Abstract
Purpose
To evaluate the effectiveness of radiation therapy and steroids for Asian patients with Graves’ ophthalmopathy using the clinical activity score (CAS), and changes in external ocular muscles and eye proptosis determined by magnetic resonance imaging (MRI).
Materials and methods
We retrospectively reviewed 48 patients who received combined orbital radiation and systemic glucocorticoids in our hospital. MRI was performed both before and 1 month after treatment in all patients. We calculated the areas of five extraocular muscles and the degree of proptosis on transverse sections, and we evaluated the activity of the disease using CAS before and 1 month after treatment and toxicity.
Results
The areas of external ocular muscles, the length of eye prominence and CAS were significantly improved by the combination of orbital radiation and steroids. The change in the area of the medial rectus muscle had a significant correlation with the change in CAS (P < 0.05). Graves’ ophthalmopathy progressed again in 4 of the 48 patients; however, there were no patients with serious side effects in a median observation period of 41.5 months.
Conclusion
Treatment with the combination of orbital radiation and systemic glucocorticoids is subjectively and objectively effective for Asian Graves’ ophthalmopathy without severe toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Graves’ ophthalmopathy (GO) is an autoimmune disorder of the orbit that is closely associated with autoimmune thyroid diseases and is clinically apparent in 25–50% of patients with Graves’ diseases. GO is the most common and most significant extrathyroidal feature of Graves’ disease, and it is, therefore, essential to effectively treat GO. Both genetic and environmental factors are involved the development of GO [1]. The main pathophysiology of GO is the presence of a hormone receptor (TSH-R) in the thyroid gland. However, this receptor is also found in normal eye tissue. The antibodies against TSH-Rs induce inflammatory and fibrotic reactions [2,3,4]. The main effector cells such as T cells, plasma cells, B cells and macrophages in GO are orbital fibroblasts present in orbital fat and muscle [5, 6], and inflammatory and fibrotic reactions cause symptomatic presentations of GO in the retro-orbit. Most patients with GO exhibit both an enlargement of the extraocular muscle and an expansion of adipose tissue. There are various symptoms due to eye compression caused by the enlargement of muscles. The classic symptoms of GO include proptosis, pain on gaze, eyelid retraction, conjunctival redness and edema, periorbital edema and spontaneous retrobulbar pain [7,8,9,10]. In some severe cases, optic neuropathy and sight loss can occur, which seriously affect the quality of life.
Orbital radiotherapy has also played an important role in relieving symptoms for decades [11,12,13]. The effectiveness and safety of orbital radiotherapy combined with systemic glucocorticoids have been investigated in some Western studies [14,15,16]. Compared with either treatment alone, orbital radiotherapy with systemic glucocorticoids might be a more effective and safe treatment modality.
The clinical activity score (CAS) has been used for evaluating activity of the disease. The CAS is determined by points for spontaneous retrobulbar pain, pain on attempted upward or downward gaze, redness of the eyelids, redness of the conjunctiva, swelling of the caruncle or plica, swelling of the eyelids and swelling of the conjunctiva [10]. These are criteria for evaluating the results of treatment. CAS was first proposed by Mourits in 1989 who treated 26 cases with hormone steroids and recorded clinical features over a long period of time [11]. In Japanese, magnetic resonance imaging (MRI) sometimes shows active inflammation even a CAS of 1 or 2.
The purpose of this study was to evaluate the early effectiveness of combined orbital radiation and systemic steroids using the CAS and MRI for the management of moderate-to-severe Asian Graves’ ophthalmopathy.
Materials and methods
Patients’ eligibility
All procedures in this study meet the standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments. After approval by the Institutional Review Board Committee in our hospital, we retrospectively reviewed patients who had been diagnosed with moderate-to-severe and active GO and had received combined orbital radiation and systemic glucocorticoids between 2011 and 2016 in our hospital. Patients who had received orbital radiation and systemic glucocorticoid treatment and met the following criteria were included: (1) diagnosis of active and moderate-to-severe GO, (2) bilateral ocular proptosis, (3) MRI T1WI having been performed both before and about 1 month after treatment, (4) 25–79 years of age, (5) no other ocular diseases of malignant tumors, and (6) no history of surgical treatment for thyroid eye disease.
