Abstract
Background
The 2012 Sendai Criteria recommend that patients with 3 cm or larger branch duct intraductal papillary mucinous neoplasms (BD-IPMN) without any additional “worrisome features” or “high-risk stigmata” may undergo close observation. Furthermore, endoscopic ultrasound (EUS) is not recommended for BD-IPMN <2 cm. These changes have generated concern among physicians treating patients with pancreatic diseases. The purposes of this study were to (i) apply the new Sendai guidelines to our institution’s surgically resected BD-IPMN and (ii) reevaluate cyst size cutoffs in identifying patients with lesions harboring high-grade dysplasia or invasive cancer.
Methods
We retrospectively reviewed 150 patients at a university medical center with preoperatively diagnosed and pathologically confirmed IPMNs. Sixty-six patients had BD-IPMN. Pathologic grade was dichotomized into low-grade (low or intermediate grade dysplasia) or high-grade/invasive (high-grade dysplasia or invasive cancers). Fisher’s exact test, chi-square test, student’s t test, linear regression, and receiver operating characteristic (ROC) analyses were performed.
Results
The median BD-IPMN size on imaging was 2.4 cm (interquartile range 1.5–3.0). Fifty-one (77 %) low-grade and 15 (23 %) high-grade/invasive BD-IPMN were identified. ROC analysis demonstrated that cyst size on preoperative imaging is a reasonable predictor of grade with an area under the curve of 0.691. Two-thirds of high-grade/invasive BD-IPMN were <3 cm (n = 10). Compared to a cutoff of 3, 2 cm was associated with higher sensitivity (73.3 vs. 33.3 %) and negative predictive value (83.3 vs. 80 %, NPV) for high-grade/invasive BD-IPMN. Mural nodules on endoscopic ultrasound (EUS) or atypical cells on endoscopic ultrasound-fine needle aspiration (EUS-FNA) were identified in all cysts <2 and only 50 % of those <3 cm. Forty percent of cysts >3 cm were removed based on size alone.
Discussion/Conclusions
Our results suggest that “larger” size on noninvasive imaging can indicate high-grade/invasive cysts, and EUS-FNA may help identify “smaller” cysts with high-grade/invasive pathology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intraductal papillary mucinous neoplasms (IPMN) are mucin-producing cystic lesions of the pancreas that originate from the ductal epithelium. They occur in 4.35 per 100,000 individuals.1 Over time, IPMNs can progress from early-stage lesions with low-grade dysplasia to more advanced pathology with high-grade dysplasia or invasive ductal adenocarcinoma.2 – 5 The cyst characteristics on noninvasive imaging (CT or MRI)6 – 9 and endoscopic ultrasound (EUS)10 – 13 associated with high-grade dysplasia or invasive cancer as well as the optimal indications for surgical intervention have been debated since they were originally described in the 1980s.14 – 22
The primary classification scheme for IPMN is centered on the distinction between main duct (MD-IPMN) and branch duct (BD-IPMN) types. MD-IPMN are cystic lesions with dilatation of the main pancreatic duct. The incidence of cancer in surgically resected MD-IPMN ranges from 41 to 60 %.23 – 25 In contrast, BD-IPMN do not involve the main pancreatic duct. The incidence of cancer in resected BD-IPMN ranges from 11 to 30 %.24 – 26
International consensus guidelines were developed in Sendai, Japan in 2005 (and published in 2006) to help guide the management of patients with IPMN.20 Due to the high incidence of high-grade dysplasia or invasive cancer in resected MD-IPMN, the original guidelines recommended that all cysts associated with a main pancreatic duct ≥1 cm in diameter be resected at the time of diagnosis. In contrast, surgical resection was only recommended for BD-IPMN, which were associated with symptoms, contained mural nodules, or had malignant cells on cyst fluid analysis. Interestingly, the 2006 consensus statement called for additional investigation into the correlation between cyst size and advanced pathology, particularly those ≥3 cm, before a strong recommendation could be made on the management of cysts based on their size. Due to this uncertainty, most physicians recommended surgical resection for BD-IPMN ≥3 cm until more information was available.
