Abstract
Purpose
To compare the detectability of unenhanced abbreviated magnetic resonance imaging (MRI) based on diffusion-weighted imaging (DWI) and abbreviated postcontrast MRI for breast cancer.
Methods
The study population consisted of 87 patients undergoing breast MRI between December 2016 and March 2017 in a clinical setting. All breast MRIs were performed using a 1.5-T MRI scanner with a 16-channel breast radiofrequency coil. The abbreviated protocols based on DWI (AP1) and postcontrast MRI (AP2) were assessed independently by two radiologists. Sensitivity and specificity were calculated. Receiver operating characteristic analysis was performed and the areas under the curves (AUCs) were compared between AP1 and AP2.
Results
The study included 87 patients with 89 breast cancer lesions ≤ 2 cm in diameter. The sensitivity/specificity for AP1 and AP2 for reader 1 was 89.9/97.6% and 95.5/90.6%, respectively, and those for reader 2 was 95.5/94.1% and 98.9/94.1%, respectively. The AUCs for AP1 and AP2 for reader 1 were 0.9629 and 0.9640 (p = 0.95), respectively, and those for reader 2 were 0.9755 and 0.9843 (p = 0.46), respectively.
Conclusions
The detectability of the unenhanced abbreviated protocol based on DWI would be comparable to that of abbreviated postcontrast MRI for breast cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A family history of breast cancer or the presence of a germline mutation in the BRCA1 or BRCA2 gene increases the risk of breast cancer considerably and is often associated with diagnosis at a young age [1]. Breast magnetic resonance imaging (MRI) appears to be more sensitive than mammography for detecting breast cancer in women with a familial or genetic predisposition [1,2,3]. Breast MRI screening shifts the distribution of breast cancer toward lower stages and reduces the fraction of interval cancers [4]. Breast MRI screening is recommended for women with an approximately 20–25% or greater lifetime risk of breast cancer, including those with a strong family history of breast or ovarian cancer and those who have been treated for Hodgkin’s disease [5]. The full diagnostic protocol (FDP) of breast MRI typically includes T1-weighted imaging, T2-weighted imaging with/without fat saturation, and dynamic contrast enhancing MRI (DCE) (one precontrast and three to six scans for postcontrast). In a recent review, the average acquisition time of FDP among six studies [4, 6,7,8,9,10] was 24 min (range 17–40 min) [11]. However, FDP still takes a long time, including preparatory time and interpretation of the results.
There have been several recent studies of abbreviated MRI protocols [4, 6,7,8,9,10, 12,13,14,15]. Abbreviated MRI, which typically uses shortened dynamic postcontrast images from the early phase, has been shown to be as effective as FDP [4, 6,7,8,9, 12, 13, 15]. The average acquisition time of abbreviated MRI among six studies was 9 min (range 3–15 min) [11]. These findings are promising for the development of screening MRI protocols that are more comfortable for high-risk women. However, the intravenous contrast media increases the cost and may increase the examination time or incidence of adverse effects. Thus, screening breast MRI based on DCE is not cost-effective for women at intermediate risk, e.g., those with dense breast tissue as the only risk factor [11]. In addition, breast MRI is contraindicated in women with allergy to contrast medium.
Diffusion-weighted imaging (DWI) has been used for breast MRI. Several studies have shown that the apparent diffusion coefficient value can differentiate between benign and malignant tumors [16]. To our knowledge, however, few studies investigated the feasibility of DWI as a tool for detection of breast cancer and the sensitivity was different between the studies [17, 18]. A recent study comparing two abbreviated protocols based on DWI with background suppression and postcontrast MRI indicated that DWI could exclude malignancy in women with suspicious screening mammograms [19]. If the tumor detection of DWI is comparable to that of an abbreviated protocol based on postcontrast MRI, unenhanced abbreviated MRI for breast cancer would be practical as a screening tool.
