Abstract
Integrated positron emission tomography/computed tomography (PET/CT) with 2-[18F]fluoro-2-deoxy-d-glucose (FDG) is a useful technique for acquisition of both glucose metabolic and anatomic imaging data using a single device in a single diagnostic session, and has opened a new field in clinical oncologic imaging. FDG-PET/CT has been used successfully for the initial staging, restaging, monitoring of the response to therapy, and prognostication of head and neck carcinoma. The present review discusses the current role of FDG-PET/CT in the management of head and neck carcinoma, focusing on its usefulness and limitations for imaging in these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the late 1990s, positron emission tomography (PET) with 2-[18F]fluoro-2-deoxy-d-glucose (FDG), which exploits the increased utilization and high uptake of glucose by malignant cells, opened a new field in clinical oncologic imaging. Intrinsically, PET images lack anatomic information, and precise localization of any suspicious lesions may be difficult. Recently, however, integrated positron emission tomography/computed tomography (PET/CT), in which a full-ring-detector clinical PET scanner and multidetector-row helical CT scanner are combined, has made it possible to acquire both metabolic and anatomic imaging data using a single device in a single diagnostic session, providing precise anatomic localization of suspicious areas of increased FDG uptake. In a clinical setting, FDG-PET/CT has achieved a significant improvement in diagnostic accuracy and exerted a considerable impact on patient management, including initial staging, optimization of treatment, restaging, monitoring of the response to therapy, and prognostication of various malignant tumors including head and neck carcinoma. Here we review the current and future roles of FDG-PET/CT in the management of head and neck carcinoma, discussing its usefulness and limitations for imaging in these patients.
Initial staging
Head and neck cancer (HNC) ranks as the sixth most common cancer worldwide, the vast majority of cases being head and neck squamous cell carcinoma (HNSCC) [1]. Most patients present with complicated locally advanced disease requiring multidisciplinary treatment plans employing combinations of surgery, radiation therapy and chemotherapy. Tumor staging is critical for therapeutic planning, and there are multiple challenges including accurate tumor localization with precise delineation of the tumor volume (T stage), neck lymph node (LN) staging (N stage), and detection of distant metastasis (M stage).
FDG-PET/CT is being used increasingly for staging of HNSCC, and has a considerable impact on treatment decision-making [2–8]. In comparison to morphological imaging methods such as computed tomography (CT) or magnetic resonance imaging (MRI), PET/CT is particularly advantageous in allowing assessment of neck nodes, potential distant metastases and synchronous second primaries in a single examination. Lonneux et al. [9] performed a multicenter prospective study to evaluate the impact of PET/CT on the initial staging and management of 233 patients with HNSCC. The group found that PET/CT improved the TNM classification of the disease and altered the management of 13.7 % of the patients, mainly due to the ability of PET/CT to detect metastatic or additional disease. In 2014, the National Comprehensive Center Network updated the clinical practice guidelines for PET/CT imaging of head and neck cancer and suggested the use of PET/CT for initial staging of oral cavity, oropharyngeal, hypopharyngeal, glottic, and supraglottic cancers for stage III–IV disease, as well as mucosal melanoma and nasopharyngeal carcinoma (World Health Organization class 2–3 and N2–3 diseases) [1].
T staging
The T stage at each site is determined by the size of the primary tumor and invasion into deep structures. Contrast-enhanced CT (ceCT) and MRI have been the primary imaging modalities for evaluation of HNSCC T stage because of their superior anatomic resolution and tissue contrast in comparison to PET/CT (Fig. 1). There is no clear recommendation for routine use of PET/CT in initial T staging. MRI remains the preferred imaging method for assessment of invasion in the nasopharynx, oral cavity, perineural areas and bone marrow, whereas CT is the modality of choice for assessment of larynx and bony cortex invasion [10–12]. Although MRI is the modality of choice for detection of perineural spread due to its high tissue contrast, on PET/CT, perineural spread can present as abnormal linear or curvilinear hypermetabolic activity along the trigeminal or facial nerves [12].
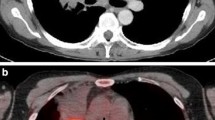
A 60-year-old man with neck node metastasis arising from hypopharyngeal cancer (pT4N2b). a Contrast-enhanced CT shows a 4-cm mass with rim enhancement and central necrosis in the left hypopharyngeal piriform fossa, suggesting hypopharyngeal cancer. Sclerotic change is observed at the proximate left thyroid cartilage (short arrow), suggesting invasion of the thyroid cartilage (cT4a). One 10 × 13-mm swollen lymph node is also seen at left level III (long arrow), suggesting the presence of nodal cancer spread. b FDG-PET and c fused PET/CT show doughnut-shaped intense FDG uptake (SUVmax: 12.8) corresponding to the hypopharyngeal mass and intense FDG uptake (SUVmax: 11.9) corresponding to the ipsilateral neck node (long arrow), confirming the hypopharyngeal cancer and neck nodal metastasis. The patient underwent resection of the primary tumor with bilateral neck dissection, and examination of the histopathological specimen revealed extensive lymph node involvement by cancer in this node (hypopharyngeal cancer, pT4N2bM0)
N staging
Pretreatment assessment of neck metastasis is important for therapeutic planning and prognostication in patients with HNSCC [13]. The likelihood of neck LN metastasis in HNSCC patients depends on the location, histology and staging of the primary tumor. Predominance of certain levels was seen for each primary site. Levels I, II, and III were at highest risk for metastasis from cancer of the oral cavity, and levels II, III, and IV were at highest risk for metastasis from carcinomas of the oropharynx, hypopharynx, and larynx [14]. Especially, the most common site of neck regional LN is level I for cancer of the oral cavity, level II for cancer of the oropharynx, and levels II and III for cancers of the hypopharynx, and larynx. At present, neck dissection with histologic examination of LNs is still the most reliable staging procedure. Preoperative nodal status is usually evaluated by clinical examinations such as palpation, ultrasonography (US), CT, and MRI. Unfortunately, CT and MRI, which evaluate morphologic parameters such as nodal size, internal architecture and contrast enhancement pattern, have been shown to have only limited value for this purpose [15]. Doppler US with fine-needle aspiration can overcome some of these limitations, but the results are dependent on the skill level of the sonographer, and this may be impractical in some cases because the number of questionable nodes may be high.
