Abstract
Purpose
To assess the benefit of combining diffusion-weighted imaging and dynamic contrast-enhanced (DCE) MR imaging in the preoperative evaluation of orbital lymphoma and other malignancies in adults.
Materials and methods
This retrospective study was institutional review board-approved and informed consent was waived. Pretreatment MR imaging was performed in 52 patients with malignant orbital lesions. Apparent diffusion coefficient (ADC) and DCE MR parameters were evaluated in 30 patients with orbital lymphoma and 22 patients with other orbital malignancies. Independent-samples t-test or Wilcoxon rank-sum test was used to compare parameters. The diagnostic capability was evaluated by using receiver operating characteristic curve (ROC) analysis.
Results
Lymphoma demonstrated significantly lower mean ADC (P < 0.001), T max (P = 0.001), enhancement ratio (ER) (P < 0.001), contrast index (CI) (P < 0.001) and significantly higher washout ratio (WR) (P = 0.002) than other malignancies in the orbit. ROC analysis indicated that T max alone or a combination with ADC and ER showed the optimal sensitivity (96.67%), a combination of ADC and WR showed optimal specificity (95.45%), while a combination of ADC and ER showed optimal accuracy (90.38%) in differentiating lymphoma from other malignancies.
Conclusion
Multiparametric MRI can help to differentiate orbital lymphoma and other malignancies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Malignant orbital lesions present with a broad spectrum of diseases: lymphoma, malignant epithelial tumors; carcinoma and malignant connective tissue tumors; or sarcoma [1,2,3]. Orbital lymphoma is treated with low-dose radiation therapy [4], whereas surgery and postoperative radiation therapy are commonly used as initial local treatment for other malignancies in the orbit [5].
Diffusion-weighted (DW) imaging and dynamic contrast-enhanced (DCE) magnetic resonance (MR) imaging have been used for preoperative evaluation of space-occupying lesions in the orbit and both of them have proved to be valuable in differential diagnosis [1, 3, 6,7,8,9,10,11,12,13,14,15,16]. DW imaging has made it possible to distinguish orbital lymphoma from other neoplastic and nonneoplastic orbital lesions [8] or orbital inflammation [1, 9, 15, 16]. DCE MR imaging shows high value in the characterization of orbital lesions [6, 11, 14,15,16]. Time intensity curve (TIC) patterns and TIC parameters prove to reflect the vascular physiology of orbital lymphoma [15, 16]. Furthermore, the combination of DW imaging and DCE MR imaging could increase the diagnostic performance of preoperative differentiation of orbital lesions in adults [14,15,16]. However, with regard to orbital lymphoma and other malignancies, the contributions of DCE MR imaging and the combined use of DW imaging and DCE MR imaging remain unclear.
Therefore, the purpose of our study was to compare the diagnostic performance of DW imaging, DCE-MR imaging, and both techniques combined for detection of lymphoma from other malignancies in the orbit.
Materials and methods
Study population
Our institutional review board approved this retrospective study and waived the informed consent requirement. From a retrospective review of the database of our institution during the period from 2013 to 2016, we identified 52 patients according to the following inclusion and exclusion criteria. The inclusion criteria for this study were: (a) patients underwent orbital MR imaging including DW imaging and DCE MR imaging before biopsy or surgery, (b) patients had no history of surgery or treatment in the affected orbit, (c) malignant tumors were pathologic findings identified from biopsy or surgery, and (d) patients were adult (age ≥ 18). The exclusion criteria for this study were as follows: (a) invasion into orbit from its vicinity, such as the skull base, paranasal sinus or nasopharynx; or (b) lesions originating from eyelids or globe. There were 52 patients who were identified on the basis of the final diagnosis of orbital lymphoma or other malignancies: 27 men (mean age 50 years; age range 19–67 years) and 25 women (mean age 48 years; age range 19–68 years). The final diagnoses of these patients are shown in Table 1 and the locations are detailed in Table 2.
