Abstract
Purpose
To compare soft-tissue cone-beam computed tomography (CBCT-P) and fiducial marker (CBCT-FM)-based image guided radiotherapy in prostate cancer patients.
Materials and methods
Sixteen prostate cancer patients were treated with volumetric modulated arc therapy. Manual alignment using CBCT-P and CBCT-FM was performed for each patient. Couch shifts were calculated and compared between methods in the left–right (x), superior–inferior (y), and anterior–posterior (z) directions.
Results
CBCT-P and CBCT-FM alignments were compared using 252 scans from the 16 patients. Mean displacement from zero was 2.4 ± 1.3, 1.7 ± 1.2, and 1.8 ± 1.1 mm for CBCT-P and 2.3 ± 1.3, 1.7 ± 1.1 and 1.8 ± 1.1 mm for CBCT-FM in the x, y and z directions, respectively. There was no difference in median displacement between CBCT-P and CBCT-FM; however, there was a significant positive correlation between CBCT-P- and CBCT-FM-based displacements in the x (r = 0.881; p < 0.001), y (r = 0.789; p < 0.001) and z (r = 0.856; p < 0.001) directions by linear regression analysis. Systematic deviations within each group were <1 mm; however, random and systematic errors were similar in the x and y directions but larger in the z direction.
Conclusion
Our study demonstrated that CBCT-FM was not superior to CBCT-P for image-guided radiotherapy in prostate cancer patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Randomised studies have shown that external beam radiotherapy (RT) improves survival in patients with localised prostate cancer [1, 2]. Furthermore, while dose escalation has been associated with improved outcomes, there is a potential for higher rates of rectal and bladder toxicities in patients treated with definitive RT [3–6]. Highly conformal techniques such as intensity modulated RT (IMRT) with image guidance (IGRT) allow precise delivery of higher doses of radiation within the target volume [7, 8].
Patient immobilization and inter- and intrafraction organ motion may potentially influence target and organs-at-risk radiation doses. Successful delivery of the prescribed dose to the prostate while sparing the rectum and bladder was correlated with the ability to accurately target the prostate during treatment [9]. Because the prostate is a movable organ, daily localisation of the gland during treatment provides target coverage verification. Electronic portal imaging devices (EPID) using mega-voltage (MV) X-rays alone are not sufficient for prostate imaging because this technique can easily determine the bone position but cannot visualise soft tissues. Several other methods have been developed to visualise and localise the prostate gland, such as ultrasound [10], computed tomography (CT) on rails [11], fiducial markers [12, 13] and cone-beam CT (CBCT) [14, 15].
One of the most common methods used for prostate localisation is implantation of fiducial markers into the prostate gland, which can be visualised on EPID and CBCT. However, the implantation of markers is an invasive procedure with the potential for discomfort, bleeding and infection. Furthermore, fiducial markers provide little information on deformation of the target, localisation of the seminal vesicles, or alteration in the neighbouring normal tissue, and may cause deformation of the prostate gland after implantation [16]. Another commonly used method for prostate visualisation is CBCT, which permits three-dimensional volumetric image acquisition while the patient is in the treatment position [17]; however, this procedure increases the time per session, workload, and the integral radiation doses [18]. Using kilovoltage (kV) CBCT, several studies have evaluated different imaging alignment techniques such as bony anatomy matching, soft-tissue matching and fiducial matching [5, 14, 15]. Although some studies have evaluated the accuracy of the prostate setup using bone anatomy corrections and online implanted fiducial-based corrections using EPIDs [19], prostate localisation using CBCT with soft-tissue- or fiducial-based corrections has not been well studied.
Based on these findings, the aim of this study was to evaluate organ motion and set-up errors during RT treatment using soft-tissue (CBCT-P) and fiducial-marker (CBCT-FM)-based corrections. This was achieved by acquiring the CBCT dataset within the same fraction before applying isocentre corrections.
Materials and methods
The dosimetric data of 16 intermediate-risk patients treated with curative RT between September 2012 and December 2012 were retrospectively analysed for this study. The treatment prescription was 78 Gy in 39 fractions using volumetric arc radiotherapy (VMAT), which was described previously [20].
Patient simulation
Three gold intraprostatic fiducial markers (24 k, 3 × 0.8 mm) were implanted into the prostate under transrectal ultrasound guidance at a median 7 days (range 3–11 days) prior to initiation of treatment planning. Patients were instructed to arrive with an empty bowel and comfortably full bladder for simulation and treatment. The patient’s immobilisation and alignment consisted of supine positioning, hands folded on chest, and knee- and leg-immobilisation devices (Med Tech Inc., Orange City, IA, USA) encompassing the foot and separating the legs. Anterior, right and left lateral permanent skin markers were placed at the isocentre coordinates.
