Abstract
The internal mammary lymph node (IMLN) chain is a pathway through which breast lymphatic drainage flows. The internal mammary lymphatic vessel runs around the internal mammary artery and veins with IMLN in the parasternal intercostal spaces. IMLN metastasis, which forms a part of clinical TNM staging, may negatively affect the prognosis of primary breast cancer patients. IMLN metastasis is clinically detected using ultrasound, computed tomography, magnetic resonance imaging, and 18F-deoxyglucose positron emission tomography computed tomography. The uptake of radioactive tracers in IMLN with clinically negative axillary lymph nodes is often identified using sentinel lymph node mapping (SLNM) in primary breast cancer patients. The indication for IMLN biopsy or resection that is clinically detected or visualized using SLNM is controversial. The clinically suspicious IMLN may be considered for ultrasound-guided fine-needle aspiration. First IMLN recurrence needs to be biopsied. Irradiation of the breast, chest wall, and/or regional nodal irradiation, including IMLN, following lumpectomy or postmastectomy is recommended. Although radiation therapy for IMLN recurrence may improve clinical outcomes, it is also associated with pulmonary and cardiac toxicities. This review covers the local anatomy of IMLN, lymph drainage and image findings of IMLN with a discussion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although breast lymphatic drainage flows into the axillary lymph node (ALN) and/or internal mammary lymph node (IMLN) chain, the importance of detecting IMLN metastasis and its treatment has long been debated [1]. IMLN metastasis forms a part of clinical TNM staging and may negatively affect the prognosis of primary breast cancer patients [2]. Therefore, radiologists need to understand the anatomy and image findings of IMLN including ultrasound (US), computed tomography (CT), magnetic resonance imaging (MR), and 18F-deoxyglucose positron emission tomography CT (FDG-PET–CT) to avoid the underdiagnosis of IMLN metastasis. The uptake of radioactive tracers in IMLN is often identified using sentinel lymph node mapping (SLNM) [3]. The indication for IMLN biopsy or resection is controversial due to the absence of prognosis improvement and the complication of bleeding [4, 5]. IMLN irradiation may improve clinical outcomes, but it is also associated with pulmonary and cardiac toxicities [6]. Thus, in treatment indications, we need to consider the risk to patients. This review discusses the anatomy, clinical staging of breast cancer patients, normal and abnormal findings for IMLN on various imaging modalities, and indications for biopsy and local treatments.
Anatomy
An intimate knowledge of the anatomy of IMLN and the surrounding vessels contributes to the detection of enlarged IMLN on each imaging modality for clinical staging in breast cancer patients. It also ensures successful and safe IMLN biopsy by preventing bleeding from vessels. The internal mammary lymphatic vessels with the internal mammary artery (IMA; internal thoracic artery) flanked by two parallel internal mammary veins (IMV) run alongside the sternal border in the anterior intercostal space (ICS) and beneath the internal intercostal muscle, ribs, costal cartilages, and intercostal nerves, but above the parietal pleura and transversus thoracis [7, 8]. Figure 1 shows an axial schema of the chest wall. The most common sites to have at least one IMLN are the second and third ICSs (Fig. 2). Although the positional relationship between IMA and IMLN is not uniform, IMLN is more likely to be laterally located in relation to the IMA in the second ICS and medially in the third and fourth ICSs [7].
