Abstract
Purpose
The purpose of this planning study was to develop an acceptable technique for highly hypofractionated intensity-modulated radiation therapy using simultaneous integrated boost technique (SIB-hHF-RT) for nonmetastatic National Comprehensive Cancer Network high-risk prostate cancer.
Materials and methods
We created SIB-hHF-RT plans for 14 nonmetastatic prostate cancer patients with MRI-detectable intraprostatic lesions (IPLs) and without intestines locating close to the seminal vesicle and prostate. We prescribed 57 Gy for IPLs and 54 Gy for the remainder of planning target volume (PTV) in 15 fractions. The IPLs were contoured based on magnetic resonance imaging, and PTV was generated by adding 6–8-mm margins to the clinical target volume. For the dose-volume constraints of organs at risk (OARs), the same constraints as 54 Gy plans were used so as not to increase the toxicity.
Results
All created plans fulfilled the dose-volume constraints of all targets and OARs. The median estimated beam-on time was 108.5 s. For patient-specific quality assurance, the global gamma passing rates (3%/2 mm) with 10% dose threshold criteria were greater than 93% in all cases and greater than 95% in 11 cases.
Conclusion
SIB-hHF-RT plans were developed that fulfill the acceptable dose-volume constraints and pass patient-specific quality assurance. We believe these plans can be applied to selected patients with nonmetastatic prostate cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is one of the most common cancer types among men [1], and external beam radiation therapy (EBRT) is a major treatment modality for nonmetastatic prostate cancer. Conventionally, EBRT of up to 78 Gy is administered in 2-Gy fractions for 8 weeks [2,3,4,5], but recently, hypofractionated radiation therapy (HF-RT) has been administered because of the low α/β value of prostate cancer [6]. HF-RT has been intensively studied in nonmetastatic prostate cancer, and guidelines for HF-RT have been issued by the American Society of Clinical Oncology, the American Urological Association, and the American Society for Radiation Oncology [7]. Previously, we performed a pilot study of highly hypofractionated radiation therapy (hHF-RT) using 54 Gy in 15 fractions (3.6 Gy per fraction) over 3 weeks, of which fractionation is in the gap between that of moderately HF-RT and of ultrahypofractionated RT or stereotactic body radiation therapy (SBRT) for the National Comprehensive Cancer Network (NCCN) for very low- to unfavorable intermediate-risk prostate cancer [8]. The tumor equivalent dose for this prescription was approximately equal to 78 Gy in 39 fractions based on an assumption that the α/β ratio for prostate cancer is 1.5 Gy. This study showed good tumor control without severe toxicity at 2 years, which is comparable to the outcomes of conventional fractionated intensity-modulated radiation therapy (IMRT).
However, additional doses may be necessary to control NCCN high-risk prostate cancer [9]. Zaorsky et al. suggested that a biologically effective dose (BED) based on an estimated α/β ratio for prostate cancer of 1.5 Gy (BED1.5) to 200 Gy was associated with increased prostate cancer control, while a dose of more than 200 Gy would not have additional clinical benefit. The BED1.5 of 54 Gy in 15 fractions was 183.6 Gy; if the irradiation plans finished in 15 fractions, 57 Gy would be necessary to achieve a BED1.5 of 200 Gy (BED1.5 201.4 Gy). Meanwhile, ultrahigh dose irradiation increases gastrointestinal (GI) and genitourinary (GU) toxicity if delivered as EBRT to the whole prostate [10,11,12]. Therefore, a boost to a small part of the prostate was considered an option to increase the radiation dose [10]. Local recurrence of prostate cancer after radiation therapy usually occurs at the same site where intraprostatic lesions (IPLs) exist at baseline [13]. This supports the usefulness of boosting the biological target volume (BTV), which was derived from biological images made using magnetic resonance imaging (MRI) and positron emission tomography (PET) [14]. In 2018, a systematic review of treatment with focal dose escalation for IPLs was published, which found favorable biochemical control and acceptable toxicity [15]. Several reports have discussed boosting IPLs in SBRT or conventionally fractionated radiation therapy with or without brachytherapy. However, to the best of our knowledge, there is no report of boosting IPLs in hHF-RT using the simultaneous integrated boost (SIB) technique.
We conducted a planning study for prostate cancer patients to develop an acceptable SIB-hHF-RT plan in which 57 Gy in 15 fractions (3.8 Gy per fraction) was prescribed for IPLs. Moreover, we used non-coplanar IMRT to decrease the dose to organs at risk (OARs) [16, 17].
