Abstract
Background
We investigated outcomes by primary tumor type in patients who underwent resection of liver metastases from gastrointestinal stromal tumors (GIST), leiomyosarcomas, and other sarcomas.
Method
Our institutional liver database was used to identify patients who underwent resection from 1998 through 2013. Histopathological, clinical, and survival data were analyzed.
Results
One hundred forty-six patients underwent resection of liver metastases from GIST (n = 49), leiomyosarcomas (n = 47), or other sarcomas (n = 50). The 5-year overall survival (OS) rates in patients with GIST, leiomyosarcomas, and other sarcomas were 55.3, 48.4, and 44.9 %, respectively, and the 10-year OS rates were 52.5, 9.2, and 23.0 %, respectively. The 5-year recurrence-free survival (RFS) rate was better for GIST (35.7 %; p = 0.003) than for leiomyosarcomas (3.4 %) and other sarcomas (21.4 %). Lung recurrence was more common for leiomyosarcomas (36 % of patients; p < 0.0001) than for other sarcomas (12 %) and GIST (2 %). For GIST, the findings support a benefit of imatinib regarding the 5-year RFS rate compared to resection alone (47.1 vs. 9.5 %; p = 0.013). For leiomyosarcoma, primary tumor location did not affect the 5-year RFS rate (intraabdominal 14.5 %; other location 0 %; p = 0.182).
Conclusion
Liver metastases from GIST, leiomyosarcomas, and other sarcomas should be assessed separately as their survival and recurrence patterns are different. This is especially important for GIST, for which imatinib is now available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The safety of liver resection is improving, allowing for broader application of resection of noncolorectal liver metastases.1 – 3 However, as the definition of technically resectable liver disease is expanding, it remains important to determine the survival benefit from liver resection given that tumors of different origins have different growth rates, invasiveness, and migratory potential.4
Among the tumors that can metastasize to the liver are gastrointestinal stromal tumors (GIST) and leiomyosarcomas (LMS). GIST, arising from interstitial cells of Cajal of the gastrointestinal tract or other intraabdominal sites, account for 90 % of gastrointestinal mesenchymal neoplasms.5 GIST may or may not possess features of smooth muscle cells but are uniformly characterized by immunoreactivity to the transmembrane tyrosine kinase receptor CD117 (KIT).6 LMS, on the other hand, generally lack expression of CD117 but reliably demonstrate high levels of smooth muscle actin and desmin.7 , 8 Distinguishing between GIST and gastrointestinal LMS (GI-LMS) was difficult before the introduction of KIT immunohistochemistry.5 Consequently, early studies of GI-LMS were comprised largely of patients with GIST, and the unique patient outcomes after resection of liver metastases from GIST and LMS remain poorly described. This problem became especially important after the introduction of imatinib mesylate (imatinib) for treatment of GIST because imatinib has improved outcomes significantly for patients with GIST, whereas targeted therapy for LMS remains experimental and LMS respond poorly to chemotherapy.9 , 10
There are a number of published studies on outcomes after resection of liver metastases from GIST and sarcomas of various origins (Table 1). However, most of these studies are limited by small patient numbers (<40 patients)11 – 18 and/or poor distinction between pathological types, some even including non-GI-LMS and sarcomas of other or indeterminate origin (SRC) in the survival analyses.1 , 2 , 19 , 20
The aim of the present study was to determine outcomes after resection of liver metastases from GIST, LMS, and SRC as unique groups in a large series of patients from a single center. We performed subanalyses to determine the survival impact of imatinib treatment in GIST, the survival impact of the location of LMS (intraabdominal vs. other location), the survival impact of radiofrequency ablation (RFA) in all pathological types, the risk factors for recurrence, and the patterns of recurrence according to pathological type.