An axial schema of the chest wall. The internal mammary artery (A) runs alongside the sternal border flanked by two parallel internal mammary veins (V) behind the internal intercostal muscle (IIM). The internal mammary lymphatic vessel (L) runs around the internal mammary artery and veins with the internal mammary lymph node in the parasternal intercostal spaces (ICS)
The internal mammary lymph node (IMLN) in the intercostal space (ICS). At least one of the IMLN is shown at the second (II), third (III) or fourth (IV) ICS on both sides. The most common sites to have at least one normal IMLN are the second and third ICSs. Although the positional relationship between IMA and IMLN is not uniform, IMLN is more likely to be laterally located in relation to the IMA in the second ICS and medially in the third and fourth ICSs. The mean number of IMLN is the highest in the third ICS
Lymph drainage
Breast lymphatic drainage flows into ALN and/or IMLN chain. Approximately, 97% of breast lymph drainage flows into ALN with the remaining 3% flowing into IMLN [9] (Fig. 3a). The breast parenchyma has three inter-communicating lymphatic plexuses involved in lymph drainage: superficial, perforating, and deep flow [8] (Fig. 3b). The superficial and perforating plexuses drain almost exclusively into ALN through the sub-areolar Sappey lymphatic network. The deep system drains into ALN and IMLN. The intermediate perforating plexus is connected to the deep plexus. Although previous studies consistently reported that medial breast cancer is strongly associated with a higher rate of IMLN metastasis, breast cancers located in any area of the breast parenchyma have the potential to metastasize through IMLN [8]. IMLN receive lymph drainage from the anterior diaphragmatic nodes, antero-superior portions of the liver, and deeper structures of the anterior chest and upper anterior abdominal walls. Furthermore, lymph drainage transversely flows between IMLN and the anterior or superior mediastinal nodes.
Schemas of breast lymphatic drainage. a Approximately 97% of breast lymph drainage flows into the axillary lymph nodes (ALN). Only 3% of breast lymph drainage flows into the internal mammary lymph node (IMLN). b The breast parenchyma has superficial, perforating, and deep drainage flows. The superficial and perforating plexuses drain almost exclusively into ALN through the sub-areolar Sappey lymphatic network. The deep system drains into ALN and IMLN. The intermediate perforating plexus is connected to the deep plexus
Staging for breast cancer and prognosis
The TNM staging system was established to reflect the extent of disease and prognosis of breast cancer patients. According to the American Joint Committee on Cancer Staging Manual, a clinical N stage is assigned by the clinical detection of lymph node metastasis, which is defined as detection by imaging modalities or a physical examination and having characteristics strongly indicating malignancy or presumed pathological macrometastasis based on fine-needle aspiration (FNA) [10]. Image modalities for a clinical N stage assessment include US, CT, MR, and PET–CT. The incidence rates of enlarged IMLN detected on US, CT, MR, and PET–CT are approximately 10, 16, 16, and 14%, respectively [11,12,13]. Enlarged IMLN on each modality, solid hypoechoic round or oval IMLN (with or without an echogenic hilum) on US, and FDG-avid IMLN with a greater uptake than the background pectoralis muscle or mediastinal blood pool level on FDG-PET–CT indicate IMLN metastasis. Regarding IMLN biopsy, there is currently no consensus for its contribution to the confirmation of metastasis [5]. Although sentinel lymph node biopsy (SLNB) is not routinely performed for IMLN, the existence of IMLN metastasis detected by SLNB, FNA, or clinical imaging upgrades the clinical and pathological N category in women with newly diagnosed breast cancer [12, 13] (Tables 1 and 2). Therefore, IMLN metastasis detected by imaging studies will upstage a patient with clinical TNM stage I or II disease to stage III [13] (Table 3). The presence of IMLN adenopathy at presentation in women with breast cancer is associated with a poor prognosis [11].
pN1b is considered when IMLN metastases are detected at SLNB with micrometastases or macrometastases, but are not clinically detected by imaging studies or a physical examination. pN2b is considered when IMLN metastases are detected by imaging studies or a physical examination in the absence of axillary lymph node metastases. However, disease-free (DFS) and overall survival did not significantly differ between the pN1b and pN2b groups due to marked improvements in the abilities of imaging modalities to detect small IMLN metastases [2]. The current classification of pN1b and pN2b is controversial and a modified N category may possibly be proposed in future.
IMLN appearance on US, CT, MR, and PET–CT
US, CT, and MR are useful imaging modalities for screening IMLN. IMLN is generally observed around internal mammary vessels on each modality.