The purpose of this preliminary study was to investigate the detectability of breast cancer between unenhanced abbreviated MRI based on DWI and abbreviated postcontrast MRI.
Materials and methods
Subjects and selection
The study was approved by our institutional review board, and the requirement for informed consent was waived due to the retrospective nature of the study. The initial study population consisted of 310 consecutive patients undergoing breast MRI between December 2016 and March 2017 for evaluation of suspicious findings on screening mammography or ultrasound, extension of breast cancer, detection of additional lesions, and evaluation of response to neo-adjuvant chemotherapy. A radiologist with 20 years of experience in breast MRI reviewed the full diagnostic MRI and medical charts of the patients. Patient enrollment is shown in Fig. 1. One hundred and thirty-one patients were excluded. The same radiologist measured the diameter of lesions in the remaining 179 patients. Of these, 82 patients with lesions > 2 cm in diameter were excluded, as invasive lesions of this size are regarded as large tumors [20] and T1 tumors are ≤ 2 cm in diameter. In this study, DCIS > 2 cm was also excluded because they were conspicuous both on DWI and postcontrast MRI. In the case of multicentric cancers, the largest tumor was taken as the representative lesion. Bilateral breast cancer was found in two patients. The lesion was counted as one lesion for each side of the breast. Therefore, the analysis included 99 lesions of breast cancer in 97 patients (Fig. 1). The first 10 lesions in 10 patients were assigned to the preparatory reading session, and the remaining 89 lesions in 87 patients were used for the main reading session. They comprised 89 breasts with breast cancer and 85 negative breasts.
Clinical manifestations of these 87 patients were as follows. Forty-six patients were symptomatic and 41 were not. The symptoms consisted of palpable tumor in 42 patients, breast pain in 2 patients and nipple discharge in 2 patients. Eighty-six patients underwent mammography examinations. Of these, seventy-five patients showed abnormal findings; mass (n = 42), microcalcifications (n = 9), mass with microcalcifications (n = 1), focal asymmetric density (n = 19), architectural distortion (n = 5) and mass with architectural distortion (n = 1). All patients demonstrated abnormal findings with ultrasound, which comprised mass (n = 78), nonmass (n = 5), mass with calcifications (n = 1) and nonmass with calcifications (n = 1).
MRI technique
Breast MRI was performed using a 1.5-T MRI scanner (Achieva; Philips Healthcare, Best, the Netherlands) with a 16-channel dedicated breast radiofrequency coil, with the patient in the prone position. The FDP included fat-suppressed T2-weighted images, T1-weighted images, DWI with background suppression, dynamic contrast enhanced (DCE) images, and sagittal postcontrast images. Scanning parameters are listed in Table 1. The total scan time was 16 min and 30 s. The contrast agent (gadobenate dimeglumine, 0.2 mmol/kg) was injected into an antecubital vein using an automated injector at a rate of 1 mL/s, followed by a 20-mL saline flush.
Image reading protocol
Two abbreviated reading protocols were performed: AP1 was based on DWI with a b factor of 1,000 s/mm2, which included fat-suppressed T2-weighted images and source and maximum intensity projection (MIP) images of DWI; and AP2, which included fat-suppressed T2-weighted images, and the source and MIP images of the second early phase (60–120 s) on DCE-MRI. ADCmap images were not included in the AP1 protocol.
The observation order of the 10 patients of AP2 protocol was the reverse of that used for the AP1 protocol in the preparatory reading session. In the main reading session, the observation order of the 87 patients of the AP1 and AP2 protocols was changed at random using a random number table. The images from the preparatory and main sessions were transferred to a picture archiving and communication system (PACS) (Rapideye; Toshiba Medical Systems Co., Tokyo, Japan). Patient information was deleted and a new number was noted on the display only.