In response to numerous studies published over the ensuing 6 years testing the ability of the 2006 Sendai Criteria to identify cysts with advanced pathology,27 – 30 the guidelines were revised in 2012.22 In brief, the new guidelines expand the preoperative imaging criteria of MD-IPMN from cysts with a main duct diameter ≥1 cm to also include those with a duct diameter ≥5 mm (in the absence of other causes of duct obstruction). Similar to the original version, the 2012 Sendai Criteria recommend that cysts with “high-risk stigmata” (HRS), defined as those that cause biliary obstruction, contain an enhancing solid component, or main pancreatic duct diameter ≥1 cm, be surgically resected. Additionally, the new guidelines include a new category of “worrisome features” (WF), which include cyst size ≥3 cm, thickened or enhancing cyst wall, nonenhancing mural nodules, abrupt change in main pancreatic duct caliber with distal pancreatic atrophy, lymphadenopathy, or main pancreatic duct size of 5–9 mm. Cysts with WF identified on noninvasive imaging are recommended to be further evaluated with EUS.
Notably, as compared to the 2006 version, the 2012 Sendai Criteria place less emphasis on cyst size. They suggest that patients with cysts ≥3 cm without WF or HRS can potentially undergo observation. Furthermore, they do not recommend evaluation of cysts without WF by EUS until they reach 2 cm on CT/MRI. These changes have generated concern among physicians treating patients with pancreatic cystic lesions as there is a growing body of evidence showing that even small (<3 cm) cysts without WF or HRS can harbor high-grade pathology31 – 33 and that CT/MRI is not sensitive at identifying these features, thus suggesting EUS be included in evaluating smaller (<2 cm) cysts.
Therefore, the aims of this study were to examine our institution’s experience with surgically resected BD-IPMN to determine the (i) association between preoperative imaging cyst size and high-grade dysplasia or invasive cancer and (ii) sensitivity of the new Sendai guidelines for identifying cysts with advanced pathology.
Methods
Data Source and Study Subjects
A total of 1482 patients who underwent pancreatic resection between July 1996 and June 2012 at the University of California, Los Angeles Medical Center, a university-based, tertiary care medical center, were identified in a prospectively maintained database. Patients were included if they had a preoperative diagnosis of a pancreatic cystic lesion on noninvasive imaging that was highly suspicious for IPMN and underwent surgical resection with pathologic confirmation. Those who underwent pancreatic resection for another indication and had an incidentally identified IPMN remote from the index lesion on surgical pathology were excluded. Using these criteria, 150 patients were identified with a preoperative diagnosis of IPMN. MD- and BD-IPMN were distinguished using the 2012 Sendai Guidelines on preoperative imaging. MD-IPMN were defined as those with dilated main pancreatic duct (MPD) >5 mm on CT/MRI or EUS without other causes of obstruction. IPMN with features of both MD- and BD-IPMN (mixed type) were also grouped with the MD-IPMNs, as these all contained MPD >5 mm which categorizes them as MD-IPMN in the 2012 Sendai Criteria.22 BD-IPMN was reserved for the remaining patients. Using this schema, 84 MD- and 66 BD-IPMNs were identified in our series; the latter are the focus of this study.
Data Acquisition
Data was extracted from the patients’ electronic medical records. Data collected included demographics, preoperative symptoms and risk factors, imaging and biopsy results, operative information, surgical pathology, and postoperative outcomes. For patients (n = 15) who did not have a radiographic cyst size reported, pathologic size was used, as radiographic and pathologic size had a high correlation (R = 0.905) in patients who had both.