Patients were scanned from top of the L1 vertebral body to below the lesser trochanter using slices 2.5 mm thick, providing all three fiducials were visible. The clinical target volume (CTV) included the prostate and the entire seminal vesicles. The planning target volume (PTV) was defined as the CTV with a margin of 5 mm posterior and 8 mm in other directions [20]. The organs at risk (OARs) included the rectum, sigmoid, bladder and femoral heads. The rectum was delineated from the anal verge to the rectosigmoid junction [21]. The femoral heads were contoured to the level of the ischial tuberosities. The plans were calculated with Elekta’s Monaco treatment planning system using the Monte Carlo algorithm and a sliding window multi-leaf collimator (MLC) delivery technique. The VMAT plans consisted of a single 360° arc. Gantry speed, MLC leaf position and dose rate varied continuously during VMAT delivery [22].
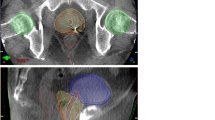
CBCT and positioning
Before treatment, patients were settled into the simulation position and the skin markers were positioned using three lasers while in the treatment room. The CBCT was delivered daily for the first 5 consecutive days and then twice weekly. Using three degrees of freedom, the prostates in the CBCT and planning CT studies were aligned in three dimensions by corresponding physicians at each setup for the first 3–5 days and by trained radiation therapists thereafter. After the delivery of CBCT, two different methods were used to identify the correct treatment position for each patient. First, manual alignment of the soft-tissue prostate (CBCT-P) using the prostate, seminal vesicles, rectum and bladder contours was performed as delineated on the reference simulation CT, and required couch shifts, without the use of rotations, were recorded for each CBCT. Second, after the delineation and reconstruction, CBCT was matched to the fiducials in the CT simulation scan, and required couch shifts, without the use of rotations, were recorded for each CBCT. Rotational corrections were found to be insignificant and were therefore ignored in this study. The recorded couch shifts were compared between the CBCT-P and CBCT-FM methods in the left–right [23] (x), superior–inferior (SI) (y) and anterior–posterior (AP) (z) directions. A verification CBCT scan was then obtained to verify the correction before the patients underwent treatment. The difference between the prostate shift and the fiducial shift in each direction was calculated. The mean values of each shift according to direction were also calculated.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 (SPSS, Chicago, IL, USA). The Pearson correlation coefficient was used to measure the correlation in couch shifts between CBCT-P and CBCT-FM. The difference between the measured couch shifts was then plotted against the average couch shift as recommended by Bland and Altman [24] when a new method of measurement is to be compared against a gold standard. If the two measures are equivalent, the difference should show a zero mean and no significant trends. The 95 % confidence interval of the error distribution was reported, and the random (σ), systematic (Σ) and group systematic (M) errors for each guidance method were calculated [25]. The distribution of the systematic error was estimated by taking the standard deviation of the mean values for each patient.
Results
The median age of the 16 patients analysed was 70 years (range 52–79 years). 608 fractions were delivered to the 16 prostate cancer patients included in our study. In addition, 304 CBTCs were delivered; of these, 52 datasets were lost because of an imaging system problem, and the remaining 252 CBCT datasets were deemed useful for the purposes of this study.
The mean displacement from zero for CBCT-P was 2.4 ± 1.3, 1.7 ± 1.2 and 1.8 ± 1.1 mm in the x, y and z directions, respectively. In comparison, the mean displacement from zero for the CBCT-FM groups was 2.3 ± 1.3, 1.7 ± 1.1 and 1.8 ± 1.1 mm in the x, y and z directions, respectively. Prostate motion was largest in the x direction for each of the groups. Differences in displacement between CBCT-P and CBCT-FM were not significant in our analysis.
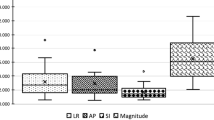
The median displacements in the x, y and z directions according to CBCT corrections are presented in Table 1. The mean vector length of total interfraction displacements was 2.0 ± 1.2 mm for CBCT-P and 1.9 ± 1.2 mm for CBT-FM (Fig. 1). The prostate movements in the x and y directions were all less than the target volume margins; however, three CBCT images in CBCT-FM and one CBCT image in CBCT-P exceeded the posterior PTV margin of 5 mm (Fig. 2).