Image interpretation
Two radiologists with 8 and 15 years of experience in breast imaging interpreted the image sets. They were aware that all patients had at least one known breast cancer but were blinded to the location nor side. Three image types were displayed on the monitor. The readers viewed the MIP images of the DWI or postcontrast MRI first to detect suspicious findings. They could rotate the MIP images in the horizontal direction. The readers then viewed the fat-suppressed T2-weighted and source images of DWI or postcontrast MRI in a synchronized manner. As the readers scrolled through both images, the line moved on the MIP images according to the slice position, which enabled the readers to refer to suspicious findings on the MIP images.
The readers were instructed to complete a checklist for each breast of the 87 patients, which included intensity of the background parenchyma, location of the suspicious lesion, and confidence regarding malignancy scored on a five-point scale: 1, no suspicious lesion detected; 2, benign lesion; 3, likely benign lesion but malignant lesion cannot be excluded; 4, likely malignant lesion; and 5, highly suspicious lesion. The point 3 or more was regarded as being positive. The level of intensity of the background parenchyma on postcontrast MRI was determined according to the Breast Imaging Reporting and Data system (BI-RADS). The BI-RADS does not define background intensity for DWI MRI; however, we used a definition similar to that of the BI-RADS. Finally, the readers were instructed to measure the observation time for each case.
The AP1 preparatory sessions were assessed first followed by the AP2 preparatory sessions, and the main sessions were then read. The AP1 and AP2 sessions were assessed at 2-week intervals.
Pathological evaluation
The pathological diagnoses were retrieved from the electronic records of our institution. All tissue specimens were examined by a pathologist with more than 10 years of experience in breast pathology. All participants underwent core-needle breast biopsy according to the location of the lesion on the screening X-ray mammogram using ultrasound (US) or conventional X-ray guidance. The final pathological diagnoses were made based on surgical specimens for patients who underwent surgery after the imaging studies. The core-needle biopsy was considered representative for patients who refused the surgery or were transferred to other hospitals. The intrinsic lesion subtype was determined based on the presence of estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2). ER and PgR were considered positive if ≥ 10% of the nuclei stained positive. HER2 was evaluated using the HercepTest (Dako, Glostrup, Denmark) and scored on a scale from 0 to 3+. Tumors with scores ≥ 3 or with a ≥ 2.2-fold increase in HER2 gene amplification, as determined by fluorescence in situ hybridization, were considered positive for HER2 overexpression.
Statistical analysis
The distributions of the intensity of background parenchyma between AP1 and AP2 were examined using Chi-square test.
The sensitivity and specificity of both readers were calculated. Receiver operating characteristic (ROC) analysis was performed. The areas under the curves (AUCs) of both readers were calculated for AP1 and AP2. The differences in the AUCs for the AP1 and AP2 protocols were calculated for each reader. The interobserver agreements on the five-point confidence of malignancy scale and the intensity of the background parenchyma were evaluated using the κ-scores. The reading time for both protocols was assessed using the paired t test.
JMP Pro 13 software (SAS Institute, Cary, NC) was used to process the data and perform the statistical analyses. In all analyses, p < 0.05 was taken to indicate statistical significance.
Results
Pathological evaluation
The pathological diagnosis was made by surgery in 63 patients, core-needle biopsy in 23 patients, and US-guided vacuum-assisted biopsy in 2 patients.
A total of 89 breast cancer lesions were detected. The histological types of the breast cancer are shown in Table 2. Most of the lesions were invasive ductal carcinoma (IDC), and ductal carcinoma in situ (DCIS) accounted for 13% of the cancer lesions. Most of the lesions were of the luminal A histological subtype and 10% were HER2-overexpressing and triple-negative subtypes (Table 2).
Interobserver agreement for intensity of the background parenchyma
The intensity level of the background parenchyma of AP2 showed a higher grade (p < 0.0001) (Table 3). The interobserver agreement for the AP1 protocol had a κ value of 0.45 (95% confidence interval (CI) 0.30, 0.61; p < 0.0001), and the κ value for the AP2 protocol was 0.56 (95% CI: 0.42, 0.69; p < 0.0001).