Statistical Analyses
Data preparation and analysis was performed with SPSS Statistics 20 (IBM, Somers, NY). For our analysis, we dichotomized pathologic grade into low-grade (low or intermediate grade dysplasia) or high-grade/invasive (high-grade dysplasia or invasive cancer) lesions. Fisher’s exact test was applied to the analysis of dichotomized categorical data sets. Pearson chi-square test was applied to data sets of more than two categories. Student’s t test was performed on continuous dichotomized quantitative data. Linear regression analysis was performed to determine the strength of correlation of imaging size to pathologic size. A multivariate logistic analysis model was performed to assess the association of risk factors nearing statistical significance upon odds of high-grade pathology. Receiver operating characteristic (ROC) analysis was generated to determine optimal size cutoff by pairing imaging size against high pathologic grade. p < 0.05 was considered statistically significant.
Results
Clinicopathologic Characteristics for All Patients
The clinicopathologic characteristics for all 66 BD-IPMN patients are listed in Table 1. The median age was 69 years (interquartile range (IQR) 58–75), and there were 42 (64 %) females.
All patients underwent an abdominal CT or MRI. EUS was performed on 62 (93 %) patients with 49 (74 %) also undergoing an EUS-guided FNA. Most cysts were identified incidentally on noninvasive imaging or as a workup for abdominal pain (43.9 and 37.9 %, respectively) and located in the head of the gland (n = 48, 72.7 %). Median cyst size on preoperative imaging was 2.4 cm (IQR 1.5–3.0). At surgery, the majority of patients underwent a Whipple procedure (71.2 %), while fewer had a distal, middle, or total pancreatectomy (18.2, 6.1, or 4.5 %, respectively).
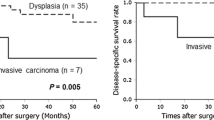
On pathologic analysis, there were 51 (77 %) low-grade (74.2 % low and 3 % intermediate grade dysplasia) and 15 (23 %) high-grade/invasive (16.7 % high-grade dysplasia and 6.1 % invasive cancer) lesions (Fig. 1).
Clinicopathologic Characteristics of Low-Grade vs. High-Grade/Invasive BD-PMN
Notably, a significantly greater percentage of low-grade IPMNs were removed from females than high-grade/invasive ones (71 vs. 40 %, respectively, p = 0.038; Table 1). High-grade/invasive IPMNs more frequently occurred in the head of the pancreas (86.7 vs. 68.6 %) and were associated with higher preoperative serum bilirubin levels (1.65 vs. 0.70 mg/dL, p = 0.125) compared to low-grade IPMNs.
Cyst Characteristics Predictive of High-Grade Pathology
We analyzed the ability of the 2012 Sendai “worrisome features” to predict high-grade/invasive BD-IPMN (Table 2). This revealed that two-thirds of high-grade/invasive IPMN were <3 cm on preoperative imaging (n = 10/15; Table 2). The sensitivity and specificity of mural nodules identified on CT/MRI or EUS for high-grade/invasive pathology were 41 (5/12) and 72 % (37/51), respectively. The sensitivity and specificity of atypical or malignant cells seen on FNA cytology for high-grade/invasive IPMN were 45 (5/11) and 84 % (32/38), respectively.
Multivariate Analysis of Factors Predictive of High-Grade Pathology
We next utilized a logistic regression model to determine the association of a combination of risk factors nearing statistical significance to high-grade pathology (Table 3). Interestingly, while the significance of sex, tobacco history, and weight loss appeared to be weakened, the association of atypical or malignant cells on FNA appeared to be enhanced in the multivariate analysis (OR 7.316, p = 0.038).
Comprehensive Analysis of Pathology Stratified on Cyst Size
A receiver operating characteristic (ROC) analysis revealed that cyst diameter (i.e., cyst size) on CT/MRI or EUS was a reasonable predictor of pathologic grade with an area under the curve (AUC) of 0.691 (Fig. 2). Therefore, a comprehensive sensitivity and specificity analysis of radiographic cyst size on pathologic grade was performed.