US
IMLN may be evaluated by expanding the extent of the standard whole-breast US examination for breast cancer. With transverse and longitudinal plane scans along the sternal border, IMLN is located around the IMA and IMVs in the ICS. In the longitudinal plane, IMLN may be seen in the ICS between palpable parasternal costal cartilages with scan of a transducer parallel to the sternal border. On US images, the ICS shows isoechoic structures filled with fat tissue between hypoechoic costal cartilages beneath the intercostal muscle. The hyperechoic line lying deep to the ICS represents the pleura. In the transverse plane, with a transducer fitted in between costal cartilages, IMLN may be found in the ICS along the sternal border. Color Doppler US is useful for detecting IMLN around these vessels, and may help to distinguish IMLN from a perforating branch of IMV. Abnormal IMLN shows a spherical or oval hypoechoic mass. However, normal IMLN is often too small to be visible (Fig. 4). The incidence of visible IMLN on US is only 10% in patients with primary breast cancer [11].
Normal ultrasound image around an invisible internal mammary lymph node in intercostal spaces (ICS). a Transverse ultrasound image shows the internal mammary artery (A) and vein (V) in the ICS (solid line). b Transverse color Doppler US image shows the internal mammary artery (A) and veins (V). c Longitudinal US image shows the intercostal muscle (M) and the ICS between 2nd and 3rd costal cartilage. d Longitudinal color Doppler US image shows internal mammary vessels
CT
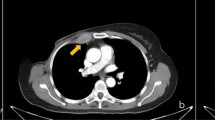
Although the size criteria for what precisely constitutes abnormal IMLN currently remain unclear, 42% of IMLN in patients with primary breast cancer present on CT (Fig. 5). Among detected IMLN, 16% are greater than 5 mm in the short axis. The presence of large IMLN is correlated with advanced disease stage based on CT evidence of distant metastases [14].
MR
Breast MR is used to diagnose and assess the extent of breast cancer and includes T1-weighted, T2-weighted, diffusion-weighted, and contrast-enhanced T1-weighted images. IMLN exists within the field of view and may be assessed on breast MR. In asymptomatic high-risk women for screening MR [15], presumed normal IMLN were visualized in 53.7% of high-risk patients. In these patients, an average of 1.4 (1–3) IMLN was detected. The average size of IMLN is 4.5 mm at their greatest diameter. Detected IMLN are more frequently observed on the left, at the 2nd and 3rd ICS, and medial to the internal mammary vessels. Figure 6 shows unilateral enlarged IMLN on an MR image.
MR image of bilateral intercostal spaces (ICS). a A T2-weighted image shows an enlarged internal mammary lymph node (IMLN) with high signal intensity (arrow) in the right intercostal spaces (ICS). b An axial image (b = 1000 s/mm2) shows abnormal IMLN (arrow) with diffusion restriction. c An axial dynamic contrast-enhanced MR image shows swollen enhanced IMLN (arrow). Normal IMLN in contralateral ICS is undetectable on each image (arrowheads)
PET–CT
PET–CT is more sensitive than CT alone for the detection of IMLN adenopathy (Fig. 7). Visual evaluations of abnormal uptake by IMLN are performed to establish whether uptake by ipsilateral IMLN is greater than that in the mediastinal blood pool or contralateral parasternal area on PET–CT [1, 16].
Left internal mammary lymph node (IMLN) recurrence 3 years after mastectomy for breast cancer. Non-enhanced (a) and enhanced (b) axial CT images both show swollen IMLN (arrow) in the left second intercostal spaces. Axial (c) and coronal (d) fused FDG-PET–CT images show hypermetabolic activity (SUV max = 2.7) within the left IMLN (arrowheads) greater than that of the mediastinal blood pool (M)
Pitfalls in image modalities
Perforating branches of IMV mimic IMLN on US. IMV sometimes shows a high signal intensity on diffusion-weighted MRI, mimicking IMLN metastasis. Osteolytic sternal metastasis resembles IMLN metastasis with sternal erosion. Inflammation or infection may be a cause of false positive FDG-avid IMLN on PET–CT. The precise identification of IMLN and the clarification of its relationship with surrounding structures are needed.