Methods
Study Population
The Institutional Review Board of The University of Texas MD Anderson Cancer Center approved the study protocol, PA14-0761. Patients who underwent resection of first-time liver metastases from GIST, LMS, or SRC from 1998 through 2013 were identified from the institutional liver database. Resection of nonliver metastatic disease prior to liver surgery was not an exclusion criterion. The following data were recorded or updated with data found in electronic patient medical records: sex, age, disease-free interval between resection of the primary tumor and presentation of liver metastases, number of liver metastases, diameter of the largest liver metastasis, type of liver resection, resection margin status (no viable tumor cells < 1 mm from the resection margin, or viable tumor cells < 1 mm from the resection margin [R1]), use of RFA, pathological type, use of chemotherapy and targeted therapy, site of recurrence, and overall survival. The patients were grouped according to pathological type: GIST, LMS, or SRC. The following groups were used for subanalyses: use of tyrosine kinase inhibitor (TKI) or not in patients with GIST, intraabdominal, or other primary tumor location in patients with LMS, and use of RFA or not in all patients.
Patient Care
Resectability and extrahepatic disease were assessed with helical computed tomography of the chest, abdomen, and pelvis with a triphasic liver protocol. Intraoperative ultrasonography was used to assess the vascular anatomy and to assess known and possibly undetected lesions. Parenchymal transection was performed using the Cavitron Ultrasonic Surgical Aspirator (Valleylab, Boulder, CO) and saline-linked cautery (Dissecting Sealer DS 3.0, Tissue Link Medical, Inc., Dover, NH) and performed with control of hepatic inflow.21 GIST diagnosis was confirmed by immunohistochemistry or PCR-based DNA sequencing analysis to detect mutations in PDGFRα (exon 18) or c-KIT (exons 9, 11, 13, and 17). Adjuvant treatment, (i.e., chemotherapy or targeted treatment) was used in 96 of the 146 patients (65.8 %). Imatinib was used to treat patients with GIST, and combinations of doxorubicin, docetaxel, gemcitabine, ifosfamide, and/or dacarbazine were used to treat patients with LMS and SRC. Radiological follow-up was performed every 4 months after resection of liver metastases to assess for recurrence.
Statistical Analysis
Continuous data were expressed as median with range and compared with Mann–Whitney U test. Categorical data were compared by Pearson Chi-squared tests or Fisher’s exact test if the expected cell count number of any cell was less than 5. A p value of less than 0.05 was considered statistically significant. Cox regression survival analyses with enter method for the covariates were conducted to determine factors associated with overall survival. Kaplan–Meier analysis was used to assess recurrence-free survival (RFS) and overall survival (OS) from the hepatectomy, and groups were compared using log–rank analyses. The statistical analyses were performed using SPSS v. 19.0 (SPSS Inc., IBM, Chicago, IL, USA).
Results
Clinicopathological Characteristics and Pathological Types
A total of 146 patients underwent resection of liver metastases from GIST (n = 49), LMS (n = 47), or SRC (n = 50) during the study period (Fig. 1). Patient characteristics by pathological type are summarized in Table 2. The median age was 55 years for patients with GIST, 57 years for those with LMS, and 54 years for those with SRC; the proportion of males was 59 % for GIST, 28 % for LMS, and 56 % for SRC. Of the 49 GIST, 20 were located in the small bowel, 14 in the stomach, 13 in the mesentery, and 2 in the large bowel. Of the 47 LMS, 14 were located in the abdomen, 10 in the uterus, 10 in the retroperitoneum, 4 in the kidneys, 3 in the adrenal glands, 3 in the vena cava, and 3 in other locations. Among the 50 SRC, there were ten cases of unclassified sarcoma; seven cases of liposarcoma; four cases each of desmoplastic small round cell sarcoma and pleomorphic sarcoma; three cases each of angiosarcoma, chondrosarcoma, fibrosarcoma, and hemangiopericytoma; two cases each of hemangioendothelioma, osteosarcoma, and spindle cell sarcoma; and one case each of clear-cell sarcoma, epithelioid sarcoma, Ewing sarcoma, myxofibrosarcoma, perivascular epithelioid cell tumor, phyllodes sarcoma, and synovial sarcoma.
RFS According to Pathological Type
The 5-year RFS rate was 35.7 % for GIST, 3.4 % for LMS, and 21.4 % for SRC, and median time to recurrence was 17.8 months for GIST, 7.9 months for LMS, and 8.8 months for SRC (p = 0.003; Fig. 2a). The liver was the most common site of recurrence for all groups (GIST, 41 % of patients had recurrence in the liver; LMS, 40 %; SRC, 34 %), and there were no differences regarding the sites of recurrence except that lung recurrence occurred in 2 % of patients with GIST, 36 % of those with LMS, and 12 % of those with SRC (p < 0.001; Table 3).