The sensitivities, specificities, and negative predictive values (NPVs) across various cyst size cutoffs for high-grade/invasive pathology are listed in Table 4 and graphically depicted in Fig. 3. The sensitivity and specificity curves intersect at a cutoff between 2 and 2.5 cm. The sensitivities and NPV for cutoffs of 2 or 2.5 cm are 73.3 and 83.3, or 53.3 and 83.3, respectively. Moreover, the NPV for all size cutoffs less than 3 cm are greater than the 3 cm emphasized in the 2006 and 2012 Sendai Criteria.
Only 50 % (5/10) of the <3 cm cyst group with high-grade/invasive pathology had WF or HRS (solid component/mural nodule or atypical FNA cytology; Table 5). The decision was made to resect the other cysts due to abdominal pain (n = 4) or patient anxiety (n = 1). Of smaller cysts, 71 % (5/7) of cysts <2.5 cm and 100 % (4/4) of cysts <2 cm had WF, HRS, or suspicious results on EUS-FNA. Interestingly, while only 25 % (1/4) of resected high-grade/invasive cysts <2 cm had mural nodules detected on EUS, 100 % (4/4) had atypical FNA cytology (Table 4). All of the <2 cm cysts occurred in patients who were asymptomatic. Moreover, 2 of the 5 (40 %) cysts >3 cm were removed based on size alone (i.e., not associated with symptoms or WF or HRS).
Discussion
Since their original description in the 1980s,34 the prevalence of dysplasia or invasive cancer in pathologically confirmed MD- or BD-IPMNs has become well-defined. However, the ability to accurately identify cysts with high-grade/invasive pathology preoperatively remains a challenge. The Sendai Consensus Guidelines, first published in 2006 and later revised in 2012, were thus developed by incorporating well-performed retrospective studies and expert opinions in order to aid in the management of patients with pancreatic cysts.20 , 22
Cyst size is a key feature highlighted in each of the Sendai versions. In 2006, it was suggested that resection in patients with BD-IPMN ≥3 cm was reasonable but additional investigation clarifying the relationship between high-grade/invasive pathology and cyst size was required before firm recommendations could be made. In response to studies published over the ensuing 6 years, the subsequent 2012 Guidelines called for observation of BD-IPMN ≥3 cm without WF or HRS. In our study, we found a ROC AUC of 0.691 for cyst size and pathologic grade (Fig. 2). This suggests a modest relationship between these cyst features. By this analysis, we found that the 3-cm size cutoff highlighted in both editions of the consensus guidelines was not optimal in our patient population. Rather, cyst size cutoffs of 2 or 2.5 cm had higher sensitivities (73.3 and 53.3 %) and NPVs (83.3 % for both) than the Sendai cutoff of 3 cm (33.3 and 80 %, respectively; Table 3). While cyst size is no longer an absolute indication for surgery, these data suggest that this characteristic still has a reasonable association with grade and should be considered when making treatment decisions.
Moreover, the 2012 Sendai Criteria also suggest that cysts <2 cm without WF or HRS on noninvasive imaging can be observed with CT/MRI at various intervals. EUS is only recommended for those BD-IPMN ≥2 cm. This change is also concerning, as the sensitivity of CT/MRI to identify mural nodules is only 24–62 %.35 , 36 In our series, only 25 % (1/4) of high-grade/invasive BD-IPMN <2 cm had nodularity detected on either EUS or CT/MRI. However, 100 % (4/4) of these cysts had atypical cells on FNA. EUS alone without FNA would have failed to identify the 3 of 4 patients with <2 cm cysts with high-grade/invasive pathology. All 4 of these patients were asymptomatic. Therefore, EUS with FNA helped determine the treatment course for these patients, especially those with smaller cysts.
