Abstract
Purpose
This study aimed to explore the clinical and prognostic significance of 18F-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) in epithelial ovarian cancer (EOC).
Methods
We retrospectively investigated 48 EOC patients who underwent preoperative 18F-FDG PET/CT and primary cytoreductive surgery at our hospital between January 2010 and June 2015. None of these patients received neoadjuvant chemotherapy. PET/CT parameters including the maximum and average standardized uptake value (SUVmax, SUVavg), the metabolic tumor volume (MTV) were measured. Tumor proliferation marker Ki67 was evaluated using immunohistochemistry. The relationships between the PET/CT parameters and chemosensitivity, tumor proliferation, and overall survival (OS) were analyzed, respectively.
Results
The median (range) SUVmax, SUVavg, and MTV values were 11.42 (3.14–20.20), 4.8 (2.55–9.47), and 150.11 (0.19–792.46), respectively. Overall, 93.8% (45/48) of patients had high-grade serous ovarian cancer. The SUVmax value had a positive correlation with the Ki67 index (P = 0.030, r = 0.314), and a higher SUVmax level was associated with chemosensitivity (P = 0.026). However, neither SUVavg nor MTV had associations with the patients’ clinicopathological parameters. None of these three PET/CT parameters were found to be potential predictors of OS.
Conclusions
Preoperative 18F-FDG PET/CT had a predictive value on chemosensitivity and proliferation after primary debulking surgery in EOC patients noninvasively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is one of the most commonly diagnosed and lethal gynecological malignancies among females worldwide [1]. About half of the patients will relapse within 16 months after primary debulking surgery and platinum-based adjuvant chemotherapy [2]. Thus, effective indicators are urgently required to earlier estimate the biological behavior and treatment efficacy of tumor cells to guide individual treatments.
18F-FDG PET/CT is an important molecular imaging modality for preoperative and therapeutic effect assessment in ovarian cancer. Previous studies have shown that semi-quantitative parameters of PET/CT, such as SUVmax, SUVavg, and MTV, are associated with patients’ clinical characteristics and prognoses in ovarian cancer [3,4,5,6,7,8,9]. Several investigators have found that higher PET/CT parameters are associated with an advanced FIGO stage and a higher tumor burden and could indicate a poorer prognosis [7,8,9,10,11]. However, other studies have failed to draw similar conclusions [12, 13].
Moreover, tumor cell proliferation and chemosensitivity are also two vital factors of malignant tumors. Nevertheless, research on the relationship between PET/CT parameters and ovarian cancer’s proliferative activity and chemosensitivity is limited. Thus, our study aimed to explore both clinical and prognostic significance of 18F-FDG PET/CT in epithelial ovarian cancer.
Materials and methods
Clinicopathological data
We retrospectively investigated 48 epithelial ovarian cancer patients who had preoperative 18F-FDG PET/CT scan and subsequent primary debulking surgery between January 2010 and June 2015 in our hospital. All patients had PET/CT scan within 2 weeks before primary surgery. Patients were excluded if they received neoadjuvant chemotherapy, or were treated for recurrent disease. The study protocol was approved by the institution’s review board.
All pathological slides were independently reviewed by two experienced gynecologic pathologists according to the WHO criteria. Immunohistochemical staining was performed for Ki67 (Roche 30-9) using a Ventana Benchmark XT autostainer (Ventana Medical Systems Inc., Tucson, AZ, USA).
The clinical data were collected from medical records, cancer registries, and pathology reports. Surgical outcome has been classified as R0, R1 and R2, according to residual disease (RD). R0 indicated no macroscopic residual disease, while R1 and R2 were defined as the maximum RD diameter of < 1 cm or ≥ 1 cm, respectively. The optimal surgery comprised R0 and R1. Chemosensitivity was defined as that we previously reported, a time interval of 6 months or longer between the completion of platinum-based chemotherapy and recurrence. Otherwise, the patient was chemoresistant [14, 15]. All of the patients were followed up until December 31, 2016. Overall survival (OS) was defined as the time interval from the date of operation to the date of death or the last follow-up.
