Abstract
Purpose
To compare oral rehydration solution (ORS) with saline infusion for preventing contrast-induced nephropathy (CIN) in a rat model.
Materials and methods
Adult male Sprague–Dawley rats (310–360 g) received intravenous indomethacin (10 mg/kg), N G-nitro-l-arginine methyl ester (10 mg/kg), and iohexol (10 mL/kg) to induce acute contrast-induced renal injury (CIN group); control rats received saline only. For hydration, rats received either continuous infusion (20 mL/kg/h) of saline or three oral doses (20 mL/kg each) of ORS. Acute renal injury was evaluated by assaying urine collected for 24 h beginning 2 h before the contrast injection, evaluating blood taken 22 h after the contrast injection, and examining the kidneys histopathologically.
Results
Hydration with saline prevented only the contrast-induced increase in plasma creatinine, whereas ORS prevented deleterious changes in plasma creatinine, blood urea nitrogen, and creatinine clearance as well as in urinary protein, albumin, and N-acetyl-d-glucosaminidase concentrations. Histopathologic changes noted in the CIN group were diminished in both saline and ORS groups.
Conclusion
Both intravenous saline administration and oral hydration with ORS decreased the severity of CIN. Hydration with ORS was comparable to intravenous saline infusion in preventing CIN-associated abnormalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Contrast-enhanced computed tomography is especially informative for the detection of diverse lesions and the characterization of their extent. Because more than 90% of the injected dose of iodine-based contrast agents used as X-ray contrast media are excreted through the kidney [1, 2], patients with reduced renal function sometimes develop contrast-induced nephropathy (CIN). Prophylactic measures include increasing hydration, decreasing the amount of contrast media, and avoiding repeated injection of contrast media at short intervals [3].
Intravenous supplemental hydration with physiologic saline before and after contrast-enhanced examination is generally recommended as a CIN-preventive measure. However, infusion therapy is difficult to conduct in outpatients or in patients undergoing emergency imaging. Because oral hydration is easier to provide than intravenous infusion—especially for outpatients, infants, and children—it is worthwhile to assess its prophylactic efficacy against CIN after contrast-enhanced imagining studies. Two articles reported comparable effects of saline infusion and oral hydration in clinical settings [4, 5]. However another study [6] demonstrated the superiority of saline infusion over oral fluids in decreasing CIN and the severity of kidney dysfunction, thus indicating no conclusive evidence supporting the efficacy of oral hydration. Therefore, the Japanese guidelines on the use of iodinated contrast media in patients with kidney disease recommend against relying on oral water intake to prevent CIN [7]. Because providing water alone does not increase the sodium content of body fluids, increasing the water intake of patients does not expand the intravascular volume or promote renal blood flow. In contrast, supplementation with an oral rehydration solution (ORS) might be effective as a CIN prophylactic, because ORS contains moderately high concentrations of sodium and glucose to stimulate sodium and water absorption [8], and thereby increased renal blood flow could be expected.
In the current study, we compared intravenous saline infusion (as the current standard of care) with oral supplementation of ORS for preventing CIN in rats.
Materials
The study population comprised male Sprague–Dawley rats (n = 28; age, 8 weeks; weight, 240–330 g; Charles River Laboratories Japan, Inc., Yokohama, Japan) that were kept in a temperature-controlled (23 ± 3 °C) animal room on a 12:12-h light: dark cycle. All rats had unlimited access to a standard commercial laboratory diet (CRF-1; Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water and were acclimatized for 4 days before the experiment. The experiment received prior approval from the Animal Care and Use Committee of our institute.
The contrast medium used in this study was iohexol (Omnipaque 300 injection syringe, 300 mg I/mL; Dai-ichi Sankyo, Co. Ltd., Tokyo, Japan). Indomethacin (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was dissolved in 100 mM sodium phosphate buffer (pH 7.4) to a concentration of 5 mg/mL. N G-nitro-l-arginine methyl ester (L-NAME; Dojindo Laboratories, Kumamoto, Japan) was dissolved in saline to a concentration of 10 mg/mL. Rats were given supplemental hydration before and after the injection of contrast medium with saline or ORS (OS-1; Otsuka Pharmaceutical Factory, Inc., Naruto, Japan). The ORS contains sodium (50 meq/L), potassium (20 meq/L), chloride (50 meq/L), magnesium (2 meq/L), phosphorus (2 mmol/L), lactate (31 meq/L), and glucose (1.8%).