Imaging protocol
All patients underwent 3.0-T MR imaging (Signa HDxt scanner; GE Healthcare) with use of an eight-channel high-resolution head coil. Our imaging protocol included axial fast spin echo (FSE) T1-weighted imaging (T1WI), axial FSE T2-weighted imaging (T2WI), and coronal FSE T1WI. The parameters were the following: T1WI: repetition time (TR), 400 ms; echo time (TE), 10 ms; T2WI: TR, 3000 ms; TE, 120 ms; number of excitations (NEX), 2; matrix, 384 × 256; field of view (FOV), 180 × 180 mm; section thickness, 3 mm; gap, 0.3 mm.
DW imaging
DW images were performed with echo planar imaging diffusion weighted imaging in the axial plane with fat suppression (TR 5000 ms, TE 64–77 ms; section thickness, 3 mm; gap, 0.3 mm; FOV, 160 × 160 mm; matrix, 128 × 128) sensitized to incoherent motion by a pair of gradient pulses. Two DW images were acquired with b values of 0, 1000 s/mm2.
DCE MR imaging
DCE MR imaging was acquired by using a fast-spoiled gradient recalled (FSPGR) sequence. The detailed imaging parameters were as follows: TR/TE, 8.4/4 ms; 1 excitation; flip angle, 15°; and a slice thickness of 3.2 mm at 0 intervals. A total of 37 scans with 16 sections were obtained. Each scan took 9 s; and there was an interval of 0–2 s between scans. Total scanning time was 344 s. Injection of gadolinium-based MR contrast agents (Magnevist; Bayer Schering, Berlin, Germany) at a dose of 0.1 mmol per kg of body weight was administered intravenously by using a power injector (Spectris Solaris; Medrad, Indianola, Pennsylvania) at a flow rate of 2 ml/s.
After obtaining DCE MR imaging data, axial, coronal, and oblique sagittal FSE T1WI was performed. Axial T1WI used fat suppression. The parameters of the pre-contrast sequence were also used in these sequences.
Imaging analysis
Two experienced head and neck radiologists (with 6 and 4 years of experience), who were blinded to diagnosis, reviewed all MR image sets, and selected a region of interest (ROI) for each study independently. DW imaging and DCE MR imaging were evaluated with a GE ADW 4.4 work-station (GE Healthcare, Milwaukee, Wisconsin) with functool 2 software.
The radiologists selected the affected side for ROI; when both sides were affected, we selected the side where the biopsy was conducted.
For DW imaging analysis, by using T2WI and contrast-enhanced axial images as a reference, the ROI was drawn manually to include the entire lesion on the apparent diffusion coefficient (ADC) map. Care was taken to avoid cystic, necrotic and hemorrhagic portions of the lesion that might influence the ADC value. Cystic components were hyperintense areas on T2WI. Necrotic components were the interior non-enhancing part on contrast-enhanced axial images. Hemorrhagic lesions were rare in the orbital lesions. To standardize the image analysis as much as possible, lesion ADC was measured in the section without the infiltrated extraocular muscles or optic nerve for reduction of the impact of normal tissue; strict attention was paid to normal lacrimal gland so that it was not included in ROI delimitation in some lesions; the edges of each lesion were excluded to reduce the partial volume effect; distortion artifacts were also carefully avoided from ROI delimitation.
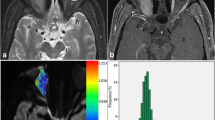
For DCE MR imaging analysis, the ROI was placed in the same location as for DW imaging. A TIC was then generated. The signal intensity (SI) was calculated from the mean pixel value on each acquired dynamic image.
-
1.
SIpre was the baseline signal intensity (SI corresponding to time T = 0) and SImax was the maximal signal intensity over the entire DCE MR imaging series. T max was measured from the time of arrival to peak time (T max = peak time − T1. Peak time was the time corresponding to the SImax). The enhancement ratio (ER) was calculated from ER = (SImax − SIpre)/SIpre [6, 11, 15,16,17,18,19,20].
-
2.
SIend was the final signal intensity over the entire DCE MR imaging series. Contrast index (CI) and washout ratio (WR) were calculated by using the following formulae, CI = (SIend − SIpre)/SIpre or WR = (SImax − SIend)/(SImax − SIpre) [6, 11, 15,16,17,18,19,20].