Subgroup analysis showed that patients with GIST who received imatinib had a higher 5-year RFS rate than patients with GIST who underwent surgical resection alone (47.1 vs. 9.5 %; p = 0.013; Fig. 3a). Patients with LMS and SRC who received adjuvant chemotherapy had similar median RFS as patients who did not receive adjuvant chemotherapy (LMS: 7.9 vs. 9.0 months, p = 0.434; SRC: 7.4 vs. 9.4 months, p = 0.805). Patients with LMS with intraabdominal primary tumors had a 5-year RFS rate (14.5 %) similar to that of patients with LMS with primary tumors at other locations (0 %; p = 0.182; Fig. 3b). Patients with any pathological tumor type who underwent RFA as part of their treatment (GIST, 27 %; LMS, 21 %; SRC, 14 %; p = 0.301) had a lower 5-year RFS rate than patients whose disease could be cleared by resection alone (10.9 vs. 24.7 %; p = 0.009; Fig. 3c). Patients who underwent resection and RFA had similar size of the largest metastasis, but more metastases than patients who underwent resection only: 45 and 55 mm (mean; p = 0.291) and two metastases vs. one metastasis (median; p = 0.005), respectively.
Kaplan–Meier plots of recurrence-free survival according to subgroup: TKI treatment in patients with GIST (a), primary tumor location in patients with LMS (b), and RFA in all patients (c). GIST gastrointestinal stromal tumor, LMS leiomyosarcoma, SRC sarcoma of other or indeterminate origin, TKI tyrosine kinase inhibitor, RFA radiofrequency ablation
OS According to Pathological Type
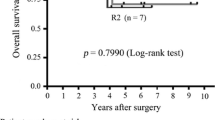
The 5-year OS rates and median survival times did not differ significantly by pathological type, although there was a clear trend toward better 5-year survival for patients with GIST (Table 3). However, the 10-year OS rate was significantly better in patients with GIST (52.5 %) than in patients with LMS (9.2 %) or SRC (23.0 %; Fig. 2b; p = 0.016). In univariate analyses (Table 4), after resection of liver metastases from GIST, age greater than 55 years (HR, 2.798; p = 0.027) was associated with reduced OS, male sex (HR, 0.447; p = 0.071) exhibited a trend toward increased OS, and concomitant RFA (HR, 2.179; p = 0.085) and R1 resection (HR, 4.100; p = 0.066) exhibited trends toward reduced OS. After resection of liver metastases from LMS, disease-free interval less than 12 months (HR, 2.253; p = 0.033) and diameter of the largest tumor more than 30 mm (HR, 2.059; p = 0.055; borderline significant) were associated with decreased OS. After resection of SRC, R1 resection (HR, 12.97; p < 0.001) was associated with decreased OS, and male sex (HR, 1.962; p = 0.076) exhibited a trend toward decreased OS (Table 4).
Discussion
In the present study, we investigated survival and factors associated with outcome after resection of liver metastases from GIST, LMS, and SRC in a large series of patients from a single center. The median RFS times for patients with GIST, LMS, and SRC were 17.8, 7.9, and 8.8 months, respectively. Lung recurrence after resection of liver metastases was more common in patients with LMS than in those with GIST or SRC. The 10-year OS rate was 52.5 % in patients with GIST, 9.2 % in those with LMS, and 23.0 % in those with SRC, indicating long-term survival benefits of surgery in selected groups. In contrast to previous studies based on small patient series and/or poorly differentiating between the different pathological types, the current study suggests unique patterns of recurrence and survival for different pathological types, indicating the importance of consideration of the tumor type when patients are evaluated for liver resection.11 – 14 , 19
The previous practice of analyzing GIST and GI-LMS together has been based on the assumption that metastasizing cells from both pathological entities possibly reach the liver through portal vein circulation. In this view, liver metastases could represent “sentinel metastases” rather than disseminated disease, and surgery may provide a survival benefit and even cure. However, this assumption is hypothetical and does not account for different tumor biology of the pathological entities. Additional reasons for analyzing these pathological entities together have been the fact that these cancers are rare and the fact that they were difficult to distinguish from one another before the routine use of immunohistochemistry or gene analyses.1 , 11
Previous studies have reported outcomes that could indicate cross-contamination between pathological groups. For example, Nunobe et al.13 and DeMatteo et al.19 did not distinguish between GIST and GI-LMS and reported 5-year survival rates of 34.0 and 40.0 %, respectively. These rates align between the 55.3 and the 21.8 % 5-year survival rates for GIST and GI-LMS in the current study. Furthermore, DeMatteo et al.19 reported similar survival after resection of GIST/GI-LMS and SRC. This could be attributed to the mixing of pathological entities but most likely reflects the fact that the study included patients treated before the use of imatinib.