Case-based image review
Primary breast cancer
IMLN biopsy during autologous free flap reconstruction [4] showed that 6% of IMLN metastases correlated with age < 40 years, lymphovascular invasion, and a negative progesterone receptor status. IMLN adenopathy identified at the initial staging with PET–CT and MR was 2.7% [1]. An inner tumor location and positive ALN status were associated with IMLN adenopathy. The incidence of clinically apparent IMLN metastases detected on US, CT, PET–CT, and/or MR was approximately 14% in patients with clinical N2 or N3 locally advanced breast cancer [12] (Fig. 8). The median size of the enlarged IMLN was 1.3 cm (range 0.5–3.0 cm). Normal IMLN was most commonly detected in the third ICS in cadavers [7]. However, enlarged IMLN involved the first, second, and third ICSs in 55, 58, and 22%, respectively [12].
Left breast cancer with internal mammary lymph node (IMLN) metastasis. Diffusion-weighted image (a), T1-weighted enhanced image (b), and enhanced chest CT image (c) show breast cancer (arrow) with a 7-mm adenopathy of IMLN (arrowhead) in the left second intercostal space. Fused FDG-PET–CT image (d) shows hypermetabolic activity (SUVmax = 10.8) within breast cancer (arrow) with intermediate activity (SUVmax = 2.7) within the left IMLN (arrowhead). After neoadjuvant systemic therapy, FDG uptake by IMLN decreased (not shown), and was presumably due to IMLN metastasis
IMLN recurrence
IMLN recurrence is rare. The incidence rates of IMLN recurrence after treatment detected on CT and PET–CT were 1.5% [17] and 0.2–1.4% [18, 19], respectively. Metastasis to the sternum is more frequent in breast cancer patients with a solitary metastatic bone lesion caused by local tumor invasion from adjacent IMLN metastasis (Fig. 9) than in those with multiple metastatic bone lesions [20].
Swelling of IMLN with breast implants
The incidence of enlarged IMLN (Fig. 10) in women with a history of breast cancer and silicone implant reconstruction on MR was 37.6% with a median short axis of 4 mm and median long axis of 7 mm [21]. The incidence of FDG avidity on PET–CT for enlarged IMLN detected with MR was 24.1% with a median standardized uptake value of 2.3 [22]. Only 1 out of 207 IMLN in patients with an adequate follow-up was malignant. In patients with breast cancer following silicone implant reconstruction, hypermetabolic activity within IMLN on FDG-PET–CT may be due to metastatic deposits, non-specific inflammation, or silicone migration [22].
A swollen internal mammary lymph node with implant rupture in a patient after left mastectomy and silicone implant reconstruction 15 years earlier. a A short-tau inversion recovery image shows a collapsed implant shell as a curvilinear structure with low signal intensity in silicone gel with high signal intensity. b An enhanced T1-weighted image shows IMLN (L) with a long-axis diameter of 4 mm between the internal mammary artery (A) and vein (V)
SLNM
The sentinel lymph node is the first node that drains cancer cells. Biopsy for axillary sentinel lymph nodes detected by SLNM is a procedure for staging and selecting a therapeutic plan for patients with clinically negative ALN. If the sentinel lymph node biopsy shows negative involvement, no further ALN surgery is indicated. In cases of unidentified sentinel ALN on SLNM, axillary dissection is recommended [10]. Although ALN frequently receives first and direct lymphatic drainage, SLNM shows ALN and IMLN or IMLN only in some patients. In approximately 20% of patients, SLNM visualizes drainage both to ALN and IMLN (Fig. 11), exclusive lymphatic drainage to IMLN (without the visualization of ALN) is only observed in approximately 8% of patients (Fig. 12). SLNM after a peritumoral injection of a radioisotope (deep lymphatic plexus) leads to markedly greater uptake in IMLN than subdermal or sub-areolar injections (superficial lymphatic plexus) [23]. IMLN is more frequently observed in younger patients with smaller and medial tumors. The rates of lymphatic drainage to IMLN differ among radiocolloids, including 99mTc-sulfur colloid, 99mTc-nanocolloid, 99mTc-antimony trisulfide 99mTc-rhenium sulfide, and 99mTc-phytate [3]. IMLN drainage on SLNM was previously shown to correlate with worse distant DFS [24].