18F-FDG PET/CT protocol
18F-FDG was produced automatically by cyclotron (Siemens CTI RDS Eclipse ST, Knoxville, TN, USA) using the Explora FDG4 module in our center. Radiochemical purity was over 95%. All patients were asked to fast at least 6 h before the 18F-FDG PET/CT procedure. The patient’s blood glucose level was below 10 mmol/L at the time of radio-tracer injection. The standard dosage of intravenous 18F-FDG administration was 7.4 MBq/kg. Before and after injection, patients were kept lying comfortably in a quiet, dimly lit room. Examination was performed 60 min after 18F-FDG injection. A Siemens Biograph 16HR PET/CT scanner (Knoxville, TN, USA) with 4 mm transaxial intrinsic spatial resolution (full width at half maximum) and 16.2 cm axial field width was used for image scanning. Whole-body PET/CT data acquisition began with low-dose CT from the inguinal region to the head, with 120 kV, a 80–250 mA automatic adjustment, a pitch of 3.6, and a 0.5 s rotation time, followed by PET emission scan in a 3-dimensional mode, with 2–3 min per bed position. The PET data were reconstructed using the ordered-subset expectation maximization technique selecting eight subsets and four iterations, a 168 × 168 matrix. The CT data were used for attenuation correction of the PET images, and coregistered images were displayed on a workstation. The reconstructed images were then converted to a semiquantitative image corrected by the injection dose and the subject’s body weight (SUV).
Imaging interpretation
All PET/CT images were independently evaluated by two experienced nuclear medicine physicians. Consensus was reached in case of discrepancy. Analysis of the 18F-FDG PET/CT images was performed using a multimodality computer platform (Syngo, Siemens, Knoxville, TN, USA). SUVmax for each patient was calculated by placing a spheroid-shaped volume of interest (VOI) within the primary ovarian lesion. The SUV was calculated as [decay-corrected activity (kBq) per milliliter of tissue volume]/[injected 18F-FDG activity (kBq) per gram of body mass]. MTV and SUVavg were measured by drawing a contour of the ovarian lesion large enough to encase the primary tumor in the axial, coronal, and sagittal PET images. We used a threshold of SUV of 2.5 [16,17,18], which is usually used in clinical settings, to define the margins around the target. The boundaries of voxels, the SUV of which exceeded a threshold of 2.5 SUV, were automatically produced. The voxels presenting an SUV intensity of greater than 2.5 within the contouring margin were incorporated to define the MTV, and the mean voxels within this contouring were measured as SUVavg. The kidneys, ureters, and the urinary bladder were manually subtracted to ensure the correct voxel.
Statistical analysis
SPSS statistical software (version 21.0, SPSS, IBM Inc., Armonk, NY, USA) was used for statistical analyses. For demographic data, a descriptive statistical analysis method was used for analysis, and the results were summarized as means with standard deviations (SD), medians with ranges, or frequencies with percentages. The categorical data were compared using Chi-square or Fisher’s exact tests. Correlations between SUVmax, SUVavg, MTV, and Ki67 were tested using Pearson’s correlation analysis. OS was analyzed using the Kaplan–Meier method, and log-rank tests were used in the univariate analyses. P < 0.05 was considered statistically significant, and all P values reported were 2-sided.
Results
Patients’ characteristics
Patients’ characteristics are described in Table 1. Among the 48 patients, the median (range) age of the patients was 52 (27–76) years, and 43 (89.6%) had advanced FIGO (stage III–IV). The majority of the patients (93.8%) had high-grade serous ovarian cancer. The median (range) Ki67 index was 70% (15–95%).
Optimal surgery was achieved for 42 patients (87.5%), including 17 debulked to R0 and 25 to R1. All patients received platinum-based adjuvant chemotherapy after surgery. Among these, 6 patients had disease progression during chemotherapy, and 32 (66.7%) were sensitive to platinum-based chemotherapy. The median (range) follow-up time was 27 (8–61) months. Nineteen patients (39.6%) relapsed during the follow-up period. At the end of the follow-up, 30 patients were still alive, seven were dead, and the other 11 were censored.
PET/CT parameters and their associations with clinicopathological characteristics and prognosis
As for preoperative PET/CT scan parameters, the median (range) SUVmax, SUVavg, and MTV values were 11.42 (3.14–20.2), 4.8 (2.55–9.47), and 150.11 (0.19–792.46), respectively. We analyzed the relationships between the clinical features and the PET/CT parameters. SUVmax, SUVavg, and MTV had no association with age, FIGO stage, CA125 level, HE4 level, ascites and postoperative residual disease.