Today, discrimination between GIST and LMS is important because patients with GIST are amenable to treatment with imatinib. In GIST, the principal pathological genetic defect has been identified as mutation in the c-KIT proto-oncogene or in the platelet-derived growth factor receptor-α (PDGFRα) gene, both leading to expression of proteins causing constitutive activation of tyrosine kinase receptors. Imatinib was initially found as an inhibitor of BCR/ABL, but subsequently also found to be a molecular antagonist of c-KIT and PDGFRα proteins, and thereby acts as a tyrosine kinase inhibitor (TKI). More than 90 % of GIST express c-KIT, and imatinib has revolutionized treatment of these tumors.22 In the current study, most patients (n = 39; 80 %) with liver metastases from GIST were treated with imatinib perioperatively, and their survival was superior to that of patients who underwent liver resection without imatinib. Liver resection before the 2001 US Food and Drug Administration approval of imatinib was the main reason for absence of treatment in the 10 patients with GIST in our study who did not receive imatinib. The effect of chemotherapy in LMS and SRC, not amenable to treatment with imatinib, is still uncertain and controversial.9 , 10 In the current study, 57.4 % of patients with LMS and 56 % of patients with SRC received some form of perioperative chemotherapy. We did not see the same survival benefit from chemotherapy in patients with LMS and SRC that we saw from imatinib in patients with GIST (data not shown).
Historically, resection of liver metastases from GIST has been controversial, and whether resection actually provided a chance for cure has been questioned. Nunobe et al.13 concluded that cure was difficult to achieve with resection in their series of 18 patients with GIST and that repeated surgical resection primarily contributed to palliation. In spite of their conclusion, they reported a 5-year survival rate of 34.0 %, reflecting long-term survivors in their series, which included both GIST and GI-LMS. Our results indicate that the 5-year GIST-specific survival rate could have been even higher had the patients stratified by pathological type.
In the era of imatinib, the role of surgery for liver metastases from GIST has been questioned. However, previous reports indicate that up to 24 % of GIST patients are poor imatinib responders and complete response to imatinib is rare.23 – 25 Xia et al.16 made the interesting observation that resection of liver metastases from GIST improved survival in patients with poor response to imatinib. Furthermore, resistance to imatinib is seen in more than 50 % of patients after 2 years of treatment.23 – 25 On the basis of our current study, we believe that resection of liver metastases from GIST, even in patients showing radiographic response to imatinib, should remain a vital component of the treatment plan.
In the current study, the liver was the most common site of recurrence after resection of metastases irrespective of pathological type, and liver metastases were seen in about 40 % of patients. In contrast, lung recurrence presented significantly more often in patients with LMS (36 %) than patients with SRC (12 %) or GIST (2 %). To our knowledge, this difference has not been noted in previous studies reporting recurrence after liver resection, possibly because of lack of differentiation in those studies between GIST and LMS.19
Because of small patient series and mixing of pathological types, it has been difficult to establish prognostic factors for survival after resection of liver metastases from GIST, LMS, and SRC.13 In studies by DeMatteo et al.19 and Pawlik et al.20, clinicopathological factors such as sex, age, tumor number, and margin status were not prognostic of survival. In contrast, in the current study, male sex was a borderline significant predictor of survival, but with opposite effects in GIST (HR, 0.447; p = 0.071) and SRC (HR, 1.962; p = 0.076). In the current study, age more than 55 years was significantly associated with worse survival for GIST (HR, 2.798; p = 0.027), but not for LMS and SRC. In patients with LMS, overall survival was positively associated with a disease-free interval of more than 12 months, which is in agreement with findings from patients with colorectal cancer liver metastases.26 In contrast, in patients with GIST and SRC, survival was not associated with disease-free interval.