Experimental procedure
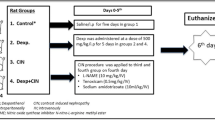
The scheme of the experiment is shown in Fig. 1. The day before the experiment, rats were stratified according to body weight and then randomly allocated into four groups of seven rats. Jugular vein catheters were inserted under isoflurane anesthesia. To prevent catheter obstruction before the experiment, normal saline was infused at 0.2 mL/h. Rats were kept individually in metabolic cages with free access to water but without feeding during the experiment.
The scheme of the experiment. To induce CIN, rats in the CIN, saline, and ORS groups received bolus injections of indomethacin and L-NAME followed by continuous infusion of contrast medium through a jugular vein catheter as indicated; rats in the control group received saline instead of CIN-inducing agents. Hydration was provided through either continuous saline infusion (saline group) or three oral doses of ORS at the indicated times. Urine was collected for 24 h, and blood samples were obtained at the end of the experiment
Because the administration of contrast media to rats with normal renal function does not induce CIN, pretreatment to reduce renal function is necessary to create a useful model system. We used L-NAME and indomethacin to inhibit NO and prostanoid production from the renal vascular endothelium, which resulted in reduced renal blood flow [9].
On the experimental day, unanesthetized, unrestrained rats in each experimental group were treated as follows. Rats in the control group (n = 7) each received a bolus injection of saline (2 mL/kg) through the catheter, followed 30 min later by a second saline bolus (1 mL/kg); after another 30-min interval, continuous saline infusion (10 mL/kg at 1.45 mL/min) was initiated. Rats in the CIN group (n = 7) received indomethacin (10 mg/2 mL/kg) followed 30 min later by L-NAME (10 mg/mL/kg) bolus injection through the catheter; continuous infusion of iohexol (10 mL/kg at 1.45 mL/min) began 30 min after L-NAME injection. Rats in the saline group (n = 7) underwent the same treatment as those in the CIN group except that saline was infused continuously at 20 mL/kg/h through the catheter for 3 h starting 2 h before the infusion of the contrast medium. Rats in the ORS group (n = 7) underwent the same treatment as those in the CIN group, except that each rat received three oral doses of ORS (20 mL/kg each) at 1-h intervals, with the first ORS dose at 105 min before the infusion of the contrast medium.
Sample collection and analysis
Heparinized blood samples (0.5 mL) were obtained from the subclavian vein of isoflurane-anesthetized rats at 22 h after the infusion of the contrast medium. Plasma was prepared from the whole-blood samples and used in biochemical analyses.
Urine was collected for 24 h beginning 2 h before the infusion of the contrast medium and was analyzed for urea nitrogen, creatinine, total protein, albumin, and N-acetyl-d-glucosaminidase (NAG) concentrations.
Plasma and urinary urea-nitrogen and creatinine as well as urinary NAG were analyzed on a clinical analyzer (model 7180; Hitachi High-Technologies Corporation, Tokyo, Japan). Creatinine clearance was calculated as (urinary creatinine × urine volume) ÷ (plasma creatinine × 24 × 60).
Histologic analysis
All rats were euthanized by bleeding under isoflurane anesthesia. At necropsy, the kidneys were weighed and then fixed in 10% neutral-buffered formalin. Paraffinated sections were stained with hematoxylin and eosin. Renal injury (that is, vacuolization of proximal convoluted tubules and acute cellular necrosis of papillary collecting tubules) was assessed semi-quantitatively by an experienced (45 years) pathologist, who was blinded to treatment group and who scored the samples on a scale of 0–5 (0, normal histology; 1, slight injury; 2, mild injury; 3, moderate injury; 4, severe injury).
Statistics
The measured values were expressed in the form of mean ± 1 standard deviation. The statistical significance of differences between the control and CIN groups was determined by using the Wilcoxon test, except for histological grade which was analyzed by using Fisher’s exact test. The Tukey procedure for multiple comparisons (non-parametric, joint ranking) was used to compare among the CIN, saline, and ORS groups. A P value of <0.05 was considered significant. All statistical analyses were performed in SAS v. 9.1.3 software (SAS Institute, Cary, NC, USA).
Results
Immediately after the infusion of the contrast medium, one rat in the CIN group experienced severe seizures and died, leaving 6 rats for analysis. We attributed the death to an anaphylactic response to the contrast medium.