Tumor locations
Tumors located at the boundary of quadrants were counted in both quadrants. Thus, the locations of the tumors were as follows: 29 lesions in the upper inner quadrant, 13 lesions in the lower inner quadrant, 42 lesions in the upper outer quadrant, 25 lesions in the lower outer quadrant, and three lesions in the subareolar area.
Interobserver agreement for the abbreviated protocols
The overall interobserver agreement for the protocols had a κ value of 0.56 [95% CI: 0.49, 0.62]. The κ value for the AP1 (DWI) protocol was 0.55 (95% CI 0.46, 0.64) and that for the AP2 (postcontrast) protocol was 0.56 (95% CI 0.48, 0.65).
Detectability of breast cancer by the abbreviated protocols
When the location of the lesion marked by the readers in the check sheet was correct and they graded it with a score ≥ 3, the diagnosis was regarded as a true positive. The sensitivity/specificity of the AP1 and AP2 protocols for reader 1 was 89.9% (80/89)/97.6% (83/85) and 95.5% (85/89)/90.6% (77/85), respectively, and those for reader 2 was 95.5% (85/89)/94.1% (80/85) and 98.9% (88/89)/94.1% (80/85), respectively (Table 4). The sensitivity of the AP2 protocol was higher than that of the AP1 protocol for both readers, suggesting that more positive findings were detected with the abbreviated protocol based on postcontrast MRI. However, the specificity of the AP2 protocol was lower than that of the AP 1 protocol for reader 1. The accuracy was not significantly different between protocols. The AUCs for the AP1 and AP2 protocols for reader 1 were 0.963 and 0.964, respectively (p = 0.95), and 0.976 and 0.984, respectively (p = 0.46), for reader 2 (Table 4).
Both readers detected most of the lesions with each protocol (Fig. 2). The diameter of tumors on postcontrast MR ranged from 0.6 to 2 cm (average 1.4 cm). The smallest tumors detected on AP1 were the two mass lesions 0.6 cm in diameter. In addition, six mass lesions 0.7 cm in diameter were detected on AP1. The nine missed tumors on AP1 by reader 1 and/or 2 ranged in size from 0.8 to 1.9 cm (median 1 cm) in diameter, of which five tumors were 1 cm or less in diameter. The four tumors missed by reader 1 and/or 2 on AP2 ranged in size from 0.8 to 1 cm (median 1 cm) in diameter.
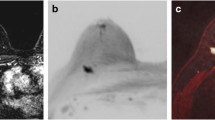
A 55-year-old woman with ductal carcinoma in situ in the left breast. Both readers identified the lesion in both protocols. a A frontal MIP image from DWI (upper) and postcontrast MRI (lower). The MIP image from DWI showed stippled lesions in the outer upper quadrant of the left breast (circle). The MIP image from the postcontrast MRI also shows the lesion (circle). b The source image from DWI (upper) showed a mass in the left breast (circle). The fat-suppressed T2-weighted image (lower) showed the mass with mixed hyper- and intermediate intensity (circle)
In terms of histopathological results, both readers missed four DCIS lesions with the AP1 protocol (Fig. 3): one with a Ki-67 index of 20%, and those of the remaining lesions were ≤ 10%. In addition, reader 1 missed two DCIS lesions (one high-grade lesion and one with a Ki-67 index of 20%) and three luminal A subtype IDCs. With the AP2 protocol, reader 1 missed four DCIS lesions that were also missed with the AP1 protocol and one luminal A subtype IDC. Reader 2 missed one DCIS lesion that reader 1 missed with both protocols.
