of life. The ability to counsel patients on their risks of surgical resection in a more granular fashion, with respect to the risk of the colorectal and hepatic components, will aid in surgical and patient informed decision making. Moreover, given the national multi-institutional source of the data and large sample size of patients with colorectal and hepatic resections, these findings may serve as national benchmarks for which major morbidity and mortality following synchronous resections can be compared. The ability to perform colorectal and hepatic resections in a synchronous fashion have several potential benefits to patient care including limiting surgical care to one general anesthetic, overall less inpatient time, and an earlier resumption of adjuvant therapy. However, these potential benefits are likely to only be possible if the patient has a relatively uncomplicated postoperative period. Therefore, the safety of (and not simply the ability) to perform synchronous colorectal and hepatic resections must be weighed against the safety of performance in an asynchronous fashion. The findings of this study have demonstrated the safety of synchronous resection when involving a minor hepatectomy at the time of high or low risk colorectal resections. However, there are insufficient data and sample size within this study to conclude confidently that synchronous colorectal resection and major hepatectomy is unsafe or significantly harmful. Although these findings support synchronous resections for stage IV colorectal cancer, intraoperative clinical judgment of patient factors, technical factors, and institutional factors will determine completion of the planned preoperative strategy for the individual patient. Further strategies and experience in high volume centers to address specific issues affecting the safety of synchronous major hepatectomy at the time of high and low risk colorectal resections are needed.
Limitations
This study has several potential limitations. First, it is a retrospective review of a prospective database. Although the American College of Surgeons has stringent quality control measures in place to maintain the integrity of the data, there are inherent limitations of such a dataset. Second, the database does not contain colectomy- or hepatectomy-specific variables. Thus, factors predictive of complications for each operative component could not be determined. Specific complications which may be graded as major morbidity such as fistula or pelvic abscess, biliary leak or fistula, hepatic failure, etc. are not available; therefore, our analysis may underestimate morbidity rates. ACS-NSQIP provides only one postoperative diagnosis code. Therefore, patients with a diagnosis of cirrhosis could not be specifically identified in this study. Third, there are also no hospital- or surgeon-specific variables to compare volume or experience. Fourth, survival analysis is limited to 30-day outcomes although 90 days is considered more clinically relevant. Fifth, as previously noted, ACS-NSQIP data lack any patient identifiers or longitudinal data; therefore, all resections and patient encounters within the dataset are assumed to be independent observations and is in general a limitation of the dataset. Although there is an assumption of independence among the patient encounters, this is not unique to this study or even this dataset, and in general there exists a trade-off between variance and independence. Also, the number of patients undergoing high risk colorectal resection and major hepatectomy in a synchronous fashion was too few for meaningful comparisons. Additionally, as the primary aim of this study was the evaluation of a new methodology for stratifying synchronous colorectal and hepatic resection, the isolated resections groups were only used for defining the cohorts by risk of major morbidity, mortality, and procedure type. Therefore, no preoperative patient characteristics were investigated or risk adjusted for. Finally, there could certainly be selection bias among the synchronous cohort, beyond the predilection for minor hepatic resection that cannot be fully explained given the data available within the ACS-NSQIP PUF. Despite these limitations, however, this report represents a large multicenter analysis evaluating the impact of risk stratification by both the colorectal and hepatic resection components of synchronous resections and the respective postoperative outcomes.
Conclusions
We have shown that a more accurate and granular representation of 30-day outcomes following synchronous colorectal and liver resection for primary and metastatic colorectal cancer can be achieved by stratification by both major and minor hepatectomy as well as high and low risk colorectal resection. Minor hepatectomies performed at the time of low and high risk colorectal resections appear to be associated with relatively low rate of major morbidity and mortality. This study was insufficiently powered to make any significant conclusions regarding synchronous resection involving major hepatectomy; therefore, cautious patient selection is warranted in candidates for synchronous major hepatectomy.