Body weight and kidney weight
Body weight at necropsy did not differ among the experimental groups (Table 1). Absolute (P < 0.05) and relative (P < 0.01) kidney weights were significantly greater in the CIN group than in the control group, but were similar between the CIN, saline, and ORS groups. Urine volume was significantly (P < 0.01) greater in the CIN group than in the control group (Table 2), probably because of osmotic diuresis. Saline infusion significantly (P < 0.05) increased urine volume compared with the control. Hydration with ORS also tended to increase urine volume (P = 0.08).
Biochemical measurements
Compared with the controls, plasma creatinine and urea nitrogen significantly (P < 0.01) increased in the CIN group, indicating acute renal injury (Table 2). Supplemental hydration buffered this effect: compared with values, creatinine levels were significantly lower in the saline and ORS groups than in the CIN rats (both P < 0.05), and blood urea nitrogen was significantly (P < 0.01) reduced in the ORS group (Table 2).
Consistent with these results, creatinine clearance significantly (P < 0.01) decreased and urinary total protein (P < 0.01), albumin (P < 0.01), and NAG (P < 0.05) significantly increased in the CIN group compared with the controls (Table 2). Both forms of supplemental hydration ameliorated these changes, but only the differences between the CIN and ORS groups were significant (creatinine clearance: P < 0.01; urine total protein: P < 0.001; urine albumin: P < 0.001; NAG: P < 0.05). Urinary total protein was significantly (P < 0.05) lower in the ORS group than in the saline group.
Renal histology
The renal cortex and medulla of control rats showed no abnormalities (Table 3). In contrast, histopathologic examination revealed vacuolization of proximal convoluted tubules in all rats that received the contrast medium (i.e., CIN, saline, and ORS groups). Acute cellular necrosis of papillary collecting tubules was observed in some rats in these groups. Renal injury was greatest in the CIN group, followed by the saline group and then the ORS group. The histological grades were significantly higher in the CIN group than in the control group (vacuolization: P < 0.001; acute cellular necrosis: P < 0.05). Although histological grade did not differ significantly among the CIN, saline, and ORS groups, acute cellular necrosis tended to be decreased in the ORS group (P = 0.08). Representative images of renal damage are shown in Fig. 2.
Representative histopathological images of a–d proximal convoluted tubules and e–g papillary collecting tubules of rats in the a, e control, b, f CIN, c, g saline, and d, h ORS groups. Arrows indicate vacuolization of proximal convoluted tubules (histopathologic grade: panel a, 0; b, 3; c, 3; and d, 2) and regions demonstrating acute cellular necrosis of papillary collecting tubules (histopathologic grade: panel e, 0; f, 3; g, 2; and h, 1) are circled
Discussion
The causes of CIN are thought to be altered renal hemodynamics and increased reabsorptive workload and oxygen consumption after the administration of contrast medium, leading to hypoxia and the formation of reactive oxygen species within the kidney, consequently resulting in renal dysfunction [10]. Clinical risk factors of CIN include pre-existing renal dysfunction, increased age, diabetes mellitus, dehydration, use of nonsteroidal anti-inflammatory drugs, frequent or high-volume use of contrast media, and the use of high-osmolar contrast media [7]. These predisposing risk factors also aggravate the mechanisms of CIN [11]. The administration of contrast media to rats with normal renal function does not induce CIN, thus requiring the use of predisposing factors and high doses of contrast media to establish the CIN model. These predisposing factors include glycerin administration [12] or 5/6-nephrectomy [13] to induce renal impairment, diabetes [14], hypercholesterolemia [15], and the inhibition of endogenous vasodilatory compounds by using L-NAME and indomethacin to block NO and prostanoid production from the renal vascular endothelium [9]. However, the cited models used ionic hyperosmotic contrast media, which are not indicated for intravascular administration in Japan currently. In addition, water deprivation leading to dehydration was necessary to induce these CIN models.
Considering the clinical prevalence of non-ionic contrast media in Japan and the objective of the current study, we compared three CIN models (glycerol administration, partial nephrectomy, and L-NAME plus indomethacin) using iohexol without water deprivation in pilot studies. Only treatment with L-NAME plus indomethacin significantly increased the plasma creatinine level compared with other preexisting insults after iohexol administration (data not shown). Therefore, we used L-NAME and indomethacin in combination with iohexol in the current study. This pretreatment enhances the acute renal dysfunction that occurs due to contrast-induced hypoperfusion and hypoxia of the outer layer of the renal medulla [9]. Although the injected volume of iohexol (10 mL/kg) was higher than that of iothalamate in the original report (6 mL/kg), the amounts of iodine administered were similar (3.0 and 2.9 g/kg, respectively).