Several studies have evaluated the diagnostic utility of FDG-PET or PET/CT for detection of neck LN metastases of HNSCC (Fig. 1). Data from those studies demonstrated variations in sensitivity, specificity and accuracy, with respective values of 67–95, 76–99 and 77–97 % [16–32] (Table 1). In a meta-analysis, Kyzas et al. [33] reviewed 32 studies of 1236 patients with HNSCC and reported that the overall sensitivity and specificity of FDG-PET for assessment of nodal disease was 80 and 86 %, respectively, but dropped to 50 and 87 %, respectively, for cN0 patients. Sun et al. [34] reviewed 24 studies of 1270 patients with HNSCC to assess nodal metastasis, and reported that the mean [95 % confidence interval (CI)] pooled per-patient, per-neck-side, and per-neck-level sensitivities/specificities of FDG-PET/CT were 91 % (82–95 %)/87 % (80–92 %), 84 % (75–90 %)/83 % (77–88 %), and 80 % (71–87 %)/96 % (94–97 %), respectively. Across 13 studies (3460 neck levels) with per-neck-level data, the sensitivity and specificity of FDG-PET/CT were 84 % (72–91 %) and 96 % (95–97 %), and those of conventional imaging (CT, MRI, and CT/MRI) were 63 % (53–72 %) and 96 % (95–97 %), respectively. Some groups have used visual assessment [16, 17, 21–23, 26, 31] and other groups have used quantitative assessment using maximum standardized uptake value (SUVmax) [18–20, 24, 25, 27–30]. To our knowledge, there have been no reports which directly compared the difference in diagnostic performance between visual and quantitative assessment, and therefore the superiority of the two methods has not been clarified. Murakami et al. [18] reported the size-based SUVmax cutoff and Jeong et al. [19] reported the level-based SUVmax cutoff.
Recently, Roh et al. [31] demonstrated that PET/CT is superior to CT/MR imaging for detection of occult cervical metastatic nodes in 91 patients who were neck palpation-negative (69 vs 39 % on a per-level basis, p < 0.001). On the other hand, several reports have indicated that FDG-PET or PET/CT offers no advantage, especially for evaluation of the N0 neck in patients with early oral cancer [17, 23], and therefore its diagnostic value remains controversial.
Although FDG-PET is a functional method based on the increased glucose metabolism of cancer cells, regardless of node size, and PET/CT can often detect metastatic LNs measuring 6–9 mm, FDG-PET has several limitations. FDG uptake by small deposits of tumor cells is often poorly depicted owing to partial volume effects. Moreover, its registration is limited to a certain LN size, because the spatial resolution of recent PET scanners is technically limited to 4–6 mm. On the other hand, FDG-PET is not 100 % specific because inflammatory reactive nodes and adjacent granulation tissue can increase uptake, yielding a false positive result. Although FDG-PET/CT does not yet have the ability to replace neck dissection as the diagnostic standard of care, in the future, the development of dual time point PET, new tumor-specific tracers and PET scanners with a higher resolution may increase the potential to detect occult LN metastases.
In summary, the spatial resolution of PET (approximately 4–6 mm) is not sufficient to allow the detection of early neck node involvement and micrometastases, and PET/CT cannot replace neck dissection as the diagnostic standard.
M staging
Approximately 4–15.4 % of patients with HNSCC have distant metastases at initial presentation [2, 6]. The most common sites of metastasis include the lung, bone and abdomen. Whole-body FDG-PET/CT is more accurate than conventional imaging for detection of metastatic foci [8]. A meta-analysis involving 15 studies with 1445 patients by Xu et al. [35] revealed that the sensitivity and specificity of FDG-PET/CT was around 87.5 % (95 % CI, 78.7–93.6 %) and 95 % (95 % CI, 93.1–96.4 %), respectively. It is very important to detect distant metastases early in the workup, as it can impact prognosis and management. Extensive surgery with curative intent may result in significant morbidity and mortality, and may be avoided in the event of documented distant metastases. FDG-PET/CT is recommended when distant spread is suspected in patients with locoregionally advanced HNSCC. However, negative findings of FDG-PET/CT do not completely rule out the presence of metastasis [35].
In the pulmonary parenchyma, FDG-PET efficiently depicts supracentimetric pulmonary nodules. However, because of the partial volume effect and respiratory movements, PET lacks sensitivity for smaller nodules. Careful scrutiny of the CT data obtained during the hybrid PET/CT examination can reveal small nodules without FDG uptake. It should be noted that free-breathing CT is less efficient than standard diagnostic thoracic CT.
In summary, FDG-PET/CT is a useful modality for detection of distant metastases, showing high sensitivity and specificity.
Carcinoma of unknown primary origin
Two to 7 % of HNSCC patients present with metastatic cervical lymphadenopathy from undefined primary sites [36], despite a complete history (nonspecific symptoms or no symptoms), thorough physical examination/office flexible fiberoptic endoscopy (small submucosal lesion), or conventional contrast-enhanced CT/MRI (small lesion obscured by normal lymphoid tissue). The majority of such primary cancers arise in the palatine tonsils or base of the tongue. Various studies have shown that PET/CT is able to identify the primary cancer in 29–54 % of cases with 62–93 % sensitivity, 33–93 % specificity, 56–89 % positive predictive value (PPV) and 25–96 % negative predictive value (NPV) [37, 38]. In recent years, Lee et al. [39] have demonstrated that FDG-PET/CT showed higher sensitivity (69 %) for detection of occult primary tumors than did ceCT (16 %) (p < 0.001) or combined ceCT and MRI (41 %, p = 0.039) in 56 patients with cervical metastasis from an unknown primary tumor.
Synchronous second primary cancer
The association between synchronous primary tumors in the aerodigestive tract is a well-known phenomenon that has been explained by the concept of “field cancerization” [40]. The mucous epithelium of the head and neck, esophagus and lung is exposed to common carcinogenic agents, leading to multiple carcinomas in these regions. Strong epidemiologic evidence implicates tobacco as the main carcinogen and alcohol as a promoter of carcinogenesis. Approximately 7.4–18 % of patients with HNSCC have a synchronous second primary malignancy [2, 6, 7]. Panendoscopy studies have shown that the prevalence of synchronous esophageal SCC ranges from 5.1 to 47.1 % [41, 42]. A meta-analysis has revealed that FDG-PET/CT had 87.5 % sensitivity and 95 % specificity for detection of synchronous primary cancer or distant metastasis [43] (Fig. 2). Negative findings of FDG-PET/CT do not completely exclude the presence of synchronous primary cancer. Strobel et al. [7] performed FDG-PET/CT for 589 patients with HNSCC, 9.5 % of whom had synchronous primary cancers, of which 84 % were detected using FDG-PET/CT. In 80 % of the patients, FDG-PET/CT led to a change of therapy because of detection of synchronous primary cancer.