Indication of biopsy and resection for IMLN
In primary breast cancer, biopsy of IMLN is more difficult than that of axillary sentinel nodes, with success rates between 70 and 100%. The exploration of IMLN is an extra procedure that carries an additional risk of intra- and post-operative complications and a less satisfactory cosmetic outcome. There is no consensus regarding sentinel IMLN biopsy for a diagnosis or prognosis when observed on pre-operative SLNM in primary breast cancer patients [5].
US-guided FNA of IMLN may be performed without the risk of injury to the vessels and pleura, and potentially influences patient management by changing staging and therapeutic decisions [12]. The aforementioned imaging characteristics suggest IMLN metastasis and such IMLN may be considered for ultrasound-guided FNA (Fig. 13) [25].
Left medial breast cancer with internal mammary lymph node (IMLN) metastasis. An enhanced chest CT image a shows the adenopathy of IMLN (arrowhead) in the left third intercostal space. A fused FDG-PET–CT image b shows hypermetabolic activity within the left IMLN (arrowhead). A US image c shows a 7-mm hypoechoic oval IMLN. d US-guided fine-needle aspiration biopsy (arrowheads denote needle) for IMLN (arrow) confirmed IMLN metastasis
IMLN was previously removed and pathologically evaluated during extended radical mastectomies. Since breast conservation has become more common, IMLN is no longer routinely sampled [24] because of the morbidity of the procedure and lack of a demonstrated survival benefit. However, IMLN needs to be biopsied in recurrent patients who presented with IMLN as the first metastatic site (Fig. 14a, b) [10].
Three years after mastectomy for triple negative breast cancer following systematic therapy, a clinician detected swelling of the parasternal area. Enhanced CT (a) and MR (not shown) images show adenopathy of IMLN with invasion to the costal cartilage. b CT-guided needle biopsy (arrowheads denote needle) confirmed recurrent involvement. c Axial dose distribution for left IMLN recurrence in a patient with breast cancer
Indication of radiation therapy for IMLN
In high-risk patients undergoing lumpectomy, whole-breast irradiation includes regional nodal irradiation (RNI). RNI generally covers the supraclavicular and infraclavicular areas, IMLN, and part of ALN. The National Cancer Institute of Canada MA.20 trial [26] showed that RNI improved 10-year DFS and reduced the recurrence rate of breast cancer. However, RNI did not improve 10-year overall survival. The European Organization for Research and Treatment of Cancer 22,922/10,925 trials [27] showed that RNI improved DFS and distant DFS, reduced breast cancer mortality, and slightly affected overall survival. The National Comprehensive Cancer Network (NCCN) panel [10] recommended RNI irradiation for patients with 4 or more positive ALNs, the strong consideration of RNI for those areas with 1–3 positive ALNs, and no routine RNI for patients with negative ALN. Moreover, RNI may be considered for patients with central/medial primary tumors or tumors that are 2 cm or more in size with other high-risk features.
Regarding RNI in postmastectomy radiation therapy (PMRT), the Early Breast Cancer Trialists’ Collaborative Group meta-analyses [28] reported that RNI reduced recurrence and breast cancer mortality. The Danish Breast Cancer Cooperative Group [29] demonstrated that IMLN irradiation increased overall survival. On the other hand, Société Francaise de Radiation Oncologique [30] showed that IMLN irradiation did not improve 10-year overall survival. The NCCN panel [10] recommended PMRT in patients with 4 or more positive ALNs, and the strong consideration of PMRT for patients with 1–3 positive ALNs. The American Society of Clinical Oncology panel [6] recommended treatment generally to both IMLN and the supraclavicular–axillary apical nodes in addition to the chest wall or reconstructed breast. Thus, PMRT plus RNI including IMLN is recommended. Due to cardiac and pulmonary toxicities, IMLN irradiation needs to be selected in consideration of the risk to the patient.