Overall, 83.3% of our patients had a high Ki67 index (> 50%), and the remaining seven patients had a moderate Ki67 index (5–50%). There was a significant positive correlation between the SUVmax value and the Ki67 index (P = 0.030, r = 0.314). Nevertheless, neither SUVavg nor MTV values were associated with Ki67 index (P = 0.051 and r = 0.283 and P = 0.586 and r = − 0.081, respectively) (Fig. 1). Furthermore, chemosensitive patients had higher SUVmax values than those who were chemoresistant. However, there were no differences in SUVavg and MTV values between these two groups (Table 2).
In addition, patients were dichotomized by the median of each PET/CT parameter into high and low level groups for prognostic analyses, respectively. There were no differences in OS between the high and low SUVmax, SUVavg, or MTV groups, respectively (Fig. 2).
Discussion
Our study demonstrated that the SUVmax value is associated with Ki67 index and chemosensitivity. However, neither SUVavg nor MTV had associations with patients’ clinicopathological parameters, and none of these three PET/CT parameters were found to be potential predictors of OS.
Compared to conventional imaging technologies such as CT, MR, and ultrasound, 18F-FDG PET/CT could not only assess anatomical or morphological features of malignant tumors, but also provide their functional or metabolic characteristics [19, 20]. PET/CT scan is used increasingly during ovarian cancer treatment for its noninvasive and multifunctional advantages [5, 10, 12, 21] (Figs. 3, 4).
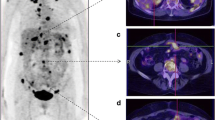
a–c Representative transaxial CT (a), PET (b) and PET/CT fused (c) ovarian tumor (white arrow showed) images of a 68-year-old chemosensitive patient with a FIGO stage IIIC. Postoperative pathology was HGSC with a 85% Ki67 value. The patient underwent six cycles’ chemotherapy after debulking surgery, and had no recurrence and metastasis until the last follow-up date. The SUVmax, SUVavg and MTV of ovarian tumor were 19.2, 6.8 and 164.96, respectively
a–c Representative transaxial CT (a), PET (b) and PET/CT fused (c) images of ovarian tumor (cross showed) in a 60-year-old chemoresistant patient staged IIIC. Postoperative pathology was HGSC with a 55% Ki67 value. The patient underwent eight cycles’ chemotherapy and recurred 12 months after cytoreductive surgery. The SUVmax, SUVavg and MTV were 6.4, 3.5 and 40.31, respectively
The Ki67 index is a vital predictive and prognostic marker for malignant tumors [22]. It is routinely used to estimate tumor proliferative activity in daily clinical practice. Several studies have shown that PET/CT parameters are correlated with the Ki67 index and thus could indicate tumor proliferation in several types of cancer [22,23,24,25,26]. Data for ovarian cancer proliferation are limited. Our study found that SUVmax had a positive correlation with the Ki67 value, while SUVavg or MTV did not. This may be because higher proliferative tumor cells have increasing glucose transporter proteins and hexokinase and glucose-6-phosphatase activities [27]. This will lead to higher 18F-FDG uptake accumulation, which could be directly reflected by SUVmax. However, SUVavg and MTV are also affected by many other factors such as viable tumor cell numbers and intra-tumor heterogeneity [28]. Thus, our research provides a noninvasive method using PET/CT scan to examine tumor cell proliferation.
Chemosensitivity is a vital prognostic factor for ovarian cancer. Previous studies have attempted to earlier estimate chemosensitivity during ovarian cancer neoadjuvant chemotherapy (NACT). Tuulia et al. [29] found that over 57% of SUVmax reduction after four cycles NACT was associated with a better histopathological response. Avril et al. [30] further indicated that PET/CT after the first cycle of NACT could earlier predict chemotherapy response instead of PET/CT after three cycles. Compared to those above, our study showed that we can predict treatment sensitivity before chemotherapy for the first time. According to the SUVmax value, we might select the proper patients for NACT or another individual treatment in a future clinical study.
Previous studies have also shown the promising prognostic significance of PET-derived parameters for ovarian cancer [6, 7, 12, 30]. Yamamoto et al. [12] demonstrated that MTV could, but SUVmax could not, serve as a prognostic factor for PFS in primary ovarian cancer patients. Konishi et al. [6] showed that SUVmax represented a prognostic factor in clear cell adenocarcinoma. Our study failed to find the prognostic value of SUV and MTV. This may be due to the limited number of patients and short follow-up time.
The limitation of our study was that it was a retrospective study with potential recall bias. More patient enrollment and further follow-up are needed in the future to strengthen our findings.