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation. The normality for any continuous variable was examined by using a Kolmogorov–Smirnov Z test. For normally distributed variables, an independent t-test was performed to examine the group effect. For variables that were not normally distributed, the Wilcoxon rank-sum test was used.
Receiver operating characteristic curve (ROC) analysis was performed to determine a cutoff value to predict lymphoma. Binary logistic regression analysis calculated the predicted probability of combination of ADC and DCE MR imaging parameters, then ROC analysis was performed to assess the diagnostic value of combined indexes.
To assess the reliability of measurements between radiologists, the interobserver variability was evaluated by using the intraclass correlation coefficient (ICC).
All tests were two-sided, and P < 0.05 was considered indicative of a significant difference. All statistical analyses were performed by using commercially available statistical software (SPSS for IBM, version 18.0, Chicago, IL, USA).
Results
DW imaging results
The lymphoma showed significantly lower mean ADC (0.694 ± 0.128 × 10−3 mm2/s) (Fig. 1) than other malignancies (1.186 ± 0.315 × 10−3 mm2/s) (Fig. 2) (t = 6.920, P < 0.001) (Table 3) (Fig. 3). By applying an ADC cutoff value of 0.888 × 10−3 mm2/s, the sensitivity (predicting lymphoma), specificity (predicting other malignancies), and diagnostic accuracy were 93.33, 81.82 and 88.46%, respectively (Table 3; Fig. 4), with an area under the ROC curve (AUC) of 0.912 [95% confidence interval (CI) 0.828, 0.996].
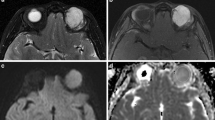
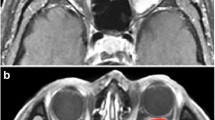
MR images in 60-year-old woman with lymphoma identified a well-defined mass molding around globe in the right intraconal space. a T1WI showed a homogeneously isointense mass. b T2WI showed a homogeneously isointense mass. c T1-weighted fat-suppressed post-contrast imaging shows mildly homogeneous enhancement of the mass. d DWI shows homogeneous isointensity with ADC = 0.672 × 10−3 mm2/s. e Time–intensity curve for this patient was characterized as a plateau pattern with T max = 122 s, ER = 0.7914, CI = 0.6930 and WR = 0.1243
MR images in a 45-year-old woman with adenoid cystic carcinoma demonstrated an oval well-defined mass in the right intraconal space. a T1WI showed a homogeneously isointense mass. b T2WI showed a homogeneously hyperintense mass. c T1-weighted fat-suppressed post-contrast image shows moderate and homogenous enhancement. d DWI shows a slightly high signal with ADC = 0.983 × 10−3 mm2/s. e Time–intensity curve for this patient was characterized as plateau pattern with T max = 121 s, ER = 1.6787, CI = 1.558 and WR = 0.0722
Box-and-whisker plots show ADC (a), T max (b), ER (c), CI (d) and WR (e) calculated for orbital lymphoma and carcinoma. The lymphoma showed significantly lower ADC, T max, ER, CI, and significantly higher WR than carcinoma. 1.00 group means orbital lymphoma; 2.00 group means orbital other malignancies
a Receiver operating curve analysis comparing the diagnostic accuracy of ADC, T max, ER, CI, and WR to differentiate orbital lymphoma from other malignancies. b Binary logistic regression and ROC analysis comparing the diagnostic accuracy of combination of DW imaging and DCE MR imaging to differentiate orbital lymphoma from other malignancies
DCE MR imaging results
Distribution of T max, ER, CI and WR between lymphoma and other malignancies is detailed in Table 3. All of them showed significant difference between lymphoma and other malignancies (P = 0.001, P < 0.001, P < 0.001, and P = 0.002, respectively) (Figs. 1, 2, 3).
Cutoff value, AUC, sensitivity (predicting lymphoma), specificity (predicting other malignancies) and accuracy of T max, ER, CI and WR are detailed in Table 4 (Fig. 4).