A 67-year-old female with invasive ductal carcinoma in the right breast and ductal carcinoma in situ in the left breast. Both readers identified the right lesion but missed the left lesion. a Frontal MIP image from DWI (upper) and postcontrast MRI (lower). Bilateral nodular lesion (arrowhead and arrow). b The source image from DWI showed the mass in the right breast (arrowhead) and another mass in the left breast (arrow). c The fat-suppressed T2-weighted image (upper) showed a spiculated mass in the left breast (arrow). The spiculated mass (arrow) was more conspicuous on the source image from postcontrast MRI (lower)
Reading time
The reading time for reader 1 was significantly shorter than that for reader 2 (mean, 13.5 s vs. 31.8 s, respectively; p < 0.0001). Reader 1’s mean reading times for the AP1 (13.2 s) and AP2 (13.5 s) protocols were not significantly different (p = 0.68), whereas reader 2’s mean reading time for the AP2 protocol (34.9 s) was significantly longer than that for the AP1 protocol (28.4 s; p = 0.001) (Table 4).
Discussion
We investigated the detectability of breast cancer on abbreviated unenhanced breast MRI based on DWI that included MIPs. Our findings indicated that this technique is comparable with an abbreviated protocol based on postcontrast MRI.
The abbreviated protocol based on postcontrast MRI (AP2) showed high sensitivity in our study, which was a little better than the sensitivities ranging from 86 to 92% in the previous reports [6, 9, 12, 13]. The population that was known to have breast cancer probably caused this result. The previous studies showed that the diagnostic accuracy of abbreviated postcontrast breast MRI, which typically uses one early phase dynamic postcontrast image, was similar to that of a full diagnostic protocol [4, 6, 7, 9, 10, 12, 13, 15]. In one of them, the AUCs for abbreviated and full diagnostic protocols were 0.931 and 0.947, respectively [12], suggesting that this technique is a potential screening tool for women at high risk of breast cancer. However, the procedure used contrast medium, which requires the presence of medical personnel to administer and monitor for adverse events, thus increasing the cost and overall acquisition time.
The detectability of breast cancer on abbreviated unenhanced MRI based on DWI was comparable to that of abbreviated postcontrast MRI. DWI has been shown to be highly effective in discriminating malignant lesions from benign in patients with known breast cancer and clinically suspicious findings [16], implying that DWI may be an effective screening tool. To our knowledge, however, there have been few studies regarding the tumor detection on DWI even in the clinical setting [17, 18]. In one study, the sensitivity was 50% and was not as high as ours. Bickelhaupt et al. [19] compared the diagnostic abilities of two abbreviated protocols using the patients with suspicious mammographic findings. The sensitivity and specificity of the abbreviated protocol based on DWI were 92 and 94%, respectively, and those of the protocol based on postcontrast MRI were 85 and 90%, respectively. The authors concluded that the abbreviated non-contrast-enhanced MRI based on DWI was highly accurate. The sensitivity and specificity on AP1 (based on DWI) in our study were similar to those of Bickelhaupt et al. In addition, the sensitivity on AP1 in our study was no worse than that of full-diagnostic postcontrast MRI (79.5 and 94%) in the screening setting [1, 3]. Thus, abbreviated unenhanced MRI based on DWI could have a potential for screening breast MRI.
We found that obtaining T2-weighted images with fat suppression was frequently a useful adjunct to DWI, although we did not compare the diagnostic abilities of DWI with T2-weighted images and DWI without T2-weighted images. Breast cancer typically shows an intermediate signal intensity and the margin was more conspicuous on the fat-suppressed T2-weighted images, enabling the readers to discriminate between cysts and tumors. In a previous study, T2-weighted images significantly increased lesion conspicuity but did not alter the detection rate of breast cancer in abbreviated postcontrast MRI [10]. We believe that T2-weighted images are a useful adjunct to DWI, whereas T2-weighted images may not be necessary for the abbreviated postcontrast protocol because the source image of postcontrast MRI shows conspicuous differences between cysts and tumors. Nevertheless, the overall examination time, including preparation, should be shorter for the abbreviated protocol based on DWI than for the abbreviated postcontrast MRI, which would improve the cost-effectiveness as screening MRI and access by the women at high or even intermediate risk of breast cancer.