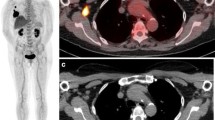
A 71-year-old man with four cancers of the oropharynx, esophagus, pancreas, and sigmoid colon. a Maximum intensity projection (MIP) of FDG-PET shows abnormal FDG uptake in the oropharynx, mediastinum, upper abdomen and pelvis. b FDG-PET/CT shows intense FDG uptake (SUVmax: 8.9) corresponding to the uvula (arrow), suggesting orophayngeal cancer. c FDG-PET/CT shows moderate FDG uptake (SUVmax: 4.5) corresponding to the esophagus (arrow), suggesting esophageal cancer. d FDG-PET/CT shows intense FDG uptake (SUVmax: 9.7) corresponding to the pancreatic body (arrow), suggesting pancreatic cancer. e FDG-PET/CT shows intense FDG uptake (SUVmax: 13.1) corresponding to the sigmoid colon (arrow), suggesting colon cancer. Examination of the histopathological specimen confirmed four cancers arising from the oropharynx, esophagus, pancreas, and sigmoid colon
However, due to the limited spatial resolution of PET/CT, small and superficially growing tumors can sometimes be invisible. Nakaminato et al. [42] demonstrated that in routine esophagogastroduodenoscopy screenings with iodine staining and FDG-PET/CT scans before initial treatment of hypopharyngeal cancer, the prevalence of esophageal cancers was 51.5 %, of which FDG-PET/CT detected only 20.7 %. FDG-PET/CT cannot replace esophagogastroduodenoscopy because of its limited ability to detect superficial esophageal cancer.
In summary, FDG-PET/CT and esophagogastroduodenoscopy are complementary, and their use in combination may be the most sensitive approach for detection of synchronous second primary tumors at an early treatable stage in patients with HNSCC.
Restaging
Despite continuing advances in surgical and non-surgical therapeutic strategies, up to 40 % of HNSCC patients suffer recurrence even after therapy [44]. However, postsurgical and radiation-induced changes in normal tissues may interfere with the early detection of recurrence by regular standard examinations of the head and neck, including physical examination, endoscopic examination, CT and MRI [45].
FDG-PET, which exploits the increased utilization of glucose by malignant cells, has made it possible to diagnose cancer recurrence and distant metastasis at the preclinical stage before it becomes evident by conventional imaging modalities [46]. Many studies have confirmed the usefulness of FDG-PET/CT as a post-treatment tool for patients with HNSCC (Figs. 3, 4). Data from those studies have demonstrated 60–100 % sensitivity, 65–98 % specificity and 66–99 % accuracy [47–73] (Table 2). Isles et al. [74] conducted a meta-analysis of 27 studies involving 1871 patients with HNSCC for whom FDG-PET or PET/CT had been conducted after radiotherapy/chemotherapy, and reported that the mean pooled sensitivity/specificity of FDG-PET or PET/CT for detection of recurrent tumors in the area affected by the primary HNSCC was 94/82 %, whereas the corresponding values for CT (67/78 %) and MRI (81/46 %) were lower; for detection of LN metastasis, the corresponding value was 74/88 %. Gupta et al. [75] conducted a meta-analysis of 51 trials involving 2335 patients to assess the diagnostic performance of post-treatment FDG-PET or PET/CT imaging for HNSCC, and reported that the mean (95 % CI) pooled sensitivity, specificity, PPV, and NPV of FDG-PET or PET/CT for detection of recurrent tumors in the primary HNSCC-affected area were 80 % (74–85 %), 88 % (85–90 %), 59 % (53–65 %), and 95 % (94–97 %), respectively. The corresponding values for neck nodes were 73 % (67–78 %), 88 % (86–89 %), 52 % (47–58 %), and 95 % (93–96 %), respectively. FDG-PET/CT shows a very high NPV and moderate PPV for evaluation of local and regional recurrence in patients with HNSCC. If the NPV remains exceptionally high, and a surveillance scan shows a negative response following definitive treatment, then remaining viable disease seems unlikely, thus offering a guide to therapeutic decision-making. A moderate PPV is due to treatment-related FDG-avid inflammation or infection in LNs, salivary gland, muscles, and soft tissue [76].
A 65-year-old man with local recurrence of maxillary sinus cancer 5 months after surgery. a CT of FDG-PET/CT shows a soft-tissue density area at the surgical site (arrow), suggesting local recurrence. b FDG-PET and c fused PET/CT show intense FDG uptake (SUVmax: 20.5) corresponding to the soft-tissue density area (arrow), confirming local recurrence. Examination of the histopathological specimen revealed cancer tissue
A 70-year-old man with neck lymph node recurrence of oral tongue cancer 6 months after surgery. a Contrast-enhanced CT shows a 7 × 7-mm left neck lymph node with enhancement in the left neck area (arrow), with an equivocal interpretation of nodal recurrence. b FDG-PET and c fused PET/CT show intense FDG uptake (SUVmax: 11.4) corresponding to the left neck node (arrow), confirming recurrence in the neck node. The histopathological specimen revealed extensive involvement of cancer in this lymph node
Although early and accurate detection of residual/recurrent disease by FDG-PET/CT can facilitate appropriate therapeutic management, the optimal timing of the first response assessment by PET/CT after definitive treatment is controversial. It is suggested that PET/CT should be performed no sooner than 2 months after completion of treatment to avoid any false-positive results; persistent local inflammation, infection, non-infectious inflammation or granulation are known to give rise to false-positive results when FDG-PET is employed. However, it may be performed sooner if recurrent disease is clinically suspected. Although Kim et al. [51] have prospectively evaluated the response to radiotherapy using FDG-PET 4 weeks after the completion of radiotherapy in patients with HNSCC, Isles et al. demonstrated that the sensitivity of FDG-PET was decreased if the interval between treatment and scan was less than 10 weeks [74]. At many institutions, an interval of 12 weeks has generally been recommended to balance the drawbacks of imaging too early versus too late [76]. Kostakoglu et al. [66] have recommended that a post-treatment scan be conducted 3–4 months after completion of treatment, followed by another scan within the first year. Paidpally et al. [77] have reported that the proportion of FDG-PET/CT studies yielding indeterminate results because of possible treatment-related inflammation stabilizes between 4 and 24 months after treatment, and that the most appropriate timing for post-therapy PET/CT is between 3 and 4 months.
It is important to detect distant metastases early in the workup, as this can change prognosis and management. Extensive surgery with curative intent may cause significant morbidity and mortality, and may be better avoided if distant metastases can be demonstrated, thus switching the focus to palliative chemoradiation options. PET/CT is recommended when distant spread is suspected in patients with locoregionally advanced HNSCC. A meta-analysis involving 10 studies with 756 patients by Gao et al. [78] revealed that the sensitivity and specificity of FDG-PET/CT was around 92 % (95 % CI, 83–96 %) and 95 % (95 % CI, 91–97 %), respectively.
In summary, FDG PET/CT is very useful for cancer restaging in patients with documented or suspected HNSCC recurrence, and is more efficient than PET alone and conventional imaging methods. An interval of 12 weeks has generally been recommended as most common, optimal timing for FDG-PET examination after finishing treatment.