Combination of DW imaging and DCE MR imaging
With binary logistic regression analysis and ROC analysis, sensitivity (predicting lymphoma), specificity (predicting other malignancies) and accuracy of ADC combined with T max were 93.33, 81.82 and 88.46%, respectively, with AUC of 0.923 (95% CI 0.846, 0.999); of ADC combined with ER were 96.67, 81.82 and 90.38%, respectively, with AUC of 0.917 (95% CI 0.823, 1.000); of ADC combined with CI were 93.33, 81.82 and 88.46%, respectively, with AUC of 0.914 (95% CI 0.827, 1.000); of ADC combined with WR were 80, 95.45 and 86.54%, respectively, with AUC of 0.939 (95% CI 0.872, 1.000) (Table 4) (Fig. 4).
ROC analysis indicated that T max alone or a combination with ADC and ER showed the optimal sensitivity (96.67%), a combination of ADC and WR showed optimal specificity (95.45%), while a combination of ADC and ER showed optimal accuracy (90.38%) in differentiating lymphoma from other malignancies in the orbit.
Interobserver agreement
There was perfect agreement interobserver agreement for mean ADC (k = 0.966), T max (k = 0.927), ER (k = 0.912), CI (k = 0.925) and WR (k = 0.800) (Table 3).
Discussion
Orbital lymphoma is the most common malignant orbital tumor in adults [8, 21], but it still poses a great clinical and radiological diagnostic challenge because its manifestation and characteristics with conventional imaging are not specific. To our knowledge, there has been no major published study on the diagnostic capabilities of DW imaging and DCE MR imaging in distinguishing lymphoma from other malignancies in the orbit. Our study found that the differences on DW imaging and DCE MR imaging were great between orbital lymphoma and other malignancies.
Our DW imaging findings of orbital lymphoma were similar to those in previous studies [8, 9, 15], which showed relatively low mean ADC values, due to their high cellularity and their high nucleus-to-cytoplasm ratio [1, 8, 9, 15, 16]. A study by Politi [8] gave mean ADC values of orbital lymphoma of 0.666 ± 0.073 × 10−3 mm2/s, whereas a study by Haradome [9] gave them as 0.54 ± 0.05 × 10−3 mm2/s. The mean ADC values of orbital lymphoma were 0.711 ± 0.152 × 10−3 mm2/s in Xu’s study [15] and 0.694 ± 0.128 × 10−3 mm2/s in our study. All of these studies [8, 9, 15, 16] demonstrated very similar distributions of orbital lymphoma ADCs. However, all of them [9, 15, 16] focused on the preoperative evaluation of the role of DW imaging in the diagnosis and management of orbital lymphoma and inflammation. Nevertheless, our study is the only one on DW imaging characteristics of orbital lymphoma and other malignancies. In previous studies [3, 7], the mean ADC values were 0.90 ± 0.37 × 10−3 or 0.84 ± 0.34 × 10−3 mm2/s in the orbital malignant group, including lymphoma. The present study showed that, after excluding orbital lymphoma, the mean ADC values of other malignancies in the orbit were 1.186 ± 0.315 × 10−3 mm2/s; also, our data confirmed that lymphoma (0.694 ± 0.128 × 10−3 mm2/s) has a significantly lower mean ADC value than that of other malignancies (1.186 ± 0.315 × 10−3 mm2/s) (P < 0.001). Politi [8] provided a threshold of 0.775 × 10−3 mm2/s with 94.4% accuracy in distinguishing orbital lymphoma from other orbital mass lesions (including primary non-lymphoma neoplasms, benign vascular lesions, inflammatory lesions and metastases). Concentrating on DW imaging characteristics of orbital lymphoma and other malignancies, we reported an ADC cutoff value of 0.888 × 10−3 mm2/s resulting in 93.33% sensitivity, 81.81% specificity and 88.46% accuracy.