As evidence of the applicability of our model system, all rats that received indomethacin, L-NAME, and iohexol had increased plasma creatinine and urea nitrogen concentrations; decreased creatinine clearance; and increased urinary total protein, albumin, and NAG.
Histopathologically, the iohexol-treated mice had acute renal injury, including endothelial vacuolization at proximal tubules and acute tubular necrosis at the renal papillae. However, we did not detect injury of the outer medulla, including medullary thick ascending limbs, which was reported to be characteristic in this CIN model [16]. We presume that the methods we used to prepare and analyze the tissue sections were not appropriate for the observation of these changes, as the kidney samples in our experiment were neither perfusion-fixed nor evaluated by electron microscopy.
Hydration might increase renal hemodynamics and the clearance of contrast media from tubules by reducing blood and urine viscosity, thus mitigating hypoxic renal damage. Both hydration methods—infusion of saline through the jugular vein and oral administration of ORS—reduced iohexol-associated renal damage. Compared with those in the CIN rats, the reductions in plasma creatinine levels were statistically significant in both the saline and ORS groups, but the improvements in the other plasma and urinary parameters were statistically significant only in the ORS group. In addition, urinary total protein excretion was statistically lower in the ORS group than in the saline group. Furthermore, semi-quantitative histopathologic analysis indicated that renal injury was greatest in the CIN group, followed by the saline group and then the ORS group. These results suggest the possible superiority of oral supplementation of ORS over saline infusion in preventing CIN. Probable explanations for these results include differences in the rate of contrast medium excretion and in the concentrations of sodium and chloride in the hydration solutions.
Our preliminary data suggested that the rate at which the contrast agent was excreted did not differ between saline infusion and ORS (unpublished data). Compared with that during ORS, plasma volume expansion would be greater under saline infusion because of its higher sodium concentration, conceivably contributing to improved renal blood flow. In contrast, given that chloride is known to promote vasoconstriction of the afferent arterioles of renal glomeruli [17], intravenous fluids with high chloride concentrations might reduce renal blood flow. Moreover, an increased glomerular filtration rate paradoxically can intensify medullary hypoxia and injury [10]. Therefore, given the results of the present study, we are currently unable to recommend one hydration method over the other. To demonstrate the relative merits of each hydration method, further experiments involving sufficient numbers of animals for statistical power and detailed quantitative analyses, including assessments of both histology and renal blood flow, are required.
In summary, our results show that oral hydration with ORS provided renal protection in the CIN model at least comparable to that provided by intravenous saline infusion. As several clinical studies show similar effects of intravenous and oral hydration protocols on preventing CIN [4, 5, 18], comparison between oral intake of water and of ORS would be an interesting research theme. ORS is reported to be more rapidly absorbed in the small intestine than water [19], and theoretically, ORS is more advantageous than water for expanding intravascular volume. Further studies are required to clarify the most suitable drink and the hydration protocol for preventing CIN.
One limitation of the current study was the relatively few rats in each group, leading to low statistical power to detect significant differences among groups. In addition, we failed to observe the tubular injury in the outer medulla that is characteristic in this CIN model, likely because kidneys were not perfusion-fixed; more detailed histopathological examinations are warranted. Finally, we did not include a comparison between oral intake of water and of rehydration solution. We plan to perform such a comparison, which likely will yield meaningful information for clinical practice.
In conclusion, we compared intravenous saline infusion (as the current standard of care) with oral supplementation of ORS in the prevention of CIN in a rat model. Both hydration protocols decreased the severity of CIN-associated abnormalities. Oral hydration with ORS provided renal protection in the CIN model that was at least comparable to that provided by intravenous saline infusion. The clinical utility of supplemental oral hydration with ORS during contrast-enhanced imaging in the clinical setting merits further evaluation.
References
Mckinstry DN, Rommel AJ, Sugerman AA. Pharmacokinetics, metabolism, and excretion of iopamidol in healthy subjects. Invest Radiol. 1984;19(Suppl):S171–4.