In cases of internal mammary node recurrence, the NCCN panel [10] recommended using radiation therapy if possible and the consideration of systemic therapy. The decision to treat locoregional recurrence must consider any prior radiation to the area and the risk of late normal tissue toxicity from the sum of the prior and planned radiation courses. CT-based treatment planning is encouraged to delineate target volumes and adjacent organ risks, particularly the heart and lung (Fig. 14c) [10]. Concurrent IMLN irradiation and anthracyclines, used increasingly in the systemic adjuvant treatment of breast cancer, may interact unfavorably on the heart and counterbalance any beneficial effect of IMLN irradiation, particularly in patients with left-sided breast cancer [31]. The cardiotoxicity of concurrent IMLN irradiation and trastuzumab currently remains unknown [32].
Conclusions
IMLN are in the intercostal spaces around the internal mammary vessels. The existence of IMLN metastasis upgrades the N factor. IMLN metastasis is clinically diagnosed on US, CT, MR, and PET–CT. Radiologists need to be familiar with the clinical image findings of IMLN for appropriate and efficient management.
References
Cheon H, Kim HJ, Lee SW, Kim DH, Lee CH, Cho SH, et al. Internal mammary node adenopathy on breast MRI and PET/CT for initial staging in patients with operable breast cancer: prevalence and associated factors. Breast Cancer Res Treat. 2016;160:523–30.
Habraken V, van Nijnatten TJ, de Munck L, Moossdorff M, Heuts EM, Lobbes MB, et al. Does the TNM classification of solitary internal mammary lymph node metastases in breast cancer still apply? Breast Cancer Res Treat. 2017;161:483–9.
Manca G, Volterrani D, Mazzarri S, Duce V, Svirydenka A, Giuliano A, et al. Sentinel lymph node mapping in breast cancer: a critical reappraisal of the internal mammary chain issue. Q J Nucl Med Mol Imaging. 2014;58:114–26.
Zhang X, Jaffer S, Bleiweiss IJ, Nayak A. The clinical significance of internal mammary lymph node (IMLN) biopsy during autologous reconstruction in breast cancer patients. Breast Cancer Res Treat. 2015;153:565–72.
Postma EL, van Wieringen S, Hobbelink MG, Verkooijen HM, van den Bongard HJ, Borel Rinkes IH, et al. Sentinel lymph node biopsy of the internal mammary chain in breast cancer. Breast Cancer Res Treat. 2012;134:735–41.
Recht A, Comen EA, Fine RE, Fleming GF, Hardenbergh PH, Ho AY, et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. Pract Radiat Oncol. 2016;6:e219–34.
Barros AC, Mori LJ, Nishimura D, Jacomo AL. Surgical anatomy of the internal thoracic lymph nodes in fresh human cadavers: basis for sentinel node biopsy. World J Surg Oncol. 2016;14:135.
Suami H, Pan WR, Mann GB, Taylor GI. The lymphatic anatomy of the breast and its implications for sentinel lymph node biopsy: a human cadaver study. Ann Surg Oncol. 2008;15:863–71.
Hultborn KA, Larsson LG, Ragnhult I. The lymph drainage from the breast to the axillary and parasternal lymph nodes, studied with the aid of colloidal Au198. Acta Radiol. 1955;43:52–64.
Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. NCCN guidelines insights: breast cancer, version 1.2017. J Natl Compr Canc Netw. 2017;15:433–51.
Jochelson MS, Lebron L, Jacobs SS, Zheng J, Moskowitz CS, Powell SN, et al. Detection of internal mammary adenopathy in patients with breast cancer by PET/CT and MRI. AJR Am J Roentgenol. 2015;205:899–904.
Dogan BE, Dryden MJ, Wei W, Fornage BD, Buchholz TA, Smith B, et al. Sonography and sonographically guided needle biopsy of internal mammary nodes in staging of patients with breast cancer. AJR Am J Roentgenol. 2015;205:905–11.
American Joint Committee on Cancer (AJCC). AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
Savaridas SL, Spratt JD, Cox J. Incidence and potential significance of internal mammary lymphadenopathy on computed tomography in patients with a diagnosis of primary breast cancer. Breast Cancer (Auckl). 2015;9:59–65.
Mack M, Chetlen A, Liao J. Incidental internal mammary lymph nodes visualized on screening breast MRI. AJR Am J Roentgenol. 2015;205:209–14.