Although the lesion detectability did not differ significantly between our abbreviated protocols, the readers missed more lesions with the AP1 (DWI) protocol. The missed tumors on AP1 ranged in size from 0.8 to 1.9 cm, of which about half tumors were 1 cm or less in diameter. Most of the missed tumors on AP2 were 1 cm or less in diameter. This was probably because DWI has lower resolution than postcontrast MRI despite the reduced intensity of the background parenchyma. Regarding the histopathological findings, the missed lesions common to both readers were DCIS. Although DCIS lesions were missed with the AP2 protocol (postcontrast), this was less frequent than with the AP1 protocol. Bickelhaupt et al. reported that two readers missed DCIS lesions with both protocols [19]. Given the current status of breast MRI, missing DCIS lesions is inevitable. A recent study indicated that surgical treatment of low-grade DCIS did not improve prognosis [21], and that BRCA1 mutation carriers did not develop DCIS [22, 23]. The importance of detecting DCIS lesions in high-risk women may be less than for regular screening. However, imaging studies cannot definitively distinguish low-grade DCIS from intermediate- or high-grade DCIS. Furthermore, our readers missed a small number of high-grade DCIS or DCIS with Ki-67 index ≥ 20%, indicating that it is still necessary to improve DCIS detection. Moreover, reader 1 missed three luminal A subtype IDCs, which raises concerns about diagnostic accuracy. Similarly, the readers in the study of Bickelhaupt et al. missed a few invasive carcinomas [19]. Our findings may be related to the reading method, which may need to be improved.
Our readers viewed images of three types: fat-suppressed T2 weighted images, source images of DWI or postcontrast MRI, and MIPs. We believe that this is a practical approach. The readers were instructed to use this method and were able to practice during the preparatory sessions. However, the reading time differed considerably between readers 1 and 2 for the main sessions. Reader 1 spent less time and missed more carcinomas, including three IDCs. It is likely that the results would have improved if reader 1 had taken more time to read the images.
Our study had several limitations. First, all patients were known to have cancer lesions and underwent breast MRI in the clinical setting. We did not include normally appearing cases as controls because these patients were not followed up in a reasonable period. This made us difficult to assess the diagnostic ability of abbreviated protocols and lead to assess the lesion detectability. Many patients were symptomatic and detected on other examinations. Although we limited the lesions in small size ≤ 2 cm in diameter, the population was quite different from the screening setting. This population would have influenced the detection of positive findings. In fact, the sensitivity and specificity values in our study were similar but slightly better than those reported by Bickelhaupt et al. [19]. Second, our patients were enrolled from a daily clinical practice and may be different from high-risk women recommended to undergo annual MR screening based on age, disease distribution, and the intensity of the background parenchyma. Third, we did not use ADCmap images. Although ADC value measurement may help to distinguish between benign and malignant lesions, it is time-consuming to measure as a screening tool. However, ADCmap images may be optional if DWI is incorporated into screening breast MRI.
In conclusion, the detectability of unenhanced abbreviated breast MRI based on DWI to detect breast cancers ≤ 2 cm in diameter was comparable to that of an abbreviated protocol based on postcontrast MRI for the population which was known to have breast cancer. The DWI protocol may have a potential for screening breast MRI; however, further validation study is mandatory to demonstrate the feasibility of DWI as a breast screening tool.
References
Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–37.
Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, Konig R, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010;28:1450–7.
Passaperuma K, Warner E, Causer PA, Hill KA, Messner S, Wong JW, et al. Long-term results of screening with magnetic resonance imaging in women with BRCA mutations. Br J Cancer. 2012;107:24–30.
Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32:2304–10.
Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89.
Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS. Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol. 2015;22:1157–62.
Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L. An abbreviated protocol for high-risk screening breast MRI saves time and resources. J Am Coll Radiol. 2016;13:R74–80.
Mango VL, Morris EA, David Dershaw D, Abramson A, Fry C, Moskowitz CS, et al. Abbreviated protocol for breast MRI: are multiple sequences needed for cancer detection? Eur J Radiol. 2015;84:65–70.
Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Abbreviated combined MR protocol: a new faster strategy for characterizing breast lesions. Clin Breast Cancer. 2016;16:207–11.
Heacock L, Melsaether AN, Heller SL, Gao Y, Pysarenko KM, Babb JS, et al. Evaluation of a known breast cancer using an abbreviated breast MRI protocol: correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol. 2016;85:815–23.
Chhor CM, Mercado CL. Abbreviated MRI protocols: wave of the future for breast cancer screening. AJR Am J Roentgenol. 2017;208:284–9.
Chen SQ, Huang M, Shen YY, Liu CL, Xu CX. Application of abbreviated protocol of magnetic resonance imaging for breast cancer screening in dense breast tissue. Acad Radiol. 2017;24:316–20.
Machida Y, Shimauchi A, Kanemaki Y, Igarashi T, Harada M, Fukuma E. Feasibility and potential limitations of abbreviated breast MRI: an observer study using an enriched cohort. Breast Cancer. 2017;24:411–9.
Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162:283–95.
Panigrahi B, Mullen L, Falomo E, Panigrahi B, Harvey S. An abbreviated protocol for high-risk screening breast magnetic resonance imaging: impact on performance metrics and BI-RADS assessment. Acad Radiol. 2017;24:1132–8.
Chen X, Li WL, Zhang YL, Wu Q, Guo YM, Bai ZL. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010;10:693.
Kuroki-Suzuki S, Kuroki Y, Nas K, Nawano S, Moriyama N, Okazaki M. Detecting breast cancer with non-contrast MR imaging: combining diffusion-weighted and STIR imaging. Magn Reson Med Sci. 2007;6:21–7.
Yabuuchi H, Matsuo Y, Sunami S, Kamitani S, Kawanami S, Setoguchi T, et al. Detection of non-palpable breast cancer in asymptomatic women by using unenhanced diffusion-weighted and T2-weighted MR imaging: comparison with mammography and dynamic contrast-enhanced MR imaging. Eur Radiol. 2011;21:11–7.
Bickelhaupt S, Laun FB, Tesdorff J, Lederer W, Daniel H, Stiever A, et al. Fast and noninvasive characterization of suspicious lesions detected at breast cancer X-ray screening: capability of diffusion-weighted MR imaging with MIPs. Radiology. 2016;278:689–97.
Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375:1438–47.
Sagara Y, Mallory MA, Wong S, Aydogan F, DeSantis S, Barry WT, et al. Survival benefit of breast surgery for low-grade ductal carcinoma in situ: a population-based cohort study. JAMA Surg. 2015;150:739–45.
Hamilton LJ, Evans AJ, Wilson AR, Scott N, Cornford EJ, Pinder SE, et al. Breast imaging findings in women with BRCA1- and BRCA2-associated breast carcinoma. Clin Radiol. 2004;59:895–902.
Schrading S, Kuhl CK. Mammographic. US and MR imaging phenotypes of familial breast cancer. Radiology. 2008;246:58–70.
Acknowledgements
We would like to thank Mr. Seiji Umano, the radiology technician who assisted with preparation of the image sets in the picture archiving and communication system server.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Takayuki Yamada: There is no conflict of interest to disclose with respect to the study. Yoshihide Kanemaki: There is no conflict of interest to disclose with respect to the study. Satoko Okamoto: There is no conflict of interest to disclose with respect to the study. Yasuo Nakajima: There is no conflict of interest to disclose with respect to the study.
IRB statement
This study was approved by our institutional review board.
About this article
Cite this article
Yamada, T., Kanemaki, Y., Okamoto, S. et al. Comparison of detectability of breast cancer by abbreviated breast MRI based on diffusion-weighted images and postcontrast MRI. Jpn J Radiol 36, 331–339 (2018). https://doi.org/10.1007/s11604-018-0731-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-018-0731-6