Treatment methods and schedule
The treatment included three courses of intravenous methylprednisolone at 500 mg in 100 ml normal saline. One course was given on three consecutive days (Fig. 1). During this period, endocrinologists observed the eye conditions of patients and recorded the results. The endocrinologists assessed symptoms in the patients and determined the CAS (0–7). A 20-Gy dose in 10 fractions was delivered to the bilateral orbits with 6 MV photon beams using a linear accelerator. Radiotherapy planning was performed by CT using a 3D radiotherapy planning system (Eclipse, Varian Medical Systems, Palo Alto, CA). The clinical target volume (CTV) included extraocular muscles and tissue at the back of the eyes. The planning target volume (PTV) was determined by adding 0.5 cm to the CTV. The lens was not included in the irradiation field certainly. Oral prednisone therapy following intravenous methylprednisolone therapy was carried out (initial daily dose of 30 mg, tapered to discontinuation). After orbital radiation and intravenous methylprednisolone treatment for about 1 month, MRI was performed again to evaluate the early effect of the treatment. At the same time, the CAS was evaluated again according to the Graves’ orbitopathy guidelines.
Method of evaluation by MRI
To determine the degree of proptosis and area of extraocular muscles by MRI, we selected the T1W1 sequence because it enables clear observation of the anatomical structure and has relatively good contrast of intensity around the eye muscles. Since it is difficult to separate the superior rectus muscle and the levator palpebrae superioris muscle, they were evaluated together as the superior muscle group. For determining the degree of proptosis, a line was drawn between the right and left ventral zygomatic borders, the so-called interzygomatic lines, on a MRI image [8]. The vertical distance from the front of the eye to that line was calculated on a cross-sectional image of MRI, the maximum display level of the optic nerve was chosen to measure the distance using this line (Fig. 2). Five muscles in the orbit were observed by MRI including the superior rectus muscle, inferior rectus muscle, medial rectus muscle, lateral rectus muscle and superior oblique rectus muscle. Based on the low intensity of muscles on MRI T1WI, we calculated the area on a coronal section using WeVIEW Z-edition software (HITACHI Japan). Measurements for the left eyes and the right eyes were conducted separately. The person performing the calculations did not have information about the symptoms of patients. Transverse and sagittal scans were used on a course parallel to the optic nerve. Coronal slices were perpendicular to the midsagittal plane [12]. This method guaranteed that scans of all muscles were obtained by the same angle to the location of the body. There were several scans that showed the muscles of the eyes. From observations of many scans of the eye muscles in several patients, a location approximately 0.5 cm behind the posterior pole of the globe in a T1W1 coronal MR image was selected to calculate the area of five extraocular muscles (Fig. 3).
Statistics
Data were analyzed using SPSS statistical software version 22.0. A paired t test was used to test the relationships between the five extraocular muscles, the degree of proptosis and the CAS before and after radiation treatment. Spearman’s correlation was used to evaluate the relationships of change in the CAS with changes in the degree of proptosis and areas of the five extraocular muscles. P < 0.05 was considered significant.
Results
Forty-eight Asian patients (19 males and 29 females) were enrolled in the present study. The median age of the patients was 60 years (range 25–78 years).
Before treatment, there were 11 patients (4 males and 7 females) with CAS of 1 or 2 but active inflammation on MRI, and there was no patient with CAS of 0. There were 37 patients (15 males and 22 females) with CAS of higher than 2. The mean CAS before treatment was 3.52 ± 1.40 (Table 1). The mean areas of the right superior ocular muscle, inferior ocular muscle, medial ocular muscle, lateral ocular muscle and superior oblique ocular muscle were 49.72 ± 22.54 mm2, 55.15 ± 22.67 mm2, 39.94 ± 15.16 mm2, 29.80 ± 10.47 mm2 and 14.83 ± 4.34 mm2, respectively (Table 2), and the mean areas of the left superior ocular muscle, inferior ocular muscle, medial ocular muscle, lateral ocular muscle and superior oblique ocular muscle were 45.91 ± 19.32 mm2, 54.92 ± 17.37 mm2, 37.53 ± 14.57 mm2, 28.81 ± 7.10 mm2 and 14.78 ± 5.30 mm2, respectively (Table 3). The mean right exophthalmos value was 21.98 ± 2.33 mm and the mean left exophthalmos value was 21.20 ± 2.61 mm (Table 4).