Zhang YJ, Oh JL, Whitman GJ, Iyengar P, Yu TK, Tereffe W, et al. Clinically apparent internal mammary nodal metastasis in patients with advanced breast cancer: incidence and local control. Int J Radiat Oncol Biol Phys. 2010;77:1113–9.
Overgaard M, Christensen JJ. Postoperative radiotherapy in DBCG during 30 years. Techniques, indications and clinical radiobiological experience. Acta Oncol. 2008;47:639–53.
Ohsumi S, Inoue T, Kiyoto S, Hara F, Takahashi M, Takabatake D, et al. Detection of isolated ipsilateral regional lymph node recurrences by F18-fluorodeoxyglucose positron emission tomography-CT in follow-up of postoperative breast cancer patients. Breast Cancer Res Treat. 2011;130:267–72.
Oh JK, Chung YA, Kim YS, Jeon HM, Kim SH, Park YH, et al. Value of F-18 FDG PET/CT in detection and prognostication of isolated extra-axillary lymph node recurrences in postoperative breast cancer. Biomed Mater Eng. 2014;24:1173–84.
Kwai AH, Stomper PC, Kaplan WD. Clinical significance of isolated scintigraphic sternal lesions in patients with breast cancer. J Nucl Med. 1988;29:324–8.
Sutton EJ, Watson EJ, Gibbons G, Goldman DA, Moskowitz CS, Jochelson MS, et al. Incidence of internal mammary lymph nodes with silicone breast implants at MR imaging after oncoplastic surgery. Radiology. 2015;277:381–7.
Soudack M, Yelin A, Simansky D, Ben-Nun A. Fluorodeoxyglucose—positive internal mammary lymph node in breast cancer patients with silicone implants: is it always metastatic cancer? Eur J Cardiothorac Surg. 2013;44:79–82.
Barros AC, Barros MA, Andrade FE, Mori LJ, Costa PA, Sheng PY, et al. Combined radioguided nonpalpable lesion localization and sentinel lymph node biopsy for early breast carcinoma. Ann Surg Oncol. 2007;14:1472–7.
Kong AL, Tereffe W, Hunt KK, Yi M, Kang T, Weatherspoon K, et al. Impact of internal mammary lymph node drainage identified by preoperative lymphoscintigraphy on outcomes in patients with stage I to III breast cancer. Cancer. 2012;118:6287–96.
Wang CL, Eissa MJ, Rogers JV, Aravkin AY, Porter BA, Beatty JD. 18F-FDG PET/CT-positive internal mammary lymph nodes: pathologic correlation by ultrasound-guided fine-needle aspiration and assessment of associated risk factors. AJR Am J Roentgenol. 2013;200:1138–44.
Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–16.
Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317–27.
EBCTCG, McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–35.
Thorsen LB, Offersen BV, Dano H, Berg M, Jensen I, Pedersen AN, et al. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol. 2016;34:314–20.
Hennequin C, Bossard N, Servagi-Vernat S, Maingon P, Dubois JB, Datchary J, et al. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2013;86:860–6.
Chen L, Gu Y, Leaw S, Wang Z, Wang P, Hu X, et al. Internal mammary lymph node recurrence: rare but characteristic metastasis site in breast cancer. BMC Cancer. 2010;10:479.
Cao L, Cai G, Chang C, Yang ZZ, Feng Y, Yu XL, et al. Early cardiac toxicity following adjuvant radiotherapy of left-sided breast cancer with or without concurrent trastuzumab. Oncotarget. 2016;7:1042–54.
Acknowledgements
This paper was presented in part at the 103rd annual meeting of the Radiological Society of North America (RSNA) in Chicago, 2017. It received a Certification of Merit for an education exhibit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Research involving human and/or animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
About this article
Cite this article
Urano, M., Denewar, F.A., Murai, T. et al. Internal mammary lymph node metastases in breast cancer: what should radiologists know?. Jpn J Radiol 36, 629–640 (2018). https://doi.org/10.1007/s11604-018-0773-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-018-0773-9