RFA was used in 30 patients (GIST: n = 13; LMS: n = 10; SRC: n = 7) and was associated (borderline significant) with poor survival in patients with GIST but not in patients with LMS or SRC. Patients who underwent RFA alone without surgical resection were not included in the present study, but Pawlik et al.20 previously reported higher rates of recurrence in patients who underwent RFA alone than in patients who underwent resection alone for GIST, LMS, or SRC.
Previous reports have shown that imatinib improves recurrence-free survival more in patients undergoing resection of liver metastases from GIST than in patients undergoing resection of liver metastases from LMS and SRC.20 In the current study, the findings supported a benefit of imatinib on recurrence-free survival in patients with liver metastases from GIST.
The current study has the following limitations. First, the study may have been underpowered for certain analyses, especially subanalyses within the pathological groups. For example, for the analysis of the impact of a positive margin (R1 resection) in patients with GIST and LMS, there were only 2 patients with R1 resection in each of these groups. Furthermore, RFS was similar after resection of liver metastases from intraabdominal primary LMS, which is drained by the portal vein, and resection of liver metastases from primary LMS at other sites, which is drained systemically (p = 0.182). However, according to the Kaplan–Meier survival plot (Fig. 3b), there could be a trend toward a more favorable outcome with the intraabdominal location of the primary LMS, and our series may have been underpowered to permit firm conclusions to be drawn. Regardless of this limitation, this surgical series is still the largest single-center experience reported to date of these pathological types. Second, while the Kaplan–Meier RFS plots showed significant separation between GIST, LMS, and SRC beginning about 1 year after resection of metastases (Fig. 2a), it was not until about 5 years after resection of metastases that the OS plots showed a survival advantage for GIST over LMS and SRC (Fig. 2b). One possible explanation for the discrepancy between the RFS and OS curves could be more aggressive treatment of recurrence in certain pathological entities. Furthermore, the number of patients with more than 5 years of follow-up was limited and may not have been sufficient to permit conclusions about whether SRC is associated with better OS than LMS between 5 and 10 years.
Conclusion
The current report suggests that patients with GIST, LMS, and SRC should be evaluated separately for resection of liver metastases. Patients with GIST treated with imatinib and resection had a clear survival benefit over patients with GIST undergoing resection alone. This benefit is most likely a synergistic effect and resection should still be the mainstay of treatment as resistance to imatinib is often observed after about 2 years of treatment. The current study demonstrates long-term survivors (5 and 10 years) after resection of liver metastases from LMS and SRC, indicating a role for surgical resection in patients with these pathological entities as well.
References
Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M, Jaeck D, Saric J, Le Treut YP, Belghiti J, Mantion G, Mentha G. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 2006; 244(4):524–35.
Page AJ, Weiss MJ, Pawlik TM. Surgical management of noncolorectal cancer liver metastases. Cancer 2014; 120(20):3111–21.
Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995; 19(1):59–71.
Sahai E. Illuminating the metastatic process. Nat Rev Cancer 2007; 7(10):737–49.
Katz SC, DeMatteo RP. Gastrointestinal stromal tumors and leiomyosarcomas. J Surg Oncol 2008; 97(4):350–9.
Pisters PW, Patel SR. Gastrointestinal stromal tumors: current management. J Surg Oncol 2010; 102(5):530–8.
Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol 2003; 27(5):625–41.
Yamaguchi U, Hasegawa T, Masuda T, Sekine S, Kawai A, Chuman H, Shimoda T. Differential diagnosis of gastrointestinal stromal tumor and other spindle cell tumors in the gastrointestinal tract based on immunohistochemical analysis. Virchows Arch 2004; 445(2):142–50.