Linear regression analysis demonstrated a significant positive correlation between CBCT-P- and CBCT-FM-based displacements in the LR (r = 0.881; p < 0.001), SI (r = 0.789; p < 0.001) and AP (r = 0.856; p < 0.001) directions (Fig. 3). According to Bland–Altman analysis, the 95 % confidence intervals were −0.21 and 0.14 for LR, −0.09 and 0.14 for SI and −0.05 and 0.14 for AP displacements (Fig. 4). Shifts of <3 mm were considered to be acceptable and the couch shift agreement within ±3 mm in the x, y and z directions was 99.8, 99.9 and 99.9 %, respectively.
Components of σ, Σ and M in the x, y and z directions are shown in Table 2. Systematic deviations of the group, representing inaccuracies during treatment preparation repeated over multiple patients, were all less than 1 mm, with AP deviation the largest contributor. Although random and systematic errors were similar in the x and y directions, the largest values for σ and Σ were observed in the AP direction.
Discussion
In this study, we demonstrated that the prostate displacements observed in CBCT corrections based on prostate-gland and fiducial markers were very similar. More than 99 % of the prostate gland displacements were within our current target volume margins. The difference between CBCT-P and CBCT-FM displacements was essentially the same in all directions. Furthermore, we demonstrated that the largest σ, Σ and M values were observed in the AP direction.
IGRT with CBCT is a reasonable technique for resolving two essential problems during prostate RT: the variation in daily patient setup and internal organ motion. Therefore, after setup corrections, the target volume receives adequate radiation doses, and the treatment margins may be reduced safely. One of the important setup techniques for prostate localisation during treatment has been fiducial marker insertion, which can be seen on EPID with MV or kV CBCT guidance. Greer et al. [19] assessed the prostate setup accuracy and setup margins using a bony-anatomy-based protocol and implanted fiducial markers. The authors found that the setup margins calculated to encompass 98 % of the prostate shifts were 11–14 mm with bony anatomy setup and 4–7 mm with fiducial markers. However, the question of whether CBCT with soft-tissue correction is enough for prostate localisation has not been addressed sufficiently. Recently, Deegan et al. [26] analysed the consistency and accuracy of three radiation therapists who performed CBCT-FM and CBCT-P in six patients receiving daily prostate RT with CBCT. The authors found that clinically acceptable limits of agreement with the mean were defined less in CBCT-FM than in CBCT-P (±2.0 vs. ±3.0 mm). In a similar study conducted by Moseley et al. [27], a comparison of localisation performance with implanted fiducial markers and CBCT was conducted with 256 CBCT images from 15 patients. The authors found a strong correlation between patient position shifts using fiducial markers in MV CBCT and soft-tissue-based corrections, as was observed in our study. Finally, the authors concluded that CBCT provided an equivalent means of patient setup correction for prostate patients with implanted gold fiducial markers.
Our comparison between CBCT-FM and CBCT-P alignments resulted in variability that exceeded the predefined acceptable limits of PTV margins for both techniques. Only three patient setups in CBCT-FM and one setup in CBCT-P exceeded the posterior PTV margin of 5 mm. Barney et al. [28] compared fiducials and kV imaging with CBCT for localisation of the prostate in 36 patients who received IMRT with daily localisation via implanted fiducials. The authors found that 28 % of treatment sessions resulted in a difference of >5.0 mm in one or more directions, and concluded that although CBCT and kV fiducial imaging were similar, more than one-quarter of the CBCT and kV shifts differed enough to affect target coverage. The considerable difference in shifts based on fiducial and CBCT techniques in this study are in concordance with results published by Moseley et al. [27], but conflict with results published by Barney et al. [28]. Moseley et al. [27] found that the percentage of shifts within a ±3-mm tolerance was 99.7 % for AP, 95.5 % for SI and 91.3 % for LR with fiducial marker matching vs. 99.5 % for AP, 70.3 % for SI and 78.4 % for LR with soft-tissue matching. However, Barney et al. [28] found that the percentage of shift agreements within ±5 mm was 72.4 % for AP, 72.7 % for SI and 97.2 % for LR. This discrepancy may be related to the sample size and the number of comparisons per patient, which may have resulted in heterogeneity within the data set. The systematic component of total interfraction prostate motion in this study was greatest in the AP direction, which was also seen in previous studies [5, 15, 27].
We also performed Bland–Altman analysis, which is a method of data plotting that is used to analyse the agreement between two different assays, instruments, or measurement techniques [24]. In this analysis, a high correlation between any two methods designed to measure the same property could be a sign that a sample with wide variation was chosen. However, a high correlation does not necessarily imply that there is good agreement between two methods. In this analysis, we found that although the 95 % confidence interval values were −0.21 and 0.14 for LR, −0.09 and 0.14 for SI and −0.05 and 0.14 for AP displacements, there were no significant trends in the data.