In 2013, Sahora et al. critically evaluated the change in Sendai criteria in a prospective study of patients treated for IPMN and found that high-grade/invasive cysts were larger than low-grade ones. Cyst size cutoff for surgical resection was important to identify 18 % of larger high-grade/invasive cysts not identified by additional Sendai criteria.37 In our series, we similarly found a trend towards larger median cyst size for high-grade/invasive vs. low-grade BD-IPMN (2.7 vs. 2.3 cm). Further, we found by ROC analysis (Fig. 2) that cyst size was a reasonable predictor of pathology. There were two of five cysts >3 cm with high-grade dysplasia or invasive cancer resected on the basis of size alone. However, this association incompletely identified smaller high-grade/invasive cysts. While cyst size may remain valuable for identifying a large proportion of high-grade/invasive lesions, there remain smaller cysts that are unidentified by size cutoffs alone.
Multiple recent studies have also highlighted the existence of many small, high-grade/invasive BD-IPMN. Jang et al. found that a 2-cm threshold for surgical resection yielded maximum sensitivity and specificity of 68 % and 60 %, respectively.33 Schmidt et al. found that malignant IPMNs were frequent in smaller lesions with a mean cyst size of 2 cm.31 Fritz et al. similarly found that in “Sendai Negative” BD-IPMN, about half were <3 cm and a quarter of these smaller lesions were of high pathologic grade.32 In our series, two-thirds of high-grade/invasive IPMN were <3 cm (Table 5). Furthermore, 50 % of these “small” lesions did not have any other Sendai 2012 WF or HRS. These patients underwent surgical resection due to associated symptoms and anxiety about the uncertain pathologic diagnosis of the cyst. A size cutoff of 2 cm was associated with the highest sensitivity and NPV for high-grade/invasive pathology. Interestingly, all size cutoffs for resection less than the 3 cm had higher NPV than the 3-cm value.
Whether or not patient clinical information can also be used to identify high-grade/invasive cysts and assist in patient management has also been the subject of much debate. Most clinicians believe that cysts which cause symptoms (“symptomatic cysts”) should be surgically resected,38 as the symptoms may be burdensome to the patient and also an indicator of high-grade or invasive lesions.27 , 39 , 40 Moreover, the 2006 and 2012 Sendai Criteria also recommend that symptomatic cysts be resected. In our series, despite the finding that patients presenting with associated symptoms were equally likely to have low-grade or high-grade/invasive IPMNs, four of the five cysts <3 cm without other WF or HRS were resected because they were associated with abdominal pain. Therefore, our data further support the Sendai guidelines, which recommend surgical resection for cysts associated with patient symptoms.
Taken together, our results suggest that “larger” size on noninvasive imaging can indicate high-grade/invasive cysts, and EUS-FNA may help identify “smaller” cysts with high-grade/invasive pathology.
Abbreviations
- IPMN:
-
Intraductal papillary mucinous neoplasms
- BD-IPMN:
-
Branch duct intraductal papillary mucinous neoplasms
- MD-IPMN:
-
Main duct intraductal papillary mucinous neoplasms
- HRS:
-
High-risk stigmata
- WF:
-
Worrisome features
- ROC:
-
Receiver operating characteristic
- NPV:
-
Negative predictive value
- EUS:
-
Endoscopic ultrasound
- FNA:
-
Fine needle aspiration
- IQR:
-
Interquartile range
- AUC:
-
Area under the curve
References
Klibansky DA, Reid-Lombardo KM, Gordon SR, Gardner TB. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clinical Gastroenterology and Hepatology 2012;10(5):555–8.
Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Annals of Surgery 2004;239(6):788–97; discussion 97–9.
Lafemina J, Katabi N, Klimstra D, Correa-Gallego C, Gaujoux S, Kingham TP, et al. Malignant progression in IPMN: a cohort analysis of patients initially selected for resection or observation. Annals of Surgical Oncology 2013;20(2):440–7.
Biankin AV, Kench JG, Biankin SA, Lee CS, Morey AL, Dijkman FP, et al. Pancreatic intraepithelial neoplasia in association with intraductal papillary mucinous neoplasms of the pancreas: implications for disease progression and recurrence. The American Journal of Surgical Pathology 2004;28(9):1184–92.