Monitoring of response to therapy
PET/CT is superior to CT for distinguishing metabolically active tumors from residual anatomic deformity after completion of chemoradiation therapy. Four studies have compared the performance of FDG-PET or PET/CT with that of ceCT for detection of persistent disease after radiotherapy with or without chemotherapy. Porceddu et al. [79] evaluated a PET-directed policy for neck management in node-positive HNSCC patients after definitive radiotherapy with or without concurrent systemic therapy. In that study, 112 patients achieving a complete response at the primary site underwent post-therapy nodal response assessment for 12 weeks using PET and diagnostic CT, and 50 CT abnormalities were observed following completion of therapy. Forty-one of these abnormalities were highlighted only on the basis of PET characteristics. None of the patients ultimately developed recurrent disease, and the false positivity rate for CT alone was 38 %. Moeller et al. [80] evaluated the extent to which FDG-PET/CT might improve assessment of the response to radiation therapy in 98 HNC patients who underwent FDG-PET/CT and ceCT imaging 8 weeks after completion of treatment. When the optimal threshold SUVmax for prediction of failure in primary tumors and nodes was taken as 6.5 and 2.8, respectively, the sensitivity, specificity, PPV, and NPV were 70, 94, 58 and 96 %, respectively, for primary tumors and 75, 76, 27, and 96 %, respectively, for neck nodes, when post-radiation FDG-PET/CT was used to discriminate responders from non-responders. They concluded that FDG-PET/CT has little merit over CT alone for assessment of the response to radiation therapy in unselected patients with locally advanced HNSCC, whereas it may improve assessment of the response to treatment in high-risk patients, such as those with human papillomavirus (HPV)-negative disease. Kitagawa et al. [81] reported 23 patients who underwent FDG-PET later than 4 weeks after treatment, whereas ceCT was performed within 2 weeks. FDG-PET identified all patients with residual disease, demonstrating sensitivity and specificity higher than those of ceCT (100 and 89 vs 75 and 59 %, respectively). Andrade et al. [82] reported performing FDG-PET/CT at 4–8 weeks, and again later than 8 weeks after treatment, thus testing two different time intervals and identifying the best timing in 28 patients with HNC. The authors found that the accuracies of PET/CT and CT were similar within 4–8 weeks, whereas beyond 8 weeks the accuracy of PET/CT was higher (100 %) than that of ceCT (55 %).
Although clinical parameters and structural imaging cannot reliably predict the presence of residual metastatic neck disease, post-treatment FDG-PET with high NPV may be justified [76]. In one of the previously cited studies [54], planned neck dissection would have been considered in 51 patients because of the presence of residual enlarged LNs, but disease was in fact present in only 7 of them. PET/CT findings in the study by Ong et al. [54] could have reduced the number of planned neck dissections by 75 % (from 51 to 13) while missing disease in 2 % (2/84 heminecks). Other investigators have suggested that negative FDG-PET/CT results after chemoradiotherapy could reduce the number of planned neck dissections by more than 80 % [49].
The potential clinical utility of PET for early response assessment during chemoradiotherapy has not been studied systematically. Data for other malignancies suggest that a significant decline in FDG uptake between baseline and interim PET after a few cycles of chemo- or chemoradiotherapy might indicate a better prognosis and a high likelihood of achieving a complete response. Only a single small study has attempted to address this issue in HNSCC [83]. Using coincidence camera imaging, that study noted an early and significant decline in FDG uptake in 47 patients with locally advanced disease after one cycle of chemotherapy or 24 Gy of radiotherapy. When dichotomized by the median SUV, individuals with lower FDG uptake showed a better rate of locoregional control. However, a closer analysis of the study results revealed that similar prognostic information could also be derived from the baseline scan alone. Therefore, although interesting, the study remained largely inconclusive. In particular, it remains unclear at what interim time point during the course of therapy a PET scan should be performed and how interim PET findings might alter patient management (good local control rates with concurrent chemoradiotherapy, lack of an established alternative therapy). In general, focal and asymmetric FDG uptake with an intensity greater than that in surrounding normal tissues (in particular, muscle) and blood vessels should be considered suggestive of residual disease. In contrast, diffuse (nonfocal) FDG uptake within the radiation field is usually an indicator of post-radiation inflammation. One of the initial trials that established concurrent chemoradiotherapy for locoregionally advanced HNSCC reported high-grade toxic effects in 82 % of patients, including grade 3 or 4 mucositis in 41 % and laryngeal toxicity in 14 % [84]. This report has obvious implications for imaging studies: laryngeal edema and treatment-induced infiltrative changes in perilaryngeal soft tissues are commonly observed on post-treatment CT, along with nonspecific contrast enhancement patterns. Likewise, increased laryngeal or oropharyngeal FDG uptake may be observed for prolonged periods after chemoradiotherapy. In most cases, this uptake will be of mild to moderate intensity and will be diffuse throughout the larynx or along the oropharyngeal walls.
There are also limited data on the role of PET in assessing the response to induction chemotherapy before subsequent concurrent chemoradiotherapy. This is a topic of growing interest to medical oncologists. It is conceivable that PET with either FDG or 18F-3′-deoxy-3′ fluorothymidine (FLT) [85] after induction chemotherapy might help in this decision. For instance, if a patient shows little or no metabolic response after induction chemotherapy, this might indicate a low likelihood of cure with subsequent chemoradiotherapy; perhaps such patients would benefit from immediate salvage surgery after induction therapy or should be enrolled for more aggressive chemoradiotherapy protocols.
In summary, further analysis is needed to assess the potential clinical utility of interim PET for early response assessment during chemoradiotherapy or the response to induction chemotherapy before subsequent concurrent chemoradiotherapy.
Prognostic value
Tumor FDG uptake has been associated with various cellular characteristics such as cell viability and proliferative activity [86]. Thus, it is expected that analyses of metabolic parameters, which are independent of morphologic changes, would offer an important opportunity to predict individual tumor behavior. High FDG uptake by a tumor may be correlated with poor outcome, and thus such patients should receive more aggressive treatment combinations.
The prognostic value of FDG-PET for prediction of clinical outcomes in patients with HNSCC has not been assessed fully and is still controversial. Several authors have demonstrated that SUVmax in the primary HNSCC tumor [87–93] or neck LN metastasis [94–97] could be predictive of outcome. However, SUVmax shows the highest intensity of FDG uptake within the region of interest or volume of interest, and cannot represent total tumor uptake for the entire tumor mass. Recently, there has been increasing interest in the use of volumetric parameters of metabolism such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG). MTV and mean SUV can be measured by contouring margins defined by thresholds. Then, TLG can be calculated by multiplying MTV by mean SUV, which weights the volumetric burden and metabolic activity of tumors. Several authors have demonstrated that MTV [93, 98–102] and TLG [98–100, 102, 103] in a primary HNSCC could be predictive of outcome. Park et al. [104] reviewed 13 studies comprising 1180 HNSCC patients and confirmed the superiority of MTV and TLG relative to SUVmax: first, patients with a high MTV showed a 3.06-fold higher risk of adverse events or a 3.51-fold higher risk of death than patients with a low MTV; second, patients with a high TLG had a 3.10-fold higher risk of events or a 3.14-fold higher risk of death than patients with a low TLG; third, patients with a high SUVmax showed a 1.83-fold higher risk of adverse events or a 2.35-fold higher risk of death than patients with a low SUVmax.