The lack of individual trends in prostate motion throughout therapy, as seen in the large majority of patients studied here, validates to some extent the use of the average prostate position during the first 4–6 days of therapy to create a new patient-specific PTV. This PTV is then used for the remainder of the therapy without daily imaging [29, 30]. If prostate motion is random as a function of time throughout IMRT treatments, the mean prostate position during the first several days of treatment could be used as a surrogate for average position throughout the course of radiotherapy. These approaches attempt to reduce Σ only, whereas daily imaging minimises both Σ and σ. A previous study using six degrees of freedom registration showed that the Σ in the prostate position was reduced by approximately 40–55 % along rotational and translational axes by averaging the prostate position for 5 days [31]. In another study using three degrees of freedom in translation only, a more modest reduction in Σ of approximately 35 % in the AP and LR directions was obtained, but no decrease in the SI direction, using the mean prostate position over 6 days [14]. Similarly, in order to reduce Σ, we performed daily CBCT for the first 5 days and analysed the average prostate positon. In addition, in this study, we treated patients with VMAT, for which intrafractional motion of the prostate was minimal relative to IMRT because of the shorter treatment time [20].
Although fiducial marker implantation for IGRT in prostate cancer patients allows the localisation of the prostate during treatment, this application is not without complications. Langenhuijsen et al. [32] reported pain and fever that resolved with oral medication in 6.2 % of patients, haematuria lasting more than 3 days in 3.8 % of patients, haematospermia in 18.5 % of patients, and rectal bleeding in 9.1 % out of 209 patients with localised prostate cancer who had four gold markers implanted under ultrasound guidance. In a study of 244 patients, Gill et al. [33] reported that 32 % of patients had at least one new symptom following the procedure: haematuria, rectal bleeding and dysuria; haematospermia in 9–13 % of patients; and one case of sepsis. The reported seed loss rate varied between less than 1–8 % [7, 34, 35]. Although underestimated, some dosimetric modifications may be required in fiducial insertion points [36].
Our study had some limitations. First, this was a retrospective study. Second, although desirable, CBCT was not performed at each fraction in order to minimise the patients’ exposure to ionising radiation. Lastly, technically deformable image registration methods were not used in our study.
Conclusions
Our study showed that CBCT-FM did not provide any significant contribution to CBCT-P. Although prostate motion in the anterior–posterior direction was higher than that in the other directions, nearly all shifts were within the limits of the target volume margins. Although fiducial marker implantation for IGRT in prostate cancer allows the localisation of the prostate during treatment, this application may cause some complications and dosimetric uncertainties. Therefore, alternative noninvasive methods of CBCT should be considered for IGRT of prostate cancer patients.
References
Rosenthal SA, Sandler HM. Treatment strategies for high-risk locally advanced prostate cancer. Nat Rev Urol. 2010;7(1):31–8.
Pisansky TM. External-beam radiotherapy for localized prostate cancer. N Engl J Med. 2006;355(15):1583–91.
Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67–74.
Al-Mamgani A, van Putten WL, Heemsbergen WD, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72(4):980–8.
Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(6):475–87.
Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233–9.
Cahlon O, Hunt M, Zelefsky MJ. Intensity-modulated radiation therapy: supportive data for prostate cancer. Semin Radiat Oncol. 2008;18(1):48–57.
Button MR, Staffurth JN. Clinical application of image-guided radiotherapy in bladder and prostate cancer. Clin Oncol (R Coll Radiol). 2010;22(8):698–706.
Peeters ST, Lebesque JV, Heemsbergen WD, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64(4):1151–61.
Morr J, DiPetrillo T, Tsai JS, et al. Implementation and utility of a daily ultrasound-based localization system with intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53(5):1124–9.
Frank SJ, Dong L, Kudchadker RJ, et al. Quantification of prostate and seminal vesicle interfraction variation during IMRT. Int J Radiat Oncol Biol Phys. 2008;71(3):813–20.
Welsh JS, Berta C, Borzillary S, et al. Fiducial markers implanted during prostate brachytherapy for guiding conformal external beam radiation therapy. Technol Cancer Res Treat. 2004;3(4):359–64.
Dehnad H, Nederveen AJ, van der Heide UA, et al. Clinical feasibility study for the use of implanted gold seeds in the prostate as reliable positioning markers during megavoltage irradiation. Radiother Oncol. 2003;67(3):295–302.