Materials and methods
This study was conducted in accordance with the Declaration of Helsinki, and our institutional ethical review board approved the research (approval numbers C1236 and R1048). Informed consent was obtained from all individual participants included in the study.
Study population
At our institution, a total of 23 patients underwent computed tomography (CT) simulation for nonmetastatic prostate cancer between March and August 2017. The period was determined because we estimated that immediate 6 months will be enough to pick up sufficient cases with various IPL characteristics. Of these patients, six had no identifiable IPLs based on MRI before the therapeutic intervention, and three had intestines close to the seminal vesicle and prostate that were considered difficult to spare from high-dose irradiation. These patients were excluded; therefore, the data from the remaining 14 patients were included in this study, and plans were created using their simulation CT. Patient characteristics are listed in Table 1 and IPL characteristics in Table 2. Because this is a planning study, we included all patients with identifiable IPLs in this study.
CT simulation
Patients were instructed to void the urinary bladder and rectum 1–1.5 h before CT simulation and were then prohibited from voiding until the end of the simulation [18]. CT simulation was performed in the supine position using a BodyFIX vacuum cushion (Elekta, Stockholm, Sweden). CT images were acquired using a LightSpeed RT scanner (GE Healthcare, Little Chalfont, UK) with a 2.5-mm slice thickness or a Siemens Somatom Definition (Siemens, Erlangen, Germany) with a 2.0-mm slice thickness.
Target and OAR delineation
The targets and OARs are shown in Fig. 1. The total volume of IPLs was contoured as the BTV in the simulation CT images according to the diffusion-weighted images (DWI) at b values of ≥ 500 s/mm2 and the T2-weighted images (T2WI) of the pretreatment MRI with ≤ 5 mm thickness. Because the prostate volume changed between the pretreatment MRI and simulation CT for patients receiving neoadjuvant androgen deprivation therapy, the BTV was contoured using an empirical algorithm, which is mapping algorithm based on the assumption that the prostate deformation is directly proportional in each direction [19]. The clinical target volume (CTV) was defined as the total volume of the prostate and proximal portion of the seminal vesicles. In patients with low- or intermediate-risk prostate cancer as determined by the D’Amico risk group [20], the CTV included the base of the seminal vesicles. In patients with prostate cancer with seminal vesicle invasion, the CTV included all seminal vesicles; in the other patients, the CTV included two-thirds of the proximal seminal vesicles. The planning target volume (PTV) was generated by adding an 8 mm margin elsewhere except for the posterior, where a 6 mm margin was applied, to the CTV. If a planner in charge judged it necessary, manual modifications of reducing the posterior margin to 5 mm were allowed to preserve the rectum from receiving an excessively high dose. The boost volume (PTV-boost) was created by adding isotropic 3-mm margins around the BTV [21], and the PTV-boost was subtracted from the PTV to define the volume in which 54 Gy was prescribed (PTV_54Gy). For OARs, the bladder, rectum, small intestine, and sigmoid colon were contoured. We used an inner bladder wall thickness of 4 mm for the bladder dose evaluation. For the rectal dose, we used an inner rectal wall thickness of 4 mm, ranging from 10 mm below the apex of the prostate to 10 mm above the tips of the seminal vesicles. The CTV, PTV, and OARs had been created using Eclipse version 13.6 (Varian Medical Systems, Palo Alto, CA) for prior radiation therapy, and the BTV, PTV-boost, and PTV_54Gy were newly generated using RayStation version 4.7 (RaySearch Laboratories, Stockholm, Sweden).