Sborov D, Chen JL. Targeted therapy in sarcomas other than GIST tumors. J Surg Oncol. 2014 Oct 20. doi:10.1002/jso.23802.
Spira AI, Ettinger DS. The use of chemotherapy in soft-tissue sarcomas. Oncologist 2002; 7(4):348–59.
Lang H, Nussbaum KT, Kaudel P, Fruhauf N, Flemming P, Raab R. Hepatic metastases from leiomyosarcoma: a single-center experience with 34 liver resections during a 15-year period. Ann Surg 2000; 231(4):500–5.
Shima Y, Horimi T, Ishikawa T, Ichikawa J, Okabayashi T, Nishioka Y, Hamada M, Shibuya Y, Ishii T, Ito M. Aggressive surgery for liver metastases from gastrointestinal stromal tumors. J Hepatobiliary Pancreat Surg 2003; 10(1):77–80.
Nunobe S, Sano T, Shimada K, Sakamoto Y, Kosuge T. Surgery including liver resection for metastatic gastrointestinal stromal tumors or gastrointestinal leiomyosarcomas. Jpn J Clin Oncol 2005; 35(6):338–41.
Rehders A, Peiper M, Stoecklein NH, Alexander A, Boelke E, Knoefel WT, Rogiers X. Hepatic metastasectomy for soft-tissue sarcomas: is it justified? World J Surg 2009; 33(1):111–7.
Sjolund K, Andersson A, Nilsson E, Nilsson O, Ahlman H, Nilsson B. Downsizing treatment with tyrosine kinase inhibitors in patients with advanced gastrointestinal stromal tumors improved resectability. World J Surg 2010; 34(9):2090–7.
Xia L, Zhang MM, Ji L, Li X, Wu XT. Resection combined with imatinib therapy for liver metastases of gastrointestinal stromal tumors. Surg Today 2010; 40(10):936–42.
Turley RS, Peng PD, Reddy SK, Barbas AS, Geller DA, Marsh JW, Tsung A, Pawlik TM, Clary BM. Hepatic resection for metastatic gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Cancer 2012; 118(14):3571–8.
Cananzi FC, Belgaumkar AP, Lorenzi B, Mudan S. Liver surgery in the multidisciplinary management of gastrointestinal stromal tumour. ANZ J Surg 2013; 84(12):937–42.
DeMatteo RP, Shah A, Fong Y, Jarnagin WR, Blumgart LH, Brennan MF. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg 2001; 234(4):540–7.
Pawlik TM, Vauthey JN, Abdalla EK, Pollock RE, Ellis LM, Curley SA. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg 2006; 141(6):537–43.
Aloia TA, Zorzi D, Abdalla EK, Vauthey JN. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg 2005; 242(2):172–7.
Joensuu H. Gastrointestinal stromal tumor (GIST). Ann Oncol 2006; 17 Suppl 10:x280–6.
Scaife CL, Hunt KK, Patel SR, Benjamin RS, Burgess MA, Chen LL, Trent J, Raymond AK, Cormier JN, Pisters PW, Pollock RE, Feig BW. Is there a role for surgery in patients with “unresectable” cKIT+ gastrointestinal stromal tumors treated with imatinib mesylate? Am J Surg 2003; 186(6):665–9.
Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, Leversha MA, Jeffrey PD, Desantis D, Singer S, Brennan MF, Maki RG, DeMatteo RP. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 2005; 11(11):4182–90.
Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003; 21(23):4342–9.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230(3):309–18.
Acknowledgments
The authors thank Stephanie Deming for editing the manuscript and Ruth J. Haynes for secretarial assistance in the preparation and submission of the manuscript.
This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672. Dr. Brudvik is supported by the Department of Hepato-Pancreato-Biliary Surgery, Oslo University Hospital, Norway, and was awarded the Unger-Vetlesen Medical Fund for 2014
Conflict of Interest
The authors report no conflicts of interest relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brudvik, K.W., Patel, S.H., Roland, C.L. et al. Survival After Resection of Gastrointestinal Stromal Tumor and Sarcoma Liver Metastases in 146 Patients. J Gastrointest Surg 19, 1476–1483 (2015). https://doi.org/10.1007/s11605-015-2845-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2845-9