After treatment, there were 43 patients (16 males and 27 females) with CAS of 0, 1 or 2 and there were 5 patients with CAS of higher than 2. The mean CAS after treatment was 1.46 ± 0.90 (Table 1). The mean areas of right superior ocular muscle, inferior ocular muscle, medial ocular muscle, lateral ocular muscle and superior oblique ocular muscle were 39.64 ± 13.24 mm2, 41.58 ± 11.73 mm2, 30.67 ± 9.48 mm2, 26.74 ± 7.30 mm2 and 12.29 ± 4.76 mm2, respectively (Table 2), and the mean areas of the left superior ocular muscle, inferior ocular muscle, medial ocular muscle, lateral ocular muscle and superior oblique ocular muscle were 37.48 ± 9.50 mm2, 40.79 ± 11.62 mm2, 29.33 ± 9.32 mm2, 25.23 ± 5.31 mm2 and 12.53 ± 4.24 mm2, respectively (Table 3).
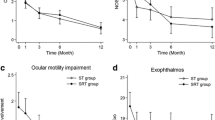
The areas of all muscles were significantly reduced by the treatment (P < 0.05). The mean right exophthalmos value was 20.62 ± 2.44 mm and the mean left exophthalmos value was 20.10 ± 2.70 mm (Table 4). The bilateral exophthalmos values and CAS were also significantly improved by the treatment (P < 0.05). Table 5 shows the relationships between values obtained from MRI images and changes in CAS. The change in the areas of the medial rectus muscle had a weak but significant correlation with change in CAS (P < 0.05); however, the changes in areas of other extraocular muscles were not correlated with changes in CAS.
There were no patients with retinopathy or a cataract in a median observation period of 41.5 months. GO progressed again in 4 patients after improved once by the treatment, and surgery was performed in 3 of those 4 patients.
Discussion
The present study showed the effectiveness of treatment for Asian GO without severe side effects. Treatment with glucocorticoids is considered to be a first-line therapy for GO, and radiation therapy can enhance the effect of steroids. VeRonique’ et al. reported the responses of GO to combined treatment with radiotherapy and steroids. About 26% of the patients had excellent responses, 50% of the patients had partial improvement depending on the residual objective and functional signs, and 19% of the patients were stable after treatment with few severe side effects [13]. Grassi et al. reported that 35 patients who had a contraindication chose only radiation therapy and had good outcomes for ocular motility and CAS without side effects [7]. However, radiotherapy could not significantly improve proptosis. They concluded that radiotherapy should be considered as a suitable alternative treatment for patients with GO who cannot tolerate steroids. It was concluded in a review that radiotherapy significantly reduced the need for further treatment including further steroid or immunosuppressive therapy [14]. Another meta-analysis of prospective studies showed that there was no significant difference between radiotherapy and sham radiotherapy based on changes in CAS. It was concluded from the results of that meta-analysis that orbital radiation as a sole treatment modality for GO remains controversial but that orbital radiation in combination with corticosteroids has greater efficacy than either treatment alone [15]. In our study, only 4 patients showed no improvement in CAS. Even in long-term observation after the treatment, GO progressed again in only 4 patients. Our results also suggest that the combination of orbital radiation and corticosteroids is a very effective treatment for Asian GO.