The structure delineation and dose distribution in a representative case. a–c The targets and organs at risk (OARs) in the axial, sagittal, and coronal planes, respectively. Each colored line defines the targets and OARs. Cyan biological target volume (BTV), red clinical target volume (CTV), orange planning target volume (PTV), purple PTV-boost, blue bladder wall (4 mm thickness), brown rectum wall (4 mm thickness). d Axial plane of dose distribution
Radiation treatment
We used a Vero4DRT System (Mitsubishi Heavy Industries, Ltd., Tokyo, Japan, and Brainlab, Munich, Germany) with 30 pairs of 5-mm-thick multileaf collimator (MLC) to administer non-coplanar IMRT. The Vero4DRT System has a gantry mounted within the O-ring structure and can rotate the gantry and O-ring simultaneously, delivering radiation therapy with a non-coplanar arc without rotating the couch, which is known as dynamic wave arc (DWA) [22]. This technique can be combined with intensity modulation and volumetric-modulated dynamic wave arc therapy (VMDWAT). VMDWAT plans were created using a single non-coplanar arc trajectory (Fig. 2). The arc trajectory was divided into four segments. The O-ring rotated from 20° to 0°, 0° to 335°, 335° to 25°, and 25° to 340° during the first, second, third, and fourth segments, and the gantry rotated clockwise from 182° to 234°, 234° to 314°, 314° to 42°, and 42° to 178° in each segment, respectively. Treatment planning was performed using RayStation version 4.7. In the plan, 6-MV photon beams and the collapsed cone dose calculation algorithm (version 3.1) was used with a calculation grid size of 2.0 mm. In the current study, 54 Gy in 15 fractions (3.6 Gy per fraction) was delivered to the PTV_54Gy and 57 Gy (3.8 Gy per fraction) to the PTV-boost simultaneously. Referring to the dose-volume indices of prior pilot study [8], new dose-volume constraints were defined as shown in Table 3. The dose-volume constraints of OARs were the same as those used in the prior study to avoid increases in GI and GU toxicity. DX% was defined as the dose to X% of the target or OAR volume, and VXGy was defined as the volume receiving at least X Gy. For patient-specific quality assurance (QA), the global gamma passing rates (3%/2 mm) with 10% dose threshold criteria between the measured or reconstructed dose distributions and planned dose distributions were evaluated using ArcCHECK (Sun Nuclear Corp., Melbourne, FL, USA) [23].
Results
The mean volumes ± standard deviation (SD) of the BTV, CTV, PTV, and PTV-boost were 2.40 ± 2.31, 25.64 ± 9.20, 78.37 ± 23.66, and 6.66 ± 5.15 mL, respectively. The axial plane of the dose distribution in a representative case is shown in Fig. 1.
Table 4 summarizes the dose-volume indices of the targets. One patient had a smaller D95% value of the PTV-boost than the ideal index; another patient had a greater D2% value of the PTV_54Gy, and four patients (28.6%) had smaller D95% values of the PTV. However, all plans had allowable indices for the targets.
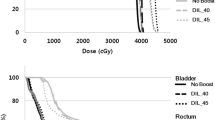
In all 14 plans, the dose indices of the OARs fulfilled the ideal dose constraints, although in some cases, the BTV was located next to the rectum. The dose-volume histograms of the bladder wall and rectum wall are shown in Fig. 3. All dose-volume constraints for the bladder wall and rectum wall were achieved in all cases. The constraints for the small intestine and sigmoid colon were also achieved in all cases (V45Gy of the small intestine: at most 0.03 mL, V48Gy of the sigmoid colon: 0 mL in all cases).
The median estimated delivery time was 108.5 s (range 104–126 s). For patient-specific QA, the mean gamma passing rates (3%/2 mm) ± SD were 95.9 ± 1.6%, greater than 93% in all cases, and greater than 95% in 11 cases.
Discussion
The purpose of this study was to develop an acceptable SIB-hHF-RT plan for nonmetastatic prostate cancer in which 57 Gy in 15 fractions was prescribed to the BTV and 54 Gy in 15 fractions was prescribed to other regions of the PTV using the SIB technique. To our knowledge, this is the first study of SIB-hHF-RT that boosts BTV.
The concept of the BTV was first suggested in 2000 [14], but it was considered difficult to achieve a BTV boost at that time, because it was difficult to reproduce the BTV position with skin markings or bone matching using on-board kV imaging. The BTV position at the time of treatment often deviates from the position in the simulation CT due to interfractional error derived from the amount of urine and rectal gas present (e.g., [24]). Recently, cone-beam computed tomography (CBCT) on a linear accelerator has become more common, and organ-matching using CBCT can be widely implemented. This technique has made it easier to reproduce the BTV position despite interfractional errors and has made BTV boosting possible.