Notwithstanding its limitation, our study involved a homologous group of patients with high-grade serous ovarian cancer, up to 93.8%. Moreover, we focused on ovarian cancer proliferation and chemosensitivity prediction using PET/CT, which is vital but was always neglected by previous studies.
Conclusions
In conclusion, our study suggests that preoperative SUVmax of 18F-FDG PET/CT has a predictive value on proliferation and chemosensitivity after debulking surgery in epithelial ovarian cancer patients noninvasively. Future prospective cooperative multicenter studies are needed to further identify the predictive significance of PET/CT in ovarian cancer.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Berek JS, Crum C, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2015;131(Suppl 2):S111–22.
Risum S, Loft A, Hogdall C, Berthelsen AK, Hogdall E, Lundvall L, et al. Standardized FDG uptake as a prognostic variable and as a predictor of incomplete cytoreduction in primary advanced ovarian cancer. Acta Oncol (Stockholm, Sweden). 2011;50(3):415–9.
Nakamura K, Hongo A, Kodama J, Hiramatsu Y. The pretreatment of maximum standardized uptake values (SUVmax) of the primary tumor is predictor for poor prognosis for patients with epithelial ovarian cancer. Acta Med Okayama. 2012;66(1):53–60.
Tanizaki Y, Kobayashi A, Shiro M, Ota N, Takano R, Mabuchi Y, et al. Diagnostic value of preoperative SUVmax on FDG-PET/CT for the detection of ovarian cancer. Int J Gynecol Cancer. 2014;24(3):454–60.
Konishi H, Takehara K, Kojima A, Okame S, Yamamoto Y, Shiroyama Y, et al. Maximum standardized uptake value of fluorodeoxyglucose positron emission tomography/computed tomography is a prognostic factor in ovarian clear cell adenocarcinoma. Int J Gynecol Cancer. 2014;24(7):1190–4.
Lee JW, Cho A, Lee JH, Yun M, Lee JD, Kim YT, et al. The role of metabolic tumor volume and total lesion glycolysis on 18F-FDG PET/CT in the prognosis of epithelial ovarian cancer. Eur J Nucl Med Mol Imaging. 2014;41(10):1898–906.
Chung HH, Kwon HW, Kang KW, Park NH, Song YS, Chung JK, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis in patients with epithelial ovarian cancer. Ann Surg Oncol. 2012;19(6):1966–72.
Fanfani F, Monterossi G, Fagotti A, Gallotta V, Costantini B, Vizzielli G, et al. Positron emission tomography–laparoscopy based method in the prediction of complete cytoreduction in platinum-sensitive recurrent ovarian cancer. Ann Surg Oncol. 2015;22(2):649–54.
Vargas HA, Burger IA, Goldman DA, Micco M, Sosa RE, Weber W, et al. Volume-based quantitative FDG PET/CT metrics and their association with optimal debulking and progression-free survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery. Eur Radiol. 2015;25(11):3348–53.
Kitajima K, Suenaga Y, Ueno Y, Maeda T, Ebina Y, Yamada H, et al. Preoperative risk stratification using metabolic parameters of 18F-FDG PET/CT in patients with endometrial cancer. Eur J Nucl Med Mol Imaging. 2015;42(8):1268–75.
Yamamoto M, Tsujikawa T, Fujita Y, Chino Y, Kurokawa T, Kiyono Y, et al. Metabolic tumor burden predicts prognosis of ovarian cancer patients who receive platinum-based adjuvant chemotherapy. Cancer Sci. 2016;107(4):478–85.
Kim CY, Jeong SY, Chong GO, Son SH, Jung JH, Kim DH, et al. Quantitative metabolic parameters measured on F-18 FDG PET/CT predict survival after relapse in patients with relapsed epithelial ovarian cancer. Gynecol Oncol. 2015;136(3):498–504.
Feng Z, Wen H, Bi R, Duan Y, Yang W, Wu X. Thrombocytosis and hyperfibrinogenemia are predictive factors of clinical outcomes in high-grade serous ovarian cancer patients. BMC Cancer. 2016;16:43.
Feng Z, Wen H, Ju X, Bi R, Chen X, Yang W, et al. Expression of hypothalamic-pituitary-gonadal axis-related hormone receptors in low-grade serous ovarian cancer (LGSC). J Ovarian Res. 2017;10(1):7.
Yang Z, Shi Q, Zhang Y, Pan H, Yao Z, Hu S, et al. Pretreatment 18F-FDG uptake heterogeneity can predict survival in patients with locally advanced nasopharyngeal carcinoma–a retrospective study. Radiat Oncol (London, England). 2015;10:4.