A previous report [16] showed that some cases were misdiagnosed only using CAS and were modified by MRI. Therefore, adding the values of muscles for evaluation of the activity of GO is necessary. That method should be less influenced by the operator. Mayer et al. [17] reported that a change in the T1 area on MR images had a significant relationship with CAS. However, there has been no common consensus or standard for diagnosis of GO by MRI. Our results suggest that the area of the medial ocular muscle on a coronal image behind the posterior pole of the globe might be more useful than proptosis in Asian for distinguishing the phase and effect of the disease. Especially, the area of the medial rectus muscle should be included in future clinical trials to objectively evaluate its efficacy and activity. In a previous study [12], the coronal section approximately 1 cm behind the posterior pole was used for evaluation, but the sizes of Asian orbits might be slightly different from those of Westerners, we selected the region approximately 0.5 cm behind the posterior pole for evaluation. In the present study, the changes in the areas of ocular muscles other than the medial ocular muscle were not correlated with changes in CAS. The reason for this might be that it was difficult to delineate borderlines of other muscles for measurement areas of extraocular muscles even by MRI. Further study is needed to determine whether changes in ocular muscles in MRI images can be used as criteria for evaluating activity of the disease, especially in patients with swelling of muscles other than the medial ocular muscle.
In some studies [18,19,20], it was shown that there was a relatively large change in the structures of eye muscles during the early treatment period. Our study also showed that the combination of radiation therapy and steroids resulted in a significant change in the maximum extraocular muscle thickness and that radiation therapy with steroids seemed to be tolerated well and effective because of the improvements in soft tissue swelling and ocular motility. Due to the limitation of our retrospective analysis for which some details and follow-up information were not sufficient, we could not analyze long-term measurements of muscles in all the patients, and further study in which all of the eye muscles are recorded by MRI throughout the course of the disease over a long-term observing period is needed. Performing MRI several times over the entire course of GO is needed for a clear evaluation of inflammation. However, we do not know the appropriate timing for evaluating the maximum effect of radiotherapy for GO because the effect of radiotherapy is due not only to its anti-inflammatory effect by modulation of various cellular components including endothelium, mononuclear cells, macrophages and granulocytes in the early phase but also to atrophy and fibrosis of muscles in the late phase. Nicosia et al. actually reported that CAS at 1 year after treatment as in the present study was better than CAS at 3–6 months after treatment [21].
Furthermore, the observation period in the present study was too short to evaluate radiation-induced carcinogenesis. A previous study with a follow-up period of 11 years after orbital radiotherapy for GO showed that there was no associated increase in secondary malignancy [22]. There were no patients who had radiation-induced carcinogenesis in the present study also. However, radiotherapy should be used with caution for young patients with GO.
In conclusion, Asian GO showed good subjective and objective responses to combined orbital radiation and systemic glucocorticoids without severe toxicity. MRI is useful for objective assessment of the activity of GO.
References
Hiromatsu Y, Eguchi H, Tani J, Kasaoka M, Teshima Y. Graves’ ophthalmopathy: epidemiology and natural history. Intern Med. 2014;53:353–60.
Ophthalmopathy T, Bahn RS. Thyrotropin receptor expression in orbital adipose/connective tissues from patients with. Thyroid. 2002;12:193–5.
Matthiesen C, Thompson JS, Thompson D, Farris B, Wilkes B, Ahmad S, et al. The efficacy of radiation therapy in the treatment of graves’ orbitopathy. Int J Radiat Oncol Biol Phys. 2012;82:117–23.
Yang D, Hiromatsu Y, Hoshino T, Inoue Y, Itoh K, Nonaka K. Dominant infiltration of T(H)1-type CD4+ T cells at the retrobulbar space of patients with thyroid-associated ophthalmopathy. Thyroid Off J Am Thyroid Assoc. 1999;9:305–10.
Dolman PJ, Rath S. Orbital radiotherapy for thyroid eye disease. Curr Opin Ophthalmol. 2012;23:427–32.
Wiersinga WM. Autoimmunity in Graves’ ophthalmopathy: the result of an unfortunate marriage between TSH receptors and IGF-1 receptors? J Clin Endocrinol Metab. 2011;96:2386–94.
Grassi P, Strianese D, Piscopo R, Pacelli R, Bonavolontà G. Radiotherapy for the treatment of thyroid eye disease—a prospective comparison: Is orbital radiotherapy a suitable alternative to steroids? Ir J Med Sci. 2017;186:647–52.