In summary, FDG-PET/CT including SUVmax, MTV, and TLG may have prognostic value in HNSCC patients; however, more studies are needed to clarify this.
Human papilloma virus-positive oropharyngeal squamous cell carcinoma
HPV-positive oropharyngeal squamous cell carcinoma (OPSCC) represents an emerging disease that differs from HPV-negative OPSCC in natural history and prognosis. HPV-positive OPSCCs are often associated with cystic LN metastases and have a higher rate of nodal involvement than do HPV-negative OPSCCs [105]. The better prognosis and outcome of HPV-positive patients would likely warrant less intense imaging follow-up during the 5-year follow-up period after treatment. However, manifestation of distant metastases later in the disease course and at unusual sites with a disseminating phenotype would require a longer follow-up with FDG-PET/CT [106].
Conclusion
FDG-PET/CT can allow combined metabolic and morphological assessment of tumors with significant improvements in diagnostic accuracy and considerable impact on patient management, initial staging, restaging, monitoring the response to therapy, and prognostication of HNSCC. Further analysis is needed to evaluate the potential clinical utility of interim PET for early response assessment during chemoradiotherapy or the response to induction chemotherapy before subsequent concurrent chemoradiotherapy.
References
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology™ 2014. Head and neck cancers v. 2. 2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#headand-neck. Accessed 26 Oct 2015
Fleming AJ Jr, Smith SP Jr, Paul CM, Hall NC, Daly BT, Agrawal A, et al. Impact of [18F]-2-fluorodeoxyglucose-positron emission tomography/computed tomography on previously untreated head and neck cancer patients. Laryngoscope. 2007;117:1173–9.
Gordin A, Golz A, Keidar Z, Daitzchman M, Bar-Shalom R, Israel O. The role of FDG-PET/CT imaging in head and neck malignant conditions: impact on diagnostic accuracy and patient care. Otolaryngol Head Neck Surg. 2007;137:130–7.
Connell CA, Corry J, Milner AD, Hogg A, Hicks RJ, Rischin D, et al. Clinical impact of, and prognostic stratification by, F-18 FDG PET/CT in head and neck mucosal squamous cell carcinoma. Head Neck. 2007;29:986–95.
Veit-Haibach P, Luczak C, Wanke I, Fischer M, Egelhof T, Beyer T, et al. TNM staging with FDG-PET/CT in patients with primary head and neck cancer. Eur J Nucl Med Mol Imaging. 2007;34:1953–62.
Kim SY, Roh JL, Yeo NK, Kim JS, Lee JH, Choi SH, et al. Combined 18F-fluorodeoxyglucose-positron emission tomography and computed tomography as a primary screening method for detecting second primary cancers and distant metastases in patients with head and neck cancer. Ann Oncol. 2007;18:1698–703.
Strobel K, Haerle SK, Stoeckli SJ, Schrank M, Soyka JD, Veit-Haibach P, et al. Head and neck squamous cell carcinoma (HNSCC)—detection of synchronous primaries with 18F-FDG-PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:919–27.
Yoo J, Henderson S, Walker-Dilks C. Evidence-based guideline recommendations on the use of positron emission tomography imaging in head and neck cancer. Clin Oncol ( Coll Radiol. 2013;25:e33–66.
Lonneux M, Hamoir M, Reychler H, Maingon P, Duvillard C, Calais G, et al. Positron emission tomography with [18F]fluorodeoxyglucose improves staging and patient management in patients with head and neck squamous cell carcinoma: a multicenter prospective study. J Clin Oncol. 2010;28:1190–5.
Becker M, Zbären P, Casselman JW, Kohler R, Dulguerov P, Becker CD. Neoplastic invasion of laryngeal cartilage: reassessment of criteria for diagnosis at MR imaging. Radiology. 2008;249:551–9.
Becker M, Burkhardt K, Dulguerov P, Allal A. Imaging of the larynx and hypopharynx. Eur J Radiol. 2008;66:460–79.
Paes FM, Singer AD, Checkver AN, Palmquist RA, De La Vega G, Sidani C. Perineural spread in head and neck malignancies: clinical significance and evaluation with 18F-FDG PET/CT. Radiographics. 2013;33:1717–36.
Snow GB, Patel P, Leemans CR, Tiwari R. Management of cervical lymph nodes in patients with head and neck cancer. Eur Arch Otorhinolaryngol. 1992;249:187–94.
Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405–9.
Castelijins JA, van den Brekel MW. Imaging of lymphadenopathy in the neck. Eur Radiol. 2002;12:727–38.
Ng SH, Yen TC, Liao CT, Chang JT, Chan SC, Ko SF, et al. 18F-FDG PET and CT/MRI in oral cavity squamous cell carcinoma: a prospective study of 124 patients with histologic correlation. J Nucl Med. 2005;46:1136–43.
Schöder H, Carlson DL, Kraus DH, Stambuk HE, Gönen M, Erdi YE, et al. 18F-FDG PET/CT for detecting nodal metastases in patients with oral cancer staged N0 by clinical examination and CT/MRI. J Nucl Med. 2006;47:755–62.
Murakami R, Uozumi H, Hirai T, Nishimura R, Shiraishi S, Ota K, et al. Impact of FDG-PET/CT imaging on nodal staging for head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2007;68:377–82.
Jeong HS, Baek CH, Son YI, Ki Chung M, Kyung Lee D, Young Choi J, et al. Use of integrated 18F-FDG PET/CT to improve the accuracy of initial cervical nodal evaluation in patients with head and neck squamous cell carcinoma. Head Neck. 2007;29:203–10.
Nabmias C, Carlson ER, Duncan LD, Blodgett TM, Kennedy J, Long MJ, et al. Positron emission tomography/computerized tomography (PET/CT) scanning for preoperative staging of patients with oral/head and neck cancer. J Oral Maxillofac Surg. 2007;65:2524–35.
Roh JL, Yeo NK, Kim JS, Lee JH, Cho KJ, Choi SH, et al. Utility of 2-[18F] fluoro-2-deoxy-d-glucose positron emission tomography and positron emission tomography/computed tomography imaging in the preoperative staging of head and neck squamous cell carcinoma. Oral Oncol. 2007;43:887–93.
Yamazaki Y, Saitoh M, Notani K, Tei K, Totsuka Y, Takinami S, et al. Assessment of cervical lymph node metastases using FDG-PET in patients with head and neck cancer. Ann Nucl Med. 2008;22:177–84.
Krabbe CA, Dijkstra PU, Pruim J, van der Laan BF, van der Wal JE, Gravendeel JP, et al. FDG PET in oral and oropharyngeal cancer. Value for confirmation of N0 neck and detection of occult metastases. Oral Oncol. 2008;44:31–6.