Edelson J, Shaw D, Palace G. Pharmacokinetics of iohexol, a new nonionic radiocontrast agent, in humans. J Pharm Sci. 1984;73:993–5.
Kitajima K, Maeda T, Watanabe S, Sugimura K. Recent issues in contrast-induced nephropathy. Int J Urol. 2011;18:686–90.
Wrobel W, Sinkiewicz W, Gordon M, Wozniak-Wisniewska A. Oral versus intravenous hydration and renal function in diabetic patients undergoing percutaneous coronary interventions. Kardiol Pol. 2010;68(9):1015–20.
Taylor AJ, Hotchkiss D, Morse RW, McCabe J. PREPARED: Preparation for Angiography in Renal Dysfunction: a randomized trial of inpatient vs outpatient hydration protocols for cardiac catheterization in mild-to-moderate renal dysfunction. Chest. 1998;114(6):1570–4.
Trivedi HS, Moore H, Nasr S, Aggarwal K, Agrawal A, Goel P, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003;93(1):C29–34.
Ohno I, Hayashi H, Aonuma K, Horio M, Kashihara N, Okada H, et al. Guidelines on the use of iodinated contrast media in patients with kidney disease 2012: digest version: JSN, JRS, and JCS Joint Working Group. Clin Exp Nephrol. 2013;17:441–79.
Atia AN, Buchman AL. Oral rehydration solutions in non-cholera diarrhea: a review. Am J Gastroenterol. 2009;104:2596–604.
Agmon Y, Peleg H, Greenfeld Z, Rosen S, Brezis M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94:1069–75.
Heyman SN, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin J Am Soc Nephrol. 2008;3(1):288–96.
Heyman SN, Rosen S, Khamaisi M, Idee JM, Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol. 2010;45(4):188–95.
Duan SB, Wang YH, Liu FY, Xu XQ, Wang P, Zou Q, et al. The protective role of telmisartan against nephrotoxicity induced by X-ray contrast media in rat model. Acta Radiol. 2009;50(7):754–9.
Sochman J, Peregrin JH, Burgelova M, Kopkan L, Kramer HJ, Cervenka L. N-acetylcysteine attenuates iodine contrast agent-induced nephropathy in 5/6-nephrectomized rats. Kidney Blood Press Res. 2010;33(2):149–56.
Vaamonde CA, Bier RT, Papendick R, Alpert H, Gouvea W, Owens B, et al. Acute and chronic renal effects of radiocontrast in diabetic rats. Role of anesthesia and risk factors. Invest Radiol. 1989;24(3):206–18.
Andrade L, Campos SB, Seguro AC. Hypercholesterolemia aggravates radiocontrast nephrotoxicity: protective role of l-arginine. Kidney Int. 1998;53(6):1736–42.
Heyman SN, Brezis M, Reubinoff CA, Greenfeld Z, Lechene C, Epstein FH, et al. Acute renal failure with selective medullary injury in the rat. J Clin Invest. 1988;82(2):401–12.
Hansen PB, Jensen BL, Skott O. Chloride regulates afferent arteriolar contraction in response to depolarization. Hypertension. 1998;32:1066–70.
Akyuz S, Karaca M, Kemaloglu Oz T, Altay S, Gungor B, Yaylak B, et al. Efficacy of oral hydration in the prevention of contrast-induced acute kidney injury in patients undergoing coronary angiography or intervention. Nephron Clin Pract. 2014;28(1–2):95–100.
Miki S, Hino K, Kondo Y. Effects of oral rehydration solution on water and electrolytes absorption in rat small intestine. Clin Nutr. 2008;3(S1):170.
Acknowledgements
For experimental accomplishment, the author received technical support of Otsuka Pharmaceutical Factory, Inc which was the affiliation of the joint author. We thank Atsushi Tosaka (Otsuka Pharmaceutical Factory, Inc) for technical assistance in animal experiment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No grant concerned.
Conflict of interest
TM, RD, and RK declare that they have no conflict of interest. KH, SM, and GE are employees of the Otsuka Pharmaceutical Factory, Inc.
About this article
Cite this article
Matsunami, T., Hino, K., Dosho, R. et al. Efficacy of oral supplemental hydration for the prevention of contrast-induced nephropathy in rats. Jpn J Radiol 35, 190–196 (2017). https://doi.org/10.1007/s11604-017-0620-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-017-0620-4