Finally, our findings suggest that future outcomes studies, inter-institutional comparisons, performance measures, risk calculators, and patient informed consent for synchronous colorectal and liver resection should consider both the extent of liver resection and the type of colorectal resection. This should be done in order to more accurately account for anticipated major morbidity, mortality, complexity of care as well as case mix variation. Reporting outcomes without such stratification may miss the opportunity to identify areas for improvement, anticipate resource needs, and/or shed further light on the remaining areas without definitive conclusions. These data provide patients and providers with clinically relevant benchmarks regarding synchronous hepatic and colorectal resection.
References
Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi:10.1002/cncr.28509.
Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi:10.3322/caac.21220.
Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. Natl Cancer Inst. 2014:based on November 2013 SEER data submission, poste. http://seer.cancer.gov/csr/1975_2011/.
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier A-M. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi:10.1097/01.sla.0000217629.94941.cf.
Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi:10.1200/JCO.2004.09.046.
Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized Phase III study.; 2008. doi:10.1200/JCO.2007.14.9930.
Van Cutsem E, Köhne C-H, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi:10.1056/NEJMoa0805019.
Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi:10.1016/S0140-6736(08)60455-9.
Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657; discussion 657–658. doi:10.1097/01.sla.0000141198.92114.f6.
Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi:10.1200/JCO.2007.11.0833.
Choti MA, Sitzmann J V, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi:10.1097/00000658-200206000-00002.
Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999. doi:10.1038/sj.bjc.6603033.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–321. doi:10.1097/00000658-199909000-00004.
Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–3491. doi:10.1245/s10434-007-9517-2.
Martin RCG, Augenstein V, Reuter NP, Scoggins CR, McMasters KM. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg. 2009;208:842–850. doi:10.1016/j.jamcollsurg.2009.01.031.
Ayez N, Burger JW a, van der Pool AE, et al. Long-term results of the “liver first” approach in patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum. 2013;56:281–287. doi:10.1097/DCR.0b013e318279b743
Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191(1):38–46. http://www.ncbi.nlm.nih.gov/pubmed/10898182.
Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397. doi:10.1097/01.SLA.0000029003.66466.B3.
Cheng W, Poon KH, Lui VC, et al. Esophageal atresia and achalasialike esophageal dysmotility. J Pediatr Surg. 2004;39(10):1581–1583. http://www.ncbi.nlm.nih.gov/pubmed/15486912.
Dimick JB, Wainess RM, Cowan JA, Upchurch Jr. GR, Knol JA, Colletti LM. National trends in the use and outcomes of hepatic resection. J Am Coll Surg. 2004;199(1):31–38. doi:10.1016/j.jamcollsurg.2004.03.005.
Chua HK, Sondenaa K, Tsiotos GG, Larson DR, Wolff BG, Nagorney DM. Concurrent vs. staged colectomy and hepatectomy for primary colorectal cancer with synchronous hepatic metastases. Dis Colon Rectum. 2004;47(8):1310–1316. http://www.ncbi.nlm.nih.gov/pubmed/15484344.
Faitot F, Faron M, Adam R, et al. Two-stage hepatectomy versus 1-stage resection combined with radiofrequency for bilobar colorectal metastases: a case-matched analysis of surgical and oncological outcomes. Ann Surg. 2014;260(5):822–828. doi:10.1097/SLA.0000000000000976.
Abbott AM, Parsons HM, Tuttle TM, Jensen EH. Short-term outcomes after combined colon and liver resection for synchronous colon cancer liver metastases: a population study. Ann Surg Oncol. 2012. doi:10.1245/s10434-012-2515-z.
Mayo SC, Pulitano C, Marques H, et al. Surgical management of patients with synchronous colorectal liver metastasis: a multicenter international analysis. J Am Coll Surg. 2013;216:707–718. doi:10.1016/j.jamcollsurg.2012.12.029.
Slesser AAP, Simillis C, Goldin R, Brown G, Mudan S, Tekkis PP. A meta-analysis comparing simultaneous versus delayed resections in patients with synchronous colorectal liver metastases. Surg Oncol. 2013;22(1):36–47. doi:10.1016/j.suronc.2012.11.002.