Xu L, Li L, Xie C, Guan M, Xue Y. Thickness of extraocular muscle and orbital fat in MRI predicts response to glucocorticoid therapy in Graves’ ophthalmopathy. Int J Endocrinol. 2017;2017:3196059.
Abalkhail S, Doi SAR, Al-Shoumer KS. The use of corticosteroids versus other treatments for Graves’ ophthalmopathy: a quantitative evaluation. Med Sci Monit 2003;9:CR477-483.
Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur Thyroid J. 2016;5:9–26.
Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–44.
Szucs-Farkas Z, Toth J, Balazs E, Galuska L, Burman KD, Karanyi Z, et al. Using morphologic parameters of extraocular muscles for diagnosis and follow-up of graves’ ophthalmopathy: diameters, areas, or volumes? Am J Roentgenol. 2002;179:1005–100.
Beckendorf V, Maalouf T, George JL, Bey P, Leclere J, Luporsi E. Place of radiotherapy in the treatment of Graves’ orbitopathy. Int J Radiat Oncol Biol Phys. 1999;43(4):805–15.
Prummel MF, Terwee CB, Gerding MN, Baldeschi L, Mourits MP, Blank L, et al. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves' ophthalmopathy. J Clin Endocrinol Metab. 2004;89(1):15–20.
Stiebel-Kalish H, Robenshtok E, Hasanreisoglu M, Ezrachi D, Shimon I, Leibovici L. Treatment modalities for Graves' ophthalmopathy: systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94(8):2708–16.
Politi LS, Godi C, Cammarata G, Ambrosi A, Iadanza A, Lanzi R, et al. Magnetic resonance imaging with diffusion-weighted imaging in the evaluation of thyroid-associated orbitopathy: getting below the tip of the iceberg. Eur Radiol. 2014;24:1118–26.
Mayer EJ, Fox DL, Herdman G, Hsuan J, Kabala J, Goddard P, et al. Signal intensity, clinical activity and cross-sectional areas on MRI scans in thyroid eye disease. Eur J Radiol. 2005;56(1):20–4.
Ohtsuka K, Sato A, Kawaguchi S, Hashimoto M, Suzuki Y. Effect of steroid pulse therapy with and without orbital radiotherapy on Graves’ ophthalmopathy. Am J Ophthalmol. 2003;135:285–90.
Ng CM, Yuen HKL, Choi KL, Chan MK, Yuen KT, Ng YW, et al. Combined orbital irradiation and systemic steroids compared with systemic steroids alone in the management of moderate-to-severe Graves’ ophthalmopathy: a preliminary study. Hong Kong Med J. 2005;11:322–30.
Kahaly GJ, Rösler HP, Pitz S, Hommel G. Low- versus high-dose radiotherapy for Graves’ ophthalmopathy: a randomized, single blind trial. J Clin Endocrinol Metab. 2000;85:102–8.
Nicosia L, Reverberi C, Agolli L, Marinelli L, De Sanctis V, Minniti G, et al. Orbital radiotherapy plus concomitant steroids in moderate-to-severe Graves' ophthalmopathy: good results after long-term follow-up. Int J Endocrinol Metab. 2019;17(1):e84427.
Wakelkamp IM, Tan H, Saeed P, Schlingemann RO, Verbraak FD, Blank LE, et al. Orbital irradiation for Graves' ophthalmopathy: is it safe? A long-term follow-up study. Ophthalmology. 2004;111(8):1557–622.
Acknowledgements
The results of the present study were presented at the 31st annual meeting of the Japanese Society of Radiation Oncology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All the procedures in this study meet the standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments. This research was conducted with the approval of the ethics review committee of Tohoku University Hospital. The reception number is 2018-1-122.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ma, Z., Ozaki, H., Ishikawa, Y. et al. Improvement of the MRI and clinical features of Asian Graves’ ophthalmopathy by radiation therapy with steroids. Jpn J Radiol 37, 612–618 (2019). https://doi.org/10.1007/s11604-019-00846-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-019-00846-y