Bassi C, Sarr MG, Lillemoe KD, Reber HA. Natural history of intraductal papillary mucinous neoplasms (IPMN): current evidence and implications for management. Journal of Gastrointestinal Surgery 2008;12(4):645–50.
Waters JA, Schmidt CM, Pinchot JW, White PB, Cummings OW, Pitt HA, et al. CT vs MRCP: optimal classification of IPMN type and extent. Journal of Gastrointestinal Surgery 2008;12(1):101–9.
Kawamoto S, Lawler LP, Horton KM, Eng J, Hruban RH, Fishman EK. MDCT of intraductal papillary mucinous neoplasm of the pancreas: evaluation of features predictive of invasive carcinoma. American Journal of Roentgenology 2006;186(3):687–95.
Nakagawa A, Yamaguchi T, Ohtsuka M, Ishihara T, Sudo K, Nakamura K, et al. Usefulness of multidetector computed tomography for detecting protruding lesions in intraductal papillary mucinous neoplasm of the pancreas in comparison with single-detector computed tomography and endoscopic ultrasonography. Pancreas 2009;38(2):131–6.
Gupta R, Mortele KJ, Tatli S, Girshman J, Glickman JN, Levy AD, et al. Pancreatic intraductal papillary mucinous neoplasms: role of CT in predicting pathologic subtypes. American Journal of Roentgenology 2008;191(5):1458–64.
Kamata K, Kitano M, Kudo M, Sakamoto H, Kadosaka K, Miyata T, et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy 2014;46(1):22–9.
Pais SA, Attasaranya S, Leblanc JK, Sherman S, Schmidt CM, DeWitt J. Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: correlation with surgical histopathology. Clinical Gastroenterology and Hepatology 2007;5(4):489–95.
Emerson RE, Randolph ML, Cramer HM. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of intraductal papillary mucinous neoplasm of the pancreas is highly predictive of pancreatic neoplasia. Diagnostic Cytopathology 2006;34(7):457–62.
Ohno E, Hirooka Y, Itoh A, Ishigami M, Katano Y, Ohmiya N, et al. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Annals of Surgery 2009;249(4):628–34.
Milchgrub S, Campuzano M, Casillas J, Albores-Saavedra J. Intraductal carcinoma of the pancreas. Cancer 1992;69(3):651–6.
Rickaert F, Cremer M, Deviere J, Tavares L, Lambilliotte JP, Schroder S, et al. Intraductal mucin-hypersecreting neoplasms of the pancreas. A clinicopathologic study of eight patients. Gastroenterology 1991;101(2):512–9.
Loftus EV, Jr., Olivares-Pakzad BA, Batts KP, Adkins MC, Stephens DH, Sarr MG, et al. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Members of the Pancreas Clinic, and Pancreatic Surgeons of Mayo Clinic. Gastroenterology 1996;110(6):1909–18.
Jang JY, Kim SW, Ahn YJ, Yoon YS, Choi MG, Lee KU, et al. Multicenter analysis of clinicopathologic features of intraductal papillary mucinous tumor of the pancreas: is it possible to predict the malignancy before surgery? Annals of Surgical Oncology 2005;12(2):124–32.
Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Annals of Surgery 1998;228(5):685–91.
Allen PJ, Jaques DP, D’Angelica M, Bowne WB, Conlon KC, Brennan MF. Cystic lesions of the pancreas: selection criteria for operative and nonoperative management in 209 patients. Journal of Gastrointestinal Surgery 2003;7(8):970–7.
Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006;6(1–2):17–32.
Tang RS, Weinberg B, Dawson DW, Reber H, Hines OJ, Tomlinson JS, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clinical Gastroenterology and Hepatology 2008;6(7):815–9; quiz 719.
Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12(3):183–97.
Salvia R, Fernandez-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Annals of Surgery 2004;239(5):678–85; discussion 85–7.