Weber JC, Bachellier P, Oussoultzoglou E, Jaeck D. Simultaneous resection of colorectal primary tumour and synchronous liver metastases. Br J Surg. 2003;90:956–962. doi:10.1002/bjs.4132.
Roxburgh CS, Richards CH, Moug SJ, Foulis AK, McMillan DC, Horgan PG. Determinants of short- and long-term outcome in patients undergoing simultaneous resection of colorectal cancer and synchronous colorectal liver metastases. Int J Colorectal Dis. 2012;27(3):363–369. doi:10.1007/s00384-011-1339-9.
Vigano L, Langella S, Ferrero A, Russolillo N, Sperti E, Capussotti L. Colorectal cancer with synchronous resectable liver metastases: monocentric management in a hepatobiliary referral center improves survival outcomes. Ann Surg Oncol. 2013;20(3):938–945. doi:10.1245/s10434-012-2628-4.
Worni M, Mantyh CR, Akushevich I, Pietrobon R, Clary BM. Is there a role for simultaneous hepatic and colorectal resections? A contemporary view from NSQIP. J Gastrointest Surg. 2012;16:2074–2085. doi:10.1007/s11605-012-1990-7.
Wise KB, Merchea A, Cima RR, Colibaseanu DT, Thomsen KM, Habermann EB. Proximal intestinal diversion is associated with increased morbidity in patients undergoing elective colectomy for diverticular disease: an ACS-NSQIP study. J Gastrointest Surg. 2014. doi:10.1007/s11605-014-2700-4.
Kwaan MR, Al-Refaie WB, Parsons HM, Chow CJ, Rothenberger D a, Habermann EB. Are right-sided colectomy outcomes different from left-sided colectomy outcomes?: study of patients with colon cancer in the ACS NSQIP database. JAMA Surg. 2013;148:504–510. doi:10.1001/jamasurg.2013.1205.
Shubert C, Habermann E, Truty M, Thomsen K, Kendrick M, Nagorney D. Defining perioperative risk after hepatectomy based on diagnosis and extent of resection. J Gastrointest Surg. 2014;18(11):1917–1928. http://www.ncbi.nlm.nih.gov/pubmed/25199947. Accessed November 18, 2014.
Andreou A, Vauthey JN, Cherqui D, et al. Improved long-term survival after major resection for hepatocellular carcinoma: a multicenter analysis based on a new definition of major hepatectomy. J Gastrointest Surg. 2013;17(1):66–77; discussion p 77. doi:10.1007/s11605-012-2005-4.
Enker WE, Merchant N, Cohen AM, et al. Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service.; 1999.
Zaheer S, Pemberton JH, Farouk R, Dozois RR, Wolff BG, Ilstrup D. Surgical treatment of adenocarcinoma of the rectum. Ann Surg. 1998;227:800–811. doi:10.1097/00000658-199806000-00003.
Krämer W. Probability & measure. Comput Stat Data Anal. 1995;20(6):703. doi:10.1016/0167-9473(95)90197-3.
Shafer G, Vovk V. The origins and legacy of Kolmogorov’s Grundbegriffe. October. 2005. doi:10.1214/088342305000000467.
Oehlert GW. A note on the delta method. Am Stat. 1992;46:27–29. doi:10.1080/00031305.1992.10475842.
Ver Hoef JM. Who invented the delta method? Am Stat. 2012;66(2):124–127. doi:10.1080/00031305.2012.687494.
Yin Z, Liu C, Chen Y, et al. Timing of hepatectomy in resectable synchronous colorectal liver metastases (SCRLM): simultaneous or delayed? Hepatology. 2013;57(6):2346–2357. doi:10.1002/hep.26283.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shubert, C.R., Habermann, E.B., Bergquist, J.R. et al. A NSQIP Review of Major Morbidity and Mortality of Synchronous Liver Resection for Colorectal Metastasis Stratified by Extent of Liver Resection and Type of Colorectal Resection. J Gastrointest Surg 19, 1982–1994 (2015). https://doi.org/10.1007/s11605-015-2895-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2895-z