The delivery of the BTV boost is also difficult due to intrafractional errors. Non-coplanar IMRT decreases the dose delivered to OARs [16, 17] but a lot of time is required to rotate the couch; thus, intrafractional prostate motion and baseline drift increase with time [25], making non-coplanar IMRT difficult to use in radiation therapy for prostate cancer with a BTV boost. However, VMDWAT using the Vero4DRT System, allows sequential non-coplanar trajectories without rotating the couch, by rotating the gantry and O-ring simultaneously. VMDWAT also can shorten the delivery time to ~ 110 s, which is much shorter than that of non-coplanar radiation therapy delivered by rotating the couch. Therefore, we consider that VMDWAT can reduce baseline drift and improve the accuracy of the BTV position. Meanwhile, the Vero4DRT System has only 5-mm MLCs, although treatment machines with thinner MLCs of 3 or 2.5 mm around the center of the field are increasingly being used. However, 5-mm MLCs are acceptable for creating dose painting plans for prostate cancer [26]. The isotropic 3-mm margins around the BTV to determine the PTV-boost were smaller than the margins around the CTV to determine PTV. This is because the dose distribution outside the PTV should be steeply reduced, but the PTV-boost in the PTV and dose distribution outside the PTV-boost was equivalent to the therapeutic dose for prostate cancer.
In the current study, we prescribed 57 Gy in 15 fractions to PTV-boost and 54 Gy in 15 fractions to the other regions of the PTV with hHF-RT. We used hHF-RT, because we think hHF-RT has some advantages over moderately HF-RT or SBRT. The advantage of hHF-RT over moderately HF-RT is the shorter overall treatment time. Although moderately HF-RT takes 4–6 weeks to complete the treatment, hHF-RT is completed over 3 weeks. One of the advantages of hHF-RT over SBRT is its robustness against random errors owing to the higher fraction number of hHF-RT. Another advantage is that hHF-RT does not necessarily require fiducial markers as previously reported [8] owing to its fractionation and short delivery time, the implantation of which is invasive and can delay the start of radiation therapy. SBRT itself takes only 1–2 weeks; however, including the period between implantation of the fiducial marker and the simulation, it takes 3–5 weeks from the implantation to complete the SBRT. This period is almost the same as that for hHF-RT itself. The dose prescription of 57 Gy to PTV-boost and 54 Gy to the other regions of the PTV was based on the following three assumptions. First, prior hHF-RT study that prescribed 54 Gy in 15 fractions using the Vero4DRT System showed good tumor control without severe toxicity at 2 years, which is comparable to outcomes following conventionally fractionated IMRT [8]. Second, as mentioned above, an increase in BED1.5 > 200 Gy was associated with increased prostate cancer control [9]. The BED1.5 of 57 Gy in 15 fractions was 201.4 Gy, achieving BED1.5 > 200 Gy. Third, many patients would not have tolerated an ultrahigh dose if it had been delivered as external beam radiation therapy to the whole of the prostate due to GI and GU toxicity [10,11,12], but a boost to a small part of the prostate was an option to increase the radiation dose [10]. In the current plans, despite a 57-Gy prescription, the dose-volume indices of the OARs were the same as in previous study, which showed no acute rectal or GU toxicity of grade ≥ 3 and late toxicity of grade ≥ 2 [8]. Therefore, the toxicity to the bladder and rectum was presumed to be acceptable. Because of the low α/β value of prostate cancer, the effect of the dose difference between PTV-boost and PTV was comparatively larger than its absolute value in a hypofractionated setting. This is an advantage of HF-RT in prostate cancer.
In these plans, a PTV D95% was difficult to achieve, and 28.6% of the patients (4 of 14 patients) had smaller indices than ideal. However, this was almost equal to that in a prior pilot study (33.3% minor deviation in PTV D95%), and it appears to be unrelated to an increased radiation dose. For patient-specific QA, the American Association of Physicists in Medicine Task Group No. 218 reported that a gamma passing rate of 3%/2 mm and 10% dose threshold should be 95% for the universal tolerance limit and 90% for the universal action limit [27]. In the present study, eleven plans passed the tolerance limit, and the remaining three plans passed the action limit. Therefore, all plans in this study were considered acceptable by these standards.
This study has some limitations. First, we used T2WI and DWI to determine IPLs but did not use PET images. To determine IPLs, some studies have used 11C choline PET [28,29,30,31] or prostate-specific membrane antigen-targeted PET [32] images. However, a combination of T2WI and DWI was used in PI-RADS version 2 to improve the detectability of prostate cancer [33, 34]. We considered the combination of T2WI and DWI as an adequate approach to detect IPLs. Second, it is unclear whether GI or GU toxicity will increase even when using allowable dose-volume constraints. In particular, the urethra is not defined as an OAR and would possibly be irradiated with the boost dose. Although the dose-volume effect on the urethra is unclear, toxicity to the urethra might increase. In addition, clinical impacts of boosting IPLs in very high-risk cases such as T3b are currently unclear. We plan to conduct a prospective clinical trial for NCCN high-risk patients to assess this toxicity and evaluate the clinical outcomes.