DCE MR imaging, a method offering information on physiological tissue microcirculation characteristics, provided noninvasive assessment of tumor vascularity and perfusion. In recent years, semi-quantitative and quantitative methods of DCE MR imaging have garnered increasing attention in the literature on diagnosis of orbital tumors [6, 11, 14,15,16]. Yuan’s and Ro’s studies [11, 14] showed that TIC is effective for differentiation of orbital malignant tumors from benign ones. These two studies suggested that DCE MR imaging had shown clinical utility in revealing high vascular permeability and high perfusion in orbital malignant lesions. Furthermore, our study suggested that DCE MR imaging was a valuable MR protocol for revealing different characteristics of local microcirculatory environments between lymphoma and other malignancies in the orbit. Lymphoma was associated with autoimmune disease or infection, which could explain why lymphoma had more blood vessels and higher permeability than other malignancies in the orbit. So, in our study, the T max, ER, CI and WR values in patients with lymphoma were significantly different from those in patients with other orbital malignancies (P = 0.001, P < 0.001, P < 0.001, and P = 0.002, respectively), and T max showed the optimal sensitivity (96.67%).
Among recent advances in MR imaging techniques, a combination of DW imaging and DCE MR imaging has improved the effectiveness of orbital MR imaging for evaluating malignant lesions [14,15,16]. As a result, these techniques have been increasingly used for clinical orbital MR imaging. According to Sun’s study [16], a combination of DW imaging and DCE MR imaging significantly improved differentiation of orbital lymphoma from orbital inflammation, compared with DW imaging alone. With the combination of DW imaging and DCE MR imaging, our study found optimal accuracy (ADC and ER) and optimal specificity (ADC and WR) could be achieved in differentiating lymphoma from other malignancies in the orbit. In the clinical setting, the high accuracy and specificity could be valuable in detecting orbital lymphoma, which is crucial for determination of a therapy plan.
In addition to the intrinsic limits of a retrospective study, our study had several limitations. First, manual ROI measurements increased variability in a multiobserver setting and decreased intraobserver reproducibility when compared with an assisted, semiautomatic or automatic lesion measurement technique [22]. In addition, instead of the 3.2-mm section thickness without gap used in the DCE MR imaging, we used a 3-mm section thickness with a 0.3-mm gap for the DW imaging, which led to the ROI placement not being located in the same section between DW imaging and DCE MR imaging. Last, we focused on the semi-quantitative analysis of DCE-MR imaging, but quantitative assessment of k trans can provide more information and improve the diagnostic efficiency of this modality.
In conclusion, DW imaging or DCE MR imaging alone was valuable for differentiation of orbital lymphoma from other malignancies. Optimal sensitivity, specificity and accuracy were yielded by combination of DW imaging and DCE MR imaging.
References
Sepahdari AR, Aakalu VK, Setabutr P, Shiehmorteza M, Naheedy JH, Mafee MF. Indeterminate orbital masses: restricted diffusion at MR imaging with echo-planar diffusion-weighted imaging predicts malignancy. Radiology. 2010;256:554–64.
Tailor TD, Gupta D, Dalley RW, Keene CD, Anzai Y. Orbital neoplasms in adults: clinical, radiologic, and pathologic review. Radiographics. 2013;33:1739–58.
Sepahdari AR, Politi LS, Aakalu VK, Kim HJ, Razek AA. Diffusion-weighted imaging of orbital masses: multi-institutional data support a 2-ADC threshold model to categorize lesions as benign, malignant, or indeterminate. AJNR Am J Neuroradiol. 2014;35:170–5.
Kharod SM, Herman MP, Morris CG, Lightsey J, Mendenhall WM, Mendenhall NP. Radiotherapy in the management of orbital lymphoma: a single institution’s experience over 4 decades. Am J Clin Oncol. 2015. doi:10.1097/COC.0000000000000229 (Epub ahead of print).
von Holstein SL, Coupland SE, Briscoe D, Le Tourneau C, Heegaard S. Epithelial tumours of the lacrimal gland: a clinical, histopathological, surgical and oncological survey. Acta Ophthalmol. 2013;91:195–206.