Suzuki Y, Atomi Y, Sugiyama M, Isaji S, Inui K, Kimura W, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas 2004;28(3):241–6.
Crippa S, Partelli S, Falconi M. Extent of surgical resections for intraductal papillary mucinous neoplasms. World journal of gastrointestinal surgery 2010;2(10):347–51.
Kanno A, Satoh K, Hirota M, Hamada S, Umino J, Itoh H, et al. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. Journal of Gastroenterology 2010;45(9):952–9.
Moriya T, Hashimoto Y, Traverso LW. The duration of symptoms predicts the presence of malignancy in 210 resected cases of pancreatic intraductal papillary mucinous neoplasms. Journal of Gastrointestinal Surgery 2011;15(5):762–70; discussion 70–1.
Tanaka M. Controversies in the management of pancreatic IPMN. Nature Reviews Gastroenterology & Hepatology 2011;8(1):56–60.
Nagai K, Doi R, Kida A, Kami K, Kawaguchi Y, Ito T, et al. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World Journal of Surgery 2008;32(2):271–8; discussion 9–80.
Yang AD, Melstrom LG, Bentrem DJ, Ujiki MB, Wayne JD, Strouch M, et al. Outcomes after pancreatectomy for intraductal papillary mucinous neoplasms of the pancreas: an institutional experience. Surgery 2007;142(4):529–34; discussion 34–7.
Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Annals of Surgery 2007;246(4):644–51; discussion 51–4.
Fritz S, Klauss M, Bergmann F, Hackert T, Hartwig W, Strobel O, et al. Small (Sendai negative) branch-duct IPMNs: not harmless. Annals of Surgery 2012;256(2):313–20.
Jang JY, Kim SW, Lee SE, Yang SH, Lee KU, Lee YJ, et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Annals of Surgical Oncology 2008;15(1):199–205.
Ohashi K, Murakami Y, Maruyama M. Four cases of mucin-producing cancer of the pancreas on specific findings of the papilla of Vater. Prog Dig Endoscopy 1982(20):348–51.
Zhong N, Zhang L, Takahashi N, Shalmiyev V, Canto MI, Clain JE, et al. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clinical Gastroenterology and Hepatology 2012;10(2):192–8, 8 e1-2.
Hirano S, Kondo S, Tanaka E, Shichinohe T, Suzuki O, Shimizu M, et al. Role of CT in detecting malignancy during follow-up of patients with branch-type IPMN of the pancreas. Hepatogastroenterology 2009;56(90):515–8.
Sahora K, Mino-Kenudson M, Brugge W, Thayer SP, Ferrone CR, Sahani D, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Annals of Surgery 2013;258(3):466–75.
Traverso LW, Moriya T, Hashimoto Y. Intraductal papillary mucinous neoplasms of the pancreas: making a disposition using the natural history. Current Gastroenterology Reports 2012;14(2):106–11.
Lee JH, Lee KT, Park J, Bae SY, Lee KH, Lee JK, et al. Predictive factors associated with malignancy of intraductal papillary mucinous pancreatic neoplasms. World Journal of Gastroenterology 2010;16(42):5353–8.
Lubezky N, Ben-Haim M, Nakache R, Lahat G, Blachar A, Brazowski E, et al. Clinical presentation can predict disease course in patients with intraductal papillary mucinous neoplasm of the pancreas. World Journal of Surgery 2010;34(1):126–32.
Funding/Support
Dr. Nguyen was supported by a T32 Training Award from the National Institute of Health (NIH T32DK07180-39) and the Gerald S. Levey Surgical Research Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, A.H., Toste, P.A., Farrell, J.J. et al. Current Recommendations for Surveillance and Surgery of Intraductal Papillary Mucinous Neoplasms May Overlook Some Patients with Cancer. J Gastrointest Surg 19, 258–265 (2015). https://doi.org/10.1007/s11605-014-2693-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2693-z