In conclusion, SIB-hHF-RT plans for 57 Gy in 15 fractions prescribed to IPLs and 54 Gy in 15 fractions to the remainder of the PTV can be developed without exceeding the dose-volume constraints for OARs stated in a prior pilot study using a 54 Gy prescription. These plans comply with patient-specific QA. We believe that these plans can be applied to patients with nonmetastatic prostate cancer. We have conducted a prospective clinical trial using this SIB-hHF-RT protocol and believe this trial will reveal the clinical significance of SIB-hHF-RT.
References
Center for Cancer Control and Information Services (2018) National Cancer Center. Projected cancer statistics. http://ganjoho.jp/en/public/statistics/short_pred.html. Accessed Mar 2021
Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, et al. Prostate cancer radiation dose response: results of the M.D. anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–105.
Peeters ST, Heemsbergen WD, Koper PC, van Putten WL, Slot A, Dielwart MF, et al. Dose-response in radiotherapy for localized prostate cancer: results of the dutch multicenter randomized phase iii trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–6.
Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the Mrc Rt01 randomised controlled trial. Lancet Oncol. 2014;15:464–73.
Aizawa R, Takayama K, Nakamura K, Inoue T, Yamasaki T, Kobayashi T, et al. Ten-year outcomes of high-dose intensity-modulated radiation therapy for nonmetastatic prostate cancer with unfavorable risk: early initiation of salvage therapy may replace long-term adjuvant androgen deprivation. Int J Clin Oncol. 2019;24:1247–55.
Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–101.
Morgan SC, Hoffman K, Loblaw DA, Buyyounouski MK, Patton C, Barocas D, et al. Hypofractionated radiation therapy for localized prostate cancer: an astro, asco, and aua evidence-based guideline. J Clin Oncol. 2018. https://doi.org/10.1016/j.juro.2018.10.001.
Nakamura K, Ikeda I, Inokuchi H, Takayama K, Inoue T, Kamba T, et al. A pilot study of highly hypofractionated intensity-modulated radiation therapy over 3 weeks for localized prostate cancer. J Radiat Res. 2018;59:656–63.
Zaorsky NG, Palmer JD, Hurwitz MD, Keith SW, Dicker AP, Den RB. What is the ideal radiotherapy dose to treat prostate cancer? A meta-analysis of biologically equivalent dose escalation. Radiother Oncol. 2015;115:295–300.
von Eyben FE, Kiljunen T, Kangasmaki A, Kairemo K, von Eyben R, Joensuu T. Radiotherapy boost for the dominant intraprostatic cancer lesion-a systematic review and meta-analysis. Clin Genitourin Cancer. 2016;14:189–97.
Aluwini S, Pos F, Schimmel E, van Lin E, Krol S, van der Toorn PP, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (Hypro): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol. 2015;16:274–83.
Aluwini S, Pos F, Schimmel E, Krol S, van der Toorn PP, de Jager H, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (Hypro): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:464–74.
Arrayeh E, Westphalen AC, Kurhanewicz J, Roach M 3rd, Jung AJ, Carroll PR, et al. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal Mri and Mrsi study. Int J Radiat Oncol Biol Phys. 2012;82:e787–93.
Ling CC, Humm J, Larson S, Amols H, Fuks Z, Leibel S, et al. Towards multidimensional radiotherapy (Md-Crt): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47:551–60.
Feutren T, Herrera FG. Prostate irradiation with focal dose escalation to the intraprostatic dominant nodule: a systematic review. Prostate Int. 2018;6:75–87.
Rossi L, Breedveld S, Heijmen BJ, Voet PW, Lanconelli N, Aluwini S. On the beam direction search space in computerized non-coplanar beam angle optimization for Imrt-Prostate Sbrt. Phys Med Biol. 2012;57:5441–58.
Price RA, Hanks GE, McNeeley SW, Horwitz EM, Pinover WH. Advantages of using noncoplanar vs. axial beam arrangements when treating prostate cancer with intensity-modulated radiation therapy and the step-and-shoot delivery method. Int J Radiat Oncol Biol Phys. 2002;53:236–43.
Norihisa Y, Mizowaki T, Takayama K, Miyabe Y, Matsugi K, Matsuo Y, et al. Detailed dosimetric evaluation of intensity-modulated radiation therapy plans created for stage C prostate cancer based on a planning protocol. Int J Clin Oncol. 2012;17:505–11.