Xian J, Zhang Z, Wang Z, Li J, Yang B, Man F, et al. Value of MR imaging in the differentiation of benign and malignant orbital tumors in adults. Eur Radiol. 2010;20:1692–702.
Razek AA, Elkhamary S, Mousa A. Differentiation between benign and malignant orbital tumors at 3-T diffusion MR-imaging. Neuroradiology. 2011;53:517–22.
Politi LS, Forghani R, Godi C, Resti AG, Ponzoni M, Bianchi S, et al. Ocular adnexal lymphoma: diffusion-weighted MR imaging for differential diagnosis and therapeutic monitoring. Radiology. 2010;256:565–74.
Haradome K, Haradome H, Usui Y, Ueda S, Kwee TC, Saito K, et al. Orbital lymphoproliferative disorders (OLPDs): value of MR imaging for differentiating orbital lymphoma from benign OPLDs. AJNR Am J Neuroradiol. 2014;35:1976–82.
Roshdy N, Shahin M, Kishk H, Ghanem AA, El-Khouly S, Mousa A, et al. MRI in diagnosis of orbital masses. Curr Eye Res. 2010;35:986–91.
Yuan Y, Kuai XP, Chen XS, Tao XF. Assessment of dynamic contrast-enhanced magnetic resonance imaging in the differentiation of malignant from benign orbital masses. Eur J Radiol. 2013;82:1506–11.
Fatima Z, Ichikawa T, Ishigame K, Motosugi U, Waqar AB, Hori M, et al. Orbital masses: the usefulness of diffusion-weighted imaging in lesion categorization. Clin Neuroradiol. 2014;24:129–34.
Zhang F, Sha Y, Qian J, Huang WH, Li XF, Wang SJ, et al. Role of magnetic resonance diffusion-weighted imaging in differentiating lacrimal masses. J Magn Reson Imaging. 2014;40:641–8.
Ro SR, Asbach P, Siebert E, Bertelmann E, Hamm B, Erb-Eigner K. Characterization of orbital masses by multiparametric MRI. Eur J Radiol. 2016;85:324–36.
Xu XQ, Hu H, Liu H, Wu JF, Cao P, Shi HB, et al. Benign and malignant orbital lymphoproliferative disorders: differentiating using multiparametric MRI at 3.0T. J Magn Reson Imaging. 2017;45:167–76.
Sun B, Song L, Wang X, Li J, Xian J, Wang F, et al. Lymphoma and inflammation in the orbit: diagnostic performance with diffusion-weighted imaging and dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2016. doi:10.1002/jmri (Epub ahead of print).
Zhang Z, Shi J, Guo J, Yan F, Fu L, Xian J. Value of MR imaging in differentiation between solitary fibrous tumor and schwannoma in the orbit. AJNR Am J Neuroradiol. 2013;34:1067–71.
Yang BT, Wang YZ, Dong JY, Wang XY, Wang ZC. MRI study of solitary fibrous tumor in the orbit. AJR Am J Roentgenol. 2012;199:506–11.
Sumi M, Nakamura T. Head and neck tumours: combined MRI assessment based on IVIM and TIC analyses for the differentiation of tumors of different histological types. Eur Radiol. 2014;24:223–31.
Yabuuchi H, Matsuo Y, Kamitani T, Setoguchi T, Okafuji T, Soeda H, et al. Parotid gland tumors: can addition of diffusion-weighted MR imaging to dynamic contrast-enhanced MR imaging improve diagnostic accuracy in characterization? Radiology. 2008;249:909–16.
Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111:997–1008.
Heye T, Merkle EM, Reiner CS, Davenport MS, Horvath JJ, Feuerlein S, et al. Reproducibility of dynamic contrast-enhanced MR imaging. Part II. Comparison of intra- and interobserver variability with manual region of interest placement versus semiautomatic lesion segmentation and histogram analysis. Radiology. 2013;266:812–21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
About this article
Cite this article
Sun, B., Song, L. Orbital malignant lesions in adults: multiparametric MR imaging. Jpn J Radiol 35, 454–462 (2017). https://doi.org/10.1007/s11604-017-0653-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-017-0653-8