Piao Y, Bold B, Tayier A, Ishida R, Omura K, Okada N, et al. Evaluation of 18F-FDG PET/CT for diagnosing cervical nodal metastases in patients with oral cavity or oropharynx carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:933–8.
Yoon DY, Hwang HS, Chang SK, Rho YS, Ahn HY, Kim JH, et al. CT, MR, US,18F-FDG PET/CT, and their combined use for the assessment of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Eur Radiol. 2009;19:634–42.
Richard C, Prevot N, Timoshenko AP, Dumollard JM, Dubois F, Martin C, et al. Preoperative combined 18-fluorodeoxyglucose positron emission tomography and computed tomography imaging in head and neck cancer: does it really improve initial N staging? Acta Otolaryngol. 2010;130:1421–4.
Iyer NG, Clark JR, Singham S, Zhu J. Role of pretreatment 18FDG-PET/CT in surgical decision-making for head and neck cancers. Head Neck. 2010;32:1202–8.
Kim SY, Kim JS, Doo H, Lee H, Lee JH, Cho KJ, et al. Combined [18F]fluorodeoxyglucose positron emission tomography and computed tomography for detecting contralateral neck metastases in patients with head and neck squamous cell carcinoma. Oral Oncol. 2011;47:376–80.
Matsubara R, Kawano S, Chikui T, Kiyosue T, Goto Y, Hirano M, et al. Clinical significance of combined assessment of the maximum standardized uptake value of F-18 FDG PET with nodal size in the diagnosis of cervical lymph node metastasis of oral squamous cell carcinoma. Acad Radiol. 2012;19:708–17.
Nguyen A, Luginbuhl A, Cognetti D, Van Abel K, Bar-Ad V, Intenzo C, et al. Effectiveness of PET/CT in the preoperative evaluation of neck disease. Laryngoscope. 2014;124:159–64.
Roh JL, Park JP, Kim JS, Lee JH, Cho KJ, Choi SH, et al. 18F fluorodeoxyglucose PET/CT in head and neck squamous cell carcinoma with negative neck palpation findings: a prospective study. Radiology. 2014;271:153–61.
Joo YH, Yoo IR, Cho KJ, Park JO, Nam IC, Kim CS, et al. The value of preoperative 18F-FDG PET/CT for assessing the contralateral neck in head and neck cancer patients with unilateral node metastasis (N1-3). Clin Otolaryngol. 2014;39:338–44.
Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JP. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst. 2008;100:712–20.
Sun R, Tang X, Yang Y, Zhang C. 18FDG-PET/CT for the detection of regional nodal metastasis in patients with head and neck cancer: a meta-analysis. Oral Oncol. 2015;51:314–20.
Xu GZ, Zhu XD, Li MY. Accuracy of whole-body PET and PET-CT in initial M staging of head and neck cancer: a meta-analysis. Head Neck. 2011;33:87–94.
Jereczek-Fossa BA, Jassem J, Orecchia R. Cervical lymph node metastases of squamous cell carcinoma from an unknown primary. Cancer Treat Rev. 2004;30:153–64.
Yabuki K, Tsukuda M, Horiuchi C, Taguchi T, Nishimura G. Role of 18F-FDG PET in detecting primary site in the patient with primary unknown carcinoma. Eur Arch Otorhinolaryngol. 2010;267:1785–92.
Wong WL, Sonoda LI, Gharpurhy A, Gollub F, Wellsted D, Goodchild K, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the assessment of occult primary head and neck cancers—an audit and review of published studies. Clin Oncol R Coll Radiol. 2012;24:190–5.
Lee JR, Kim JS, Roh JL, Lee JH, Baek JH, Cho KJ, et al. Detection of occult primary tumors in patients with cervical metastases of unknown primary tumors: comparison of 18F FDG PET/CT with contrast-enhanced CT or CT/MR imaging—prospective study. Radiology. 2015;274:764–71.
Van Rees BP, Cleton-Jansen AM, Cense HA, Polak MM, Clement MJ, Drillenburg P, et al. Molecular evidence of field cancerization in a patient with 7 tumors of the aerodigestive tract. Hum Pathol. 2000;31:269–71.
Su YY, Fang FM, Chuang HC, Luo SD, Chien CY. Detection of metachronous esophageal squamous carcinoma in patients with head and neck cancer with use of transnasal esophagoscopy. Head Neck. 2010;32:780–5.
Nakaminato S, Toriihara A, Makino T, Kawano T, Kishimoto S, Shibuya H. Prevalence of esophageal cancer during the pretreatment of hypopharyngeal cancer patients: routinely performed esophagogastroduodenoscopy and FDG-PET/CT findings. Acta Oncol. 2012;51:645–52.
Xu GZ, Guan DJ, He ZY. 18FDG-PET/CT for detecting distant metastases and second primary cancers in patients with head and neck cancer. A meta-analysis. Oral Oncol. 2011;47:560–5.
Ang KK, Trotti A, Brown BW, Garden AS, Foote RL, Morrison WH, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:571–8.
Lell M, Baum U, Greess H, Nömayr A, Nkenke E, Koester M, et al. Head and neck tumors: imaging recurrent tumor and post-therapeutic changes with CT and MRI. Eur J Radiol. 2000;33:239–47.
Salaun PY, Abgral R, Querellou S, Couturier O, Valette G, Bizais Y, et al. Does 18fluoro-fluorodeoxyglucose positron emission tomography improve recurrence detection in patients treated for head and neck squamous cell carcinoma with negative clinical follow-up? Head Neck. 2007;29:1115–20.
Branstetter BF 4th, Blodgett TM, Zimmer LA, Snyderman CH, Johnson JT, Raman S, et al. Head and neck malignancy: is PET/CT more accurate than PET or CT alone? Radiology. 2005;235:580–6.
Ryan WR, Fee WE Jr, Le QT, Pinto HA. Positron-emission tomography for surveillance of head and neck cancer. Laryngoscope. 2005;115:645–50.
Nayak JV, Walvekar RR, Andrade RS, Daamen N, Lai SY, Argiris A, et al. Deferring planned neck dissection following chemoradiation for stage IV head and neck cancer: the utility of PET-CT. Laryngoscope. 2007;117:2129–34.
Halpern BS, Yeom K, Fueger BJ, Lufkin RB, Czernin J, Allen-Auerbach M. Evaluation of suspected local recurrence in head and neck cancer: a comparison between PET and PET/CT for biopsy proven lesions. Eur J Radiol. 2007;62:199–204.
Kim SY, Lee SW, Nam SY, Im KC, Kim JS, Oh SJ, et al. The feasibility of 18F-FDG PET scans 1 month after completing radiotherapy of squamous cell carcinoma of the head and neck. J Nucl Med. 2007;48:373–8.