Mizowaki T, Cohen GN, Fung AY, Zaider M. Towards integrating functional imaging in the treatment of prostate cancer with radiation: the registration of the Mr spectroscopy imaging to ultrasound/Ct images and its implementation in treatment planning. Int J Radiat Oncol Biol Phys. 2002;54:1558–64.
D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74.
Tong X, Chen X, Li J, Xu Q, Lin MH, Chen L, et al. Intrafractional prostate motion during external beam radiotherapy monitored by a real-time target localization system. J Appl Clin Med Phys. 2015;16:5013.
Mizowaki T, Takayama K, Nagano K, Miyabe Y, Matsuo Y, Kaneko S, et al. Feasibility evaluation of a new irradiation technique: three-dimensional unicursal irradiation with the Vero4drt (Mhi-Tm2000). J Radiat Res. 2013;54:330–6.
Hirashima H, Nakamura M, Miyabe Y, Mukumoto N, Uto M, Nakamura K, et al. Geometric and dosimetric quality assurance using logfiles and a 3d helical diode detector for dynamic wavearc. Phys Med. 2017;43:107–13.
Ikeda I, Mizowaki T, Sawada Y, Nakata M, Norihisa Y, Ogura M, et al. Assessment of interfractional prostate motion in patients immobilized in the prone position using a thermoplastic shell. J Radiat Res. 2014;55:168–74.
Langen KM, Willoughby TR, Meeks SL, Santhanam A, Cunningham A, Levine L, et al. Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2008;71:1084–90.
Abe E, Mizowaki T, Norihisa Y, Narita Y, Matsuo Y, Narabayashi M, et al. Impact of multileaf collimator width on intraprostatic dose painting plans for dominant intraprostatic lesion of prostate cancer. J Appl Clin Med Phys. 2010;11:3193.
Miften M, Olch A, Mihailidis D, Moran J, Pawlicki T, Molineu A, et al. Tolerance limits and methodologies for imrt measurement-based verification Qa: recommendations of Aapm task group no. 218. Med Phys. 2018;45:e53–83.
Muldermans JL, Romak LB, Kwon ED, Park SS, Olivier KR. Stereotactic body radiation therapy for oligometastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2016;95:696–702.
Kuang Y, Wu L, Hirata E, Miyazaki K, Sato M, Kwee SA. Volumetric modulated arc therapy planning for primary prostate cancer with selective intraprostatic boost determined by 18f-choline pet/Ct. Int J Radiat Oncol Biol Phys. 2015;91:1017–25.
Chang JH, Lim Joon D, Davis ID, Lee ST, Hiew CY, Esler S, et al. Comparison of [(11)C]choline positron emission tomography with T2- and diffusion-weighted magnetic resonance imaging for delineating malignant intraprostatic lesions. Int J Radiat Oncol Biol Phys. 2015;92:438–45.
Abdellatif A, Craig J, Jensen M, Mulligan M, Mosalaei H, Bauman G, et al. Experimental assessments of intrafractional prostate motion on sequential and simultaneous boost to a dominant intraprostatic lesion. Med Phys. 2012;39:1505–17.
Schild MH, Schild SE, Wong WW, Vora SA, Keole SR, Vargas CE, et al. A prospective trial of intensity modulated radiation therapy (Imrt) incorporating a simultaneous integrated boost for prostate cancer: long-term outcomes compared with standard image guided Imrt. Int J Radiat Oncol Biol Phys. 2017;97:1021–5.
Barrett T, Turkbey B, Choyke PL. Pi-Rads version 2: What you need to know. Clin Radiol. 2015;70:1165–76.
Kajihara H, Hayashida Y, Murakami R, Katahira K, Nishimura R, Hamada Y, et al. Usefulness of diffusion-weighted imaging in the localization of prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:399–403.
Funding
This work was supported by JSPS KAKENHI Grant Number JP16K10390, and partially supported by the Practical Research for Innovative Cancer Control (Grant No. JP18ck0106427h0001) of the Japan Agency for Medical Research and Development.
Author information
Authors and Affiliations
Contributions
RA and TM contributed to the study conception and design. RA, KN, RA, KT and TM collected the data. HI and MN performed patient-specific quality assurance. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ashida, R., Nakamura, K., Aizawa, R. et al. Highly hypofractionated intensity-modulated radiation therapy for nonmetastatic prostate cancer with a simultaneous integrated boost to intraprostatic lesions: a planning study. Jpn J Radiol 40, 210–218 (2022). https://doi.org/10.1007/s11604-021-01186-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-021-01186-6