Snir JA, Battista JJ, Bauman G, et al. Evaluation of inter-fraction prostate motion using kilovoltage cone beam computed tomography during radiotherapy. Clin Oncol (R Coll Radiol). 2011;23(9):625–31.
Bylund KC, Bayouth JE, Smith MC, et al. Analysis of interfraction prostate motion using megavoltage cone beam computed tomography. Int J Radiat Oncol Biol Phys. 2008;72(3):949–56.
Nichol AM, Brock KK, Lockwood GA, et al. A magnetic resonance imaging study of prostate deformation relative to implanted gold fiducial markers. Int J Radiat Oncol Biol Phys. 2007;67(1):48–56.
Jaffray DA, Siewerdsen JH. Cone-beam computed tomography with a flat-panel imager: initial performance characterization. Med Phys. 2000;27(6):1311–23.
Ding GX, Duggan DM, Coffey CW, et al. A study on adaptive IMRT treatment planning using kV cone-beam CT. Radiother Oncol. 2007;85(1):116–25.
Greer PB, Dahl K, Ebert MA, et al. Comparison of prostate set-up accuracy and margins with off-line bony anatomy corrections and online implanted fiducial-based corrections. J Med Imaging Radiat Oncol. 2008;52(5):511–6.
Onal C, Arslan G, Parlak C, et al. Comparison of IMRT and VMAT plans with different energy levels using Monte-Carlo algorithm for prostate cancer. Jpn J Radiol. 2014;32(4):224–32.
Onal C, Topkan E, Efe E, et al. Comparison of rectal volume definition techniques and their influence on rectal toxicity in patients with prostate cancer treated with 3D conformal radiotherapy: a dose-volume analysis. Radiat Oncol. 2009;4:14.
Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35(1):310–7.
Berkovic P, De Meerleer G, Delrue L, et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin Genitourin Cancer. 2013;11(1):27–32.
Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346(8982):1085–7.
van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14(1):52–64.
Deegan T, Owen R, Holt T, et al. Assessment of cone beam CT registration for prostate radiation therapy: fiducial marker and soft tissue methods. J Med Imaging Radiat Oncol. 2015;59(1):91–8.
Moseley DJ, White EA, Wiltshire KL, et al. Comparison of localization performance with implanted fiducial markers and cone-beam computed tomography for on-line image-guided radiotherapy of the prostate. Int J Radiat Oncol Biol Phys. 2007;67(3):942–53.
Barney BM, Lee RJ, Handrahan D, et al. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT). Int J Radiat Oncol Biol Phys. 2011;80(1):301–5.
Nijkamp J, Pos FJ, Nuver TT, et al. Adaptive radiotherapy for prostate cancer using kilovoltage cone-beam computed tomography: first clinical results. Int J Radiat Oncol Biol Phys. 2008;70(1):75–82.
Vargas C, Yan D, Kestin LL, et al. Phase II dose escalation study of image-guided adaptive radiotherapy for prostate cancer: use of dose-volume constraints to achieve rectal isotoxicity. Int J Radiat Oncol Biol Phys. 2005;63(1):141–9.
Hoogeman MS, van Herk M, de Bois J, et al. Strategies to reduce the systematic error due to tumor and rectum motion in radiotherapy of prostate cancer. Radiother Oncol. 2005;74(2):177–85.
Langenhuijsen JF, van Lin EN, Kiemeney LA, et al. Ultrasound-guided transrectal implantation of gold markers for prostate localization during external beam radiotherapy: complication rate and risk factors. Int J Radiat Oncol Biol Phys. 2007;69(3):671–6.
Gill S, Li J, Thomas J, et al. Patient-reported complications from fiducial marker implantation for prostate image-guided radiotherapy. Br J Radiol. 1015;2012(85):1011–7.
Poggi MM, Gant DA, Sewchand W, et al. Marker seed migration in prostate localization. Int J Radiat Oncol Biol Phys. 2003;56(5):1248–51.
Fawaz ZS, Yassa M, Nguyen DH, et al. Fiducial marker implantation in prostate radiation therapy: complication rates and technique. Cancer Radiother. 2014;18(8):736–9.
Crijns W, Van Herck H, Defraene G, et al. Dosimetric adaptive IMRT driven by fiducial points. Med Phys. 2014;41(6):061716.
Acknowledgments
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
About this article
Cite this article
Yildirim, B.A., Onal, C. & Dolek, Y. Is it essential to use fiducial markers during cone-beam CT-based radiotherapy for prostate cancer patients?. Jpn J Radiol 35, 3–9 (2017). https://doi.org/10.1007/s11604-016-0590-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-016-0590-y