Lee JC, Kim JS, Lee JH, Nam SY, Choi SH, Lee SW, et al. F-18 FDG-PET as a routine surveillance tool for the detection of recurrent head and neck squamous cell carcinoma. Oral Oncol. 2007;43:686–92.
Comoretto M, Balestreri L, Borsatti E, Cimitan M, Franchin G, Lise M. Detection and restaging of residual and/or recurrent nasopharyngeal carcinoma after chemotherapy and radiation therapy: comparison of MR imaging and FDG PET/CT. Radiology. 2008;249:203–11.
Ong SC, Schöder H, Lee NY, Patel SG, Carlson D, Fury M, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for locoregional advanced head and neck cancer. J Nucl Med. 2008;49:532–40.
Krabbe CA, Pruim J, Dijkstra PU, Balink H, van der Laan BF, de Visscher JG, et al. 18F-FDG PET as a routine posttreatment surveillance tool in oral and oropharyngeal squamous cell carcinoma: a prospective study. J Nucl Med. 2009;50:1940–7.
Rabalais AG, Walvekar R, Nuss D, McWhorter A, Wood C, Fields R, et al. Positron emission tomography-computed tomography surveillance for the node-positive neck after chemoradiotherapy. Laryngoscope. 2009;119:1120–4.
Gourin CG, Watts T, Williams HT, Patel VS, Bilodeau PA, Coleman TA. Identification of distant metastases with PET-CT in patients with suspected recurrent head and neck cancer. Laryngoscope. 2009;119:703–6.
Kao J, Vu HL, Genden EM, Mocherla B, Park EE, Packer S, et al. The diagnostic and prognostic utility of positron emission tomography/computed tomography-based follow-up after radiotherapy for head and neck cancer. Cancer. 2009;115:4586–94.
Abgral R, Querellou S, Potard G, Le Roux PY, Le Duc-Pennec A, Marianovski R, et al. Does 18F-FDG PET/CT improve the detection of posttreatment recurrence of head and neck squamous cell carcinoma in patients negative for disease on clinical follow-up? J Nucl Med. 2009;50:24–9.
Yao M, Smith RB, Hoffman HT, Funk GF, Lu M, Menda Y, et al. Clinical significance of postradiotherapy [18F]-fluorodeoxyglucose positron emission tomography imaging in management of head-and-neck cancer—a long-term outcome report. Int J Radiat Oncol Biol Phys. 2009;74:9–14.
Kim SY, Kim JS, Yi JS, Lee JH, Choi SH, Nam SY, et al. Evaluation of 18F-FDG PET/CT and CT/MRI with histopathologic correlation in patients undergoing salvage surgery for head and neck squamous cell carcinoma. Ann Surg Oncol. 2011;18:2579–84.
Zundel MT, Michel MA, Schultz CJ, Maheshwari M, Wong SJ, Campbell BH, et al. Comparison of physical examination and fluorodeoxyglucose positron emission tomography/computed tomography 4–6 months after radiotherapy to assess residual head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:e825–32.
Zhang I, Branstetter BF 4th, Beswick DM, Maxwell JH, Gooding WE, Ferris RL. The benefit of early PET/CT surveillance in HPV-associated head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137:1106–11.
Yi JS, Kim JS, Lee JH, Choi SH, Nam SY, Kim SY, et al. 18F-FDG PET/CT for detecting distant metastases in patients with recurrent head and neck squamous cell carcinoma. J Surg Oncol. 2012;106:708–12.
Beswick DM, Gooding WE, Johnson JT, Branstetter BF 4th. Temporal patterns of head and neck squamous cell carcinoma recurrence with positron-emission tomography/computed tomography monitoring. Laryngoscope. 2012;122:1512–7.
Kostakoglu L, Fardanesh R, Posner M, Som P, Rao S, Park E, et al. Early detection of recurrent disease by FDG-PET/CT leads to management changes in patients with squamous cell cancer of the head and neck. Oncologist. 2013;18:1108–17.
Ho AS, Tsao GJ, Chen FW, Shen T, Kaplan MJ, Colevas AD, et al. Impact of positron emission tomography/computed tomography surveillance at 12 and 24 months for detecting head and neck cancer recurrence. Cancer. 2013;119:1349–56.
Rangaswamy B, Fardanesh MR, Genden EM, Park EE, Fatterpekar G, Patel Z, et al. Improvement in the detection of locoregional recurrence in head and neck malignancies: F-18 fluorodeoxyglucose-positron emission tomography/computed tomography compared to high-resolution contrast-enhanced computed tomography and endoscopic examination. Laryngoscope. 2013;123:2664–9.
Dunsky KA, Wehrmann DJ, Osman MM, Thornberry BM, Varvares MA. PET-CT and the detection of the asymptomatic recurrence or second primary lesions in the treated head and neck cancer patient. Laryngoscope. 2013;123:2161–4.
Kim JW, Roh JL, Kim JS, Lee JH, Cho KJ, Choi SH, et al. 18F-FDG PET/CT surveillance at 3–6 and 12 months for detection of recurrence and second primary cancer in patients with head and neck squamous cell carcinoma. Br J Cancer. 2013;109:2973–9.
Koshkareva Y, Branstetter BF 4th, Gaughan JP, Ferris RL. Predictive accuracy of first post-treatment PET/CT in HPV-related oropharyngeal squamous cell carcinoma. Laryngoscope. 2014;124:1843–7.
Robin P, Abgral R, Valette G, Le Roux PY, Keromnes N, Rousset J, et al. Diagnostic performance of FDG PET/CT to detect subclinical HNSCC recurrence 6 months after the end of treatment. Eur J Nucl Med Mol Imaging. 2015;42:72–8.
Suenaga Y, Kitajima K, Ishihara T, Sasaki R, Otsuki N, Nibu KI, et al. FDG-PET/contrast-enhanced CT as a post-treatment tool in head and neck squamous cell carcinoma: comparison with FDG-PET/non-contrast-enhanced CT and contrast-enhanced CT. Eur Radiol. 2015 [Epub ahead of print].
Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow-up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol. 2008;33:210–22.
Gupta T, Master Z, Kannan S, Agarwal JP, Ghsoh-Laskar S, Rangarajan V, et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38:2083–95.
Kawabe J, Higashiyama S, Yoshida A, Kotani K, Shiomi S. The role of FDG PET-CT in the therapeutic evaluation for HNSCC patients. Jpn J Radiol. 2012;30:463–70.
Paidpally V, Tahari AK, Lam S, Alluri K, Marur S, Koch W, et al. Addition of 18F-FDG PET/CT to clinical assessment predicts overall survival in HNSCC: a retrospective analysis with follow-up for 12 years. J Nucl Med. 2013;54:2039–45.
Gao S, Li S, Yang X, Tang Q. 18FDG PET-CT for distant metastases in patients with recurrent head and neck cancer after definitive treatment. A meta-analysis. Oral Oncol. 2014;50:163–7.