Kurtipek E, Cayci M, Duzgun N, Esme H, Terzi Y, Bakdik S, et al. 18F-FDG PET/CT mean SUV and metabolic tumor volume for mean survival time in non-small cell lung cancer. Clin Nucl Med. 2015;40(6):459–63.
Lee HJ, Lee JJ, Park JY, Kim JH, Kim YM, Kim YT, et al. Prognostic value of metabolic parameters determined by preoperative 18F-FDG PET/CT in patients with uterine carcinosarcoma. J Gynecol Oncol. 2017;28(4):e43.
Czernin J, Weber WA, Herschman HR. Molecular imaging in the development of cancer therapeutics. Annu Rev Med. 2006;57:99–118.
Jager PL, de Korte MA, Lub-de Hooge MN, van Waarde A, Koopmans KP, Perik PJ, et al. Molecular imaging: what can be used today. Cancer Imaging. 2005;5 Spec No A:S27–32.
Bono Y, Mizumoto Y, Nakamura M, Iwadare J, Obata T, Fujiwara H. FDG-PET-positive ovarian thecoma with GLUT5 expression: five cases. J Obstet Gynaecol Res. 2017;43(3):599–603.
Del Gobbo A, Pellegrinelli A, Gaudioso G, Castellani M, Zito Marino F, Franco R, et al. Analysis of NSCLC tumour heterogeneity, proliferative and 18F-FDG PET indices reveals Ki67 prognostic role in adenocarcinomas. Histopathology. 2016;68(5):746–51.
Hu SL, Yang ZY, Zhou ZR, Yu XJ, Ping B, Zhang YJ. Role of SUV(max) obtained by 18F-FDG PET/CT in patients with a solitary pancreatic lesion: predicting malignant potential and proliferation. Nucl Med Commun. 2013;34(6):533–9.
Abgral R, Leboulleux S, Deandreis D, Auperin A, Lumbroso J, Dromain C, et al. Performance of 18fluorodeoxyglucose-positron emission tomography and somatostatin receptor scintigraphy for high Ki67 (≧ 10%) well-differentiated endocrine carcinoma staging. J Clin Endocrinol Metab. 2011;96(3):665–71.
Ueda S, Tsuda H, Saeki T, Omata J, Osaki A, Shigekawa T, et al. Early metabolic response to neoadjuvant letrozole, measured by FDG PET/CT, is correlated with a decrease in the Ki67 labeling index in patients with hormone receptor-positive primary breast cancer: a pilot study. Breast Cancer (Tokyo, Japan). 2011;18(4):299–308.
Onishi R, Noguchi M, Kaida H, Moriya F, Chikui K, Kurata S, et al. Assessment of cell proliferation in renal cell carcinoma using dual-phase 18F-fluorodeoxyglucose PET/CT. Oncol Lett. 2015;10(2):822–8.
Aloj L, Caraco C, Jagoda E, Eckelman WC, Neumann RD. Glut-1 and hexokinase expression: relationship with 2-fluoro-2-deoxy-d-glucose uptake in A431 and T47D cells in culture. Cancer Res. 1999;59(18):4709–14.
Tanimoto K, Yoshikawa K, Obata T, Ikehira H, Shiraishi T, Watanabe K, et al. Role of glucose metabolism and cellularity for tumor malignancy evaluation using FDG-PET/CT and MRI. Nucl Med Commun. 2010;31(6):604–9.
Vallius T, Peter A, Auranen A, Carpen O, Kemppainen J, Matomaki J, et al. 18F-FDG-PET/CT can identify histopathological non-responders to platinum-based neoadjuvant chemotherapy in advanced epithelial ovarian cancer. Gynecol Oncol. 2016;140(1):29–35.
Avril N, Sassen S, Schmalfeldt B, Naehrig J, Rutke S, Weber WA, et al. Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. J Clin Oncol. 2005;23(30):7445–53.
Funding
This work was funded by the project of the Science and Technology Commission of Shanghai Municipality for XH Wu (15411962000) and for ZX Jiang (15411968200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The ethics committee of our institution does not require patient consent for retrospective review of imaging studies.
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Liu, S., Feng, Z., Wen, H. et al. 18F-FDG PET/CT can predict chemosensitivity and proliferation of epithelial ovarian cancer via SUVmax value. Jpn J Radiol 36, 544–550 (2018). https://doi.org/10.1007/s11604-018-0755-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-018-0755-y