Abstract
Manganese-based cathode material has a wide range of applications in aqueous zinc-ion batteries, but its structural stability is poor. We can improve the microstructure of manganese-based cathode materials by simple mechanical ball milling methods, thus increasing the discharge capacity of batteries. However, the capacity of manganese-based cathode material batteries decreases rapidly during cycling, which is caused by the dissolution of manganese-based materials in the electrolyte. To ameliorate this problem, in this work, MnSO4 is pre-added to the ZnSO4 electrolyte, which can effectively inhibit the dissolution of the manganese-based material and maintain the conversion reaction inside the battery, providing better cycle life and higher discharge capacity. In addition, with the increase of MnSO4 additive concentration in a certain range, the discharge capacity and cycle life of the batteries were improved (the battery pre-added with 0.5 M MnSO4 solution had the highest reversible specific capacity of up to 521.91 mAh·g−1 at a current density of 100 mA·g−1 and 109.12 mAh·g−1 even at 1 A·g−1, the capacity retention rate remained at 83.8% after cycling for more than 8000 cycles), which provides an idea for the electrolyte regulation of aqueous zinc-ion batteries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the energy scarcity and environmental pollution caused by non-renewable energy sources such as coal and oil, new energy sources are urgently needed to alleviate this pressure [1]. It is well known that lithium-ion batteries have dominated the new energy storage market for many years, but the increasing scarcity of lithium resources, uneven geographic distribution, and the flammability and toxicity of the electrolyte are unfavorable factors that affect the sustainable development of lithium-ion batteries [2, 3]. In contrast, the emergence of mild aqueous ion batteries (potassium, calcium, magnesium, zinc, etc.) has received much attention [4,5,6,7], among which aqueous zinc-ion batteries (AZBs) have been favored by researchers due to their environmentally friendly, low-cost, resource-abundant, and high theoretical specific capacity (820 mAh·g−1) advantages [8,9,10]. The cathode materials play a key role in the overall performance of aqueous zinc-ion batteries. Currently, there are three main types of cathode materials: manganese-based oxides [11,12,13], vanadium-based oxides [14, 15], and Prussian blue analogs [16, 17]. Manganese-based oxide materials are polycrystalline and have the advantages of low preparation cost, abundant reserves, and simple fabrication, among which manganese dioxide has been recognized as an ideal cathode material for aqueous zinc-ion batteries. However, during the cycling process, manganese-based materials will inevitably lead to the collapse of the electrode structure due to inherent structural defects [18], which in turn affects the capacity and cycling performance of the battery. In a previous study [19], we constructed a hybrid cathode material of β-MnO2/3D graphene-carbon nanotube cross-links by a simple mechanical ball milling method, which improved the inherent stability of the cathode material, but it still suffers from the problem of faster capacity decay, which depends on the electrolyte modulation to maintain the structural stability during the reaction process.
Cao et al. [20] found excellent electrode performance of MnO2/nano-graphite flake complexes in ZnSO4 + MnSO4 electrolyte: the discharge capacity reached 230 mAh·g−1 at 100 mA·g−1, and the capacity retention rate was 80.8% after 1000 cycles at 1 A·g−1. Pan et al. [21] added MnSO4 additive to the ZnSO4 electrolyte to regulate the balance of Mn2+, which well suppressed the dissolution of the α-MnO2 nanofiber cathode, enabling the battery to exhibit excellent multiplicity performance and capacity retention of up to 92% even after 5000 cycles. Soundharrajan et al. [22] established an electrochemical equilibrium between Zn2+ and Mn2+ in a battery with ZnMn2O4 as the cathode by using 1 M ZnSO4 + 0.1 M MnSO4 electrolyte, which promoted excellent performance. Zhang et al. [23] formulated a Zn(CF3SO3)2 + Mn(CF3SO3)2 electrolyte, which was able to form a protective porous manganese oxide layer on the surface of the β-MnO2 cathode, and the battery had 225 mAh·g−1 high reversible capacity and long-term recyclability, maintained 94% capacity after 2000 cycles.
Based on this, we can know that the cathode materials formed by Mn-based oxides or mixtures with C mass have excellent electrochemical performance under appropriate electrolyte solution. In this paper, we take the mixture of β-MnO2/3D graphene-carbon nanotube cross-links (abbreviation: β-MnO2/3D GPE-CNT) as a target to investigate the effect of the pre-addition of different concentrations of MnSO4 solution (0.1 M, 0.3 M, 0.5 M, 0.6 M) in 2 M ZnSO4 on the electrochemical performance of this cathode material.
Experimental materials and methods
The detailed preparation process of β-MnO2/3D GPE-CNT is shown in Fig. 1. Before use, the ball milling jar was first cleaned. A mixture of β-MnO2 powder and 3D graphene-carbon nanotube with a mass ratio of 9:1 was put into the ball milling jar of the high-energy ball mill, as shown in Fig. 1 a, and the mass ratio of stainless steel balls to the mixture was 50:1. The use of several different sizes of stainless steel balls had better ball milling effects. Set the automatic power on and off intervals of 1 min, the total time of 5 h (the actual ball milling process 2.5 h). The obtained β-MnO2/3D GPE-CNT powders were collected by natural cooling for 1 h after ball milling. The microscopic particle morphology and arrangement of the materials were investigated by field emission scanning electron microscopy (FSEM, S-4800) and transmission electron microscopy (TEM, JEM-F200); the phase structure of the materials was characterized by X-ray diffraction (XRD, SmartLab); and the particle size distribution of the specimens was tested by a laser particle size analyzer (Malvern 3000); The specific surface area of the specimens was tested by a specific surface pore size analyzer (JW-BK200B).
The β-MnO2/3D GPE-CNT cathode material collected from the above ball milling was mixed with conductive carbon (Super P) and PVDF at the mass ratio of 7:2:1 and put into an onyx mortar to be fully milled, and then dripped into the appropriate amount of N-methyl-2-pyrrolidone (NMP) solution to develop into a slurry. Then the slurry was uniformly coated on the stainless steel foil with a coating machine, and then placed in a vacuum drying oven at 110 °C for 12 h. After drying, the slurry was cut into round positive electrode sheets with a diameter of about 14 mm, and the average mass density of the active substance on the positive electrode sheets was about 0.46 mg·cm−2. Then the CR2032 coin batteries were assembled using 2 M ZnSO4 with the addition of 0.1 M, 0.3 M, 0.5 M, and 0.6 M MnSO4 as electrolyte in the order of negative electrode shell, zinc flake, fiberglass diaphragm, positive electrode flake, gasket, and positive electrode shell, respectively. The assembled batteries were allowed to stand for more than 6 h; galvanostatic charge–discharge (GCD) tests were performed in a Neware battery test system. The multiplicity performance and cycle life of the batteries under different electrolytes were tested at different current densities in the range of 100 mA·g−1 to 1 A·g−1 under a voltage window of 1.0 ~ 1.8 V, respectively (BTS, CT-4008, Neware). Cyclic voltammetry (CV) curves were tested in the voltage range of 0.8 ~ 1.8 V at a scan rate of 0.1 ~ 0.5 mv·s−1 on a CHI660E electrochemical workstation, and electrochemical impedance spectroscopy (EIS) analysis was performed in the frequency range of 0.01 Hz ~ 10 kHz.
Results and discussion
Micro-morphology and analysis
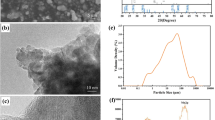
The XRD patterns of the original sample of β-MnO2 as well as the β-MnO2/3D GPE-CNT hybrids are shown in Fig. 2. It can be seen that the XRD patterns related to MnO2 match the standard card (JCPDS: 39–0375) of the original sample of β-MnO2, and there are obvious diffraction peaks at 2θ = 29.31°, 35.02°, 56.21°, and 61.54°, corresponding to the (201), (103), (402), and (103) facets, respectively, which indicates that ball milling before and after does not significantly change the phase structure of the samples [19].
The microstructures of pure β-MnO2 and β-MnO2/3D GPE-CNT hybrids were measured by SEM and TEM, and the results are shown in Fig. 3. As can be seen in Fig. 3 a and b, the pure β-MnO2 samples exhibit a tightly packed appearance with an angular blocky particle structure. The ball-milled hybrids, on the other hand, showed a distribution of spherical particles, and the agglomeration phenomenon was obviously reduced. In addition, under the same magnification, the SEM images of the ball-milled β-MnO2/3D GPE-CNT hybrids observed significantly more particles, which indicated that ball milling greatly reduced the particle size of the cathode material. This would lead to an increase in the specific surface area of the material a better contact reaction with the electrolyte [25], which can be verified in the next experiment. Figure 3 c shows the TEM image of the β-MnO2/3D GPE-CNT hybrids, which shows that the 3D GPE-CNT and β-MnO2 form a whole, and the β-MnO2 particles are more dispersed to cover the surface of the 3D network [20]. The atomic arrangement in Fig. 3 d shows that a few lattice distortions appeared after ball milling, which are defects caused by collision and extrusion during the ball milling process. Figure 3 e–g can further show that the elements C, O, and Mn are more uniformly distributed in the hybrids, which further proves the uniformly dispersed distribution of β-MnO2 after ball milling.
Further specific surface area analysis of pure β-MnO2 and β-MnO2/3D GPE-CNT hybrids yielded results of 14.24 m2·g−1 and 22.38 m2·g−1, respectively, and the particle size distribution of the specimens was tested using a laser particle size analyzer, with an average particle size of 20.33 μm, which is in line with the phenomenon observed above in the experimental results.
Electrochemical performance and analysis
Figure 4 a shows the capacity decay curves of the cells with different concentrations of MnSO4 solution pre-added to the electrolyte at 100 mA·g−1. The discharge specific capacity of the β-MnO2/3D GPE-CNT cathode material with 2 M ZnSO4 + 0.1 M MnSO4 solution as the electrolyte reaches 221.91 mAh·g−1 after full activation is higher than the specific capacity when ZnSO4 solution is used as electrolyte alone, and the capacity of the batteries with MnSO4 solution added decreases very slowly after the batteries are stabilized. This indicates that the capacity decay of the battery can be well suppressed after adding MnSO4 in the electrolyte. This is because the dissolution of the cathode material can be effectively inhibited by pre-adding appropriate metal cations to the solution according to the chemical dissolution-equilibrium principle of the cathode material and the active substance in the electrolyte, so pre-adding MnSO4 to the ZnSO4 electrolyte can inhibit the dissolution of MnO2 by establishing the dissolution equilibrium of Mn2+ in the electrolyte and cathode materials. This principle was confirmed by Pan [21] et al. They examined the concentration of Mn2+ in the electrolyte by technical device and found that the concentration of Mn2+ in the ZnSO4 electrolyte was directly proportional to the rate of capacity decay of the batteries, which indicated that the capacity decay was due to the dissolution of the anode MnO2 in the electrolyte, and when the capacity was stabilized, the concentration of Mn2+ was relatively stable, which was due to the fact that the concentration of Mn2+ in the electrolyte has reached equilibrium with the dissolution of anode MnO2, which inhibits the dissolution of the anode material.
a Capacity decay curves at 100 mA·g−1 with the addition of different concentrations of MnSO4; b charge–discharge curves when the specific capacity reaches the maximum; c charge–discharge curves when the electrolyte is 2 M ZnSO4 + 0.5 M MnSO4; d cycling performance at 1A·g−1 when the electrolyte is 2 M ZnSO4 + 0.5 M MnSO4 at 1A·g−1 cycling performance; e CV curves with the addition of 0.5 M MnSO4; f Tafel curves fitting the peaks in e
Continuing to increase the concentration of MnSO4 solution in the electrolyte to 0.3 M, it can be seen from the constant-current GCD curve shown in Fig. 4 a that the maximum discharge specific capacity of the battery after activation reached 365.76 mAh·g−1, and the capacity retention rate was also improved very well, which indicates that increasing the concentration of Mn2+ can not only deepen the internal chemical reaction of the battery, but also better inhibit the dissolution of cathode material in the electrolyte, effectively slowing down the capacity degradation of the battery [23]. This is shown until the concentration of MnSO4 solution is increased to 0.5 M. Under this condition, the specific capacity of the battery rises rapidly after more than ten turns of full activation, and reaches an astonishing 521.91 mAh·g−1 after 27 turns of cyclic charging and discharging, which is much higher than what is reported in related literatures [20, 24, 25]. The capacity can be well maintained after the battery is stabilized. After 100 turns of constant current charging and discharging, the discharge specific capacity is maintained at 416.48 mAh·g−1. However, when the concentration of MnSO4 solution is increased to 0.6 M, the capacity of the battery does not have a rapid and substantial increase in the process of activation as that of 0.5 M, but instead, it exhibits a similar nature of capacity decay as that of 0.3 M. This can be verified from the relevant studies [21, 23, 27]: appropriate concentration of MnSO4 added to the electrolyte can effectively inhibit the collapse of the anode MnO2 structure due to the dissolution of anode materials in the electrolyte to participate in the reaction, thus improving the specific capacity of discharge as well as the capacity retention rate, but the concentration of MnSO4 in the electrolyte is too high which will not be conducive to the participation of the anode materials in the reaction. Figure 4 b shows the charge–discharge curves of batteries with different MnSO4 concentrations as electrolyte when the specific capacity reaches the highest value at 100 mA·g−1, and it can be seen that the discharge curves have inflection points at about 1.4 V and 1.3 V. With the increase of the MnSO4 concentration in the electrolyte, the discharge platforms at 1.4 V and 1.3 V become more obvious, and the consistency of the shapes of the discharge curves is better, which show the good stability of the battery. The Coulomb efficiency is above 96%, showing high reversibility.
The charging and discharging curves of the battery at different current densities when the electrolyte is 2 M ZnSO4 + 0.5 M MnSO4 solution are shown in Fig. 4 c. The contribution of the voltage range below 1.3 V to the capacity decreases as the current density increases, and this section reacts more slowly and is the main capacity contributing stage. The discharge specific capacity of the battery reaches 432.33 mAh·g−1 at 200 mA·g−1 and 316.75 mAh·g−1 at 300 mA·g−1, and 209.55 mAh·g−1 even at 500 mA·g−1 current density. The results show that the batteries with 0.5 M MnSO4 solution as electrolyte additive have excellent multiplication performance. To further investigate the cycling stability of the batteries, the batteries were charged and discharged cyclically at a high current density of 1 A·g−1; as shown in Fig. 4 d, the discharge specific capacity of the batteries reached about 80 mAh·g−1 after full activation. The overall trend of the battery capacity in the subsequent cycles showed a slow oscillation, and there was no obvious attenuation until 7377 cycles. On the contrary, the discharge specific capacity of the battery increased before 7377, reached a maximum of 109.12 mAh·g−1 at 7377 cycles, the discharge specific capacity decayed only after 7377 cycles. Overall, the batteries with β-MnO2/3D GPE-CNT as cathode have excellent cycling stability and capacity retention when 0.5 M MnSO4 solution is added to the electrolyte, with a high discharge specific capacity of up to 109.12 mAh·g−1 even at a high current density of 1 A·g−1. After 8000 cycles of charging and discharging, the specific capacity is still 91.49 mAh·g−1, which is 83.8% of the highest discharge specific capacity.
The electrochemical performance of β-MnO2/3D GPE-CNT batteries at 0.5 M