Porceddu SV, Pryor DI, Burmeister E, Burmeister BH, Poulsen MG, Foote MC, et al. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node-positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck. 2011;33:1675–82.
Moeller BJ, Rana V, Cannon BA, Williams MD, Sturgis EM, Ginsberg LE, et al. Prospective risk-adjusted [18F]fluorodeoxyglucose positron emission tomography and computed tomography assessment of radiation response in head and neck cancer. J Clin Oncol. 2009;27:2509–15.
Kitagawa Y, Nishizawa S, Sano K, Ogasawara T, Nakamura M, Sadato N, et al. Prospective comparison of 18F-FDG PET with conventional imaging modalities (MRI, CT, and 67 Ga scintigraphy) in assessment of combined intra-arterial chemotherapy and radiotherapy for head and neck carcinoma. J Nucl Med. 2003;44:198–206.
Andrade RS, Heron DE, Degirmenci B, Filho PA, Branstetter BF, Seethala RR, et al. Posttreatment assessment of response using FDG-PET/CT for patients treated with definitive radiation therapy for head and neck cancers. Int J Radiat Oncol Biol Phys. 2006;65:1315–22.
Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Eng J Med. 2003;349:2091–8.
Brun E, Kjellén E, Tennvall J, Ohlsson T, Sandell A, Perfekt R, et al. FDG PET studies during treatment: prediction of therapy outcome in head and neck squamous cell carcinoma. Head Neck. 2002;24:127–35.
Kishino T, Hoshikawa H, Nishiyama Y, Yamamoto Y, Mori N. Usefulness of 3′-deoxy-3′-18F-fluorothymidine PET for predicting early response to chemoradiotherapy in head and neck cancer. J Nucl Med. 2012;53:1521–7.
Minn H, Clavo AC, Grenman R, Wahl RL. In vitro comparison of cell proliferation kinetics and uptake of tritiated fluorodeoxyglucose and l-methionine in squamous-cell carcinoma of the head and neck. J Nucl Med. 1995;36:252–8.
Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-d-glucose. Int J Radiat Oncol Biol Phys. 2004;59:1295–300.
Roh JL, Pae KH, Choi SH, Kim JS, Lee S, Kim SB, et al. 2-[18F]-Fluoro-2-deoxy-d-glucose positron emission tomography as guidance for primary treatment in patients with advanced-stage resectable squamous cell carcinoma of the larynx and hypopharynx. Eur J Surg Oncol. 2007;33:790–5.
Machtay M, Natwa M, Andrel J, Hyslop T, Anne PR, Lavarino J, et al. Pretreatment FDG-PET standardized uptake value as a prognostic factor for outcome in head and neck cancer. Head Neck. 2009;31:195–201.
Torizuka T, Tanizaki Y, Kanno T, Futatsubashi M, Naitou K, Ueda Y, et al. Prognostic value of 18F-FDG PET in patients with head and neck squamous cell cancer. AJR Am J Roentgenol. 2009;192:W156–60.
Suzuki K, Nishioka T, Homma A, Tsuchiya K, Yasuda M, Aoyama H, et al. Value of fluorodeoxyglucose positron emission tomography before radiotherapy for head and neck cancer: does the standardized uptake value predict treatment outcome? Jpn J Radiol. 2009;27:237–42.
Zhang B, Li X, Lu X. Standardized uptake value is of prognostic value for outcome in head and neck squamous cell carcinoma. Acta Otolaryngol. 2010;130:756–62.
Tang C, Murphy JD, Khong B, La TH, Kong C, Fischbein NJ, et al. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;83:1514–20.
Demirci U, Coskun U, Akdemir UO, Benekli M, Kapucu O, Ozkan S, et al. The nodal standard uptake value (SUV) as a prognostic factor in head and neck squamous cell cancer. Asian Pac J Cancer Prev. 2011;12:1817–20.
Inokuchi H, Kodaira T, Tachibana H, Nakamura T, Tomita N, Nakahara R, et al. Clinical usefulness of [18F] fluoro-2-deoxy-D-glucose uptake in 178 head-and-neck cancer patients with nodal metastasis treated with definitive chemoradiotherapy: consideration of its prognostic value and ability to provide guidance for optimal selection of patients for planned neck dissection. Int J Radiat Oncol Biol Phys. 2011;79:747–55.
Kubicek GJ, Champ C, Fogh S, Wang F, Reddy E, Intenzo C, et al. FDG-PET staging and importance of lymph node SUV in head and neck cancer. Head Neck Oncol. 2010;16:19–25.
Kitajima K, Suenaga Y, Kanda T, Miyawaki D, Yoshida K, Ejima Y, et al. Prognostic value of FDG PET imaging in patients with laryngeal cancer. PLoS One. 2014;9(5):e96999.
Chan SC, Chang JT, Lin CY, Ng SH, Wang HM, Liao CT, et al. Clinical utility of 18F-FDG PET parameters in patients with advanced nasopharyngeal carcinoma: predictive role for different survival endpoints and impact on prognostic stratification. Nucl Med Commun. 2011;32:989–96.
Paidpally V, Chirindel A, Lam S, Agrawal N, Quon H, Subramaniam RM. FDGPET/CT imaging biomarkers in head and neck squamous cell carcinoma. Imaging Med. 2012;4:633–47.
Lim R, Eaton A, Lee NY, Setton J, Ohri N, Rao S, et al. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med. 2012;53:1506–13.
Park GC, Kim JS, Roh JL, Choi SH, Nam SY, Kim SY. Prognostic value of metabolic tumor volume measured by 18F-FDG PET/CT in advanced-stage squamous cell carcinoma of the larynx and hypopharynx. Ann Oncol. 2013;24:208–24.
Abd El-Hafez YG. Moustafa HM, Khalil HF, Liao CT, Yen TC. Total lesion glycolysis: a possible new prognostic parameter in oral cavity squamous cell carcinoma. Oral Oncol. 2013;49:261–8.
Chang KP, Tsang NM, Liao CT, Hsu CL, Chung MJ, Lo CW, et al. Prognostic significance of 18F-FDG PET parameters and plasma Epstein-Barr virus DNA load in patients with nasopharyngeal carcinoma. J Nucl Med. 2012;53:21–8.
Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55:884–90.
Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30:898–903.
Subramaniam RM, Alluri KC, Tahari AK, Aygun N, Quon H. PET/CT imaging and human papilloma virus-positive oropharyngeal squamous cell cancer: evolving clinical imaging paradigm. J Nucl Med. 2014;55:431–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no financial support or relationship to declare that may pose a conflict of interest.
About this article
Cite this article
Kitajima, K., Suenaga, Y. & Sugimura, K. Present and future role of FDG-PET/CT imaging in the management of head and neck carcinoma. Jpn J Radiol 33, 776–789 (2015). https://doi.org/10.1007/s11604-015-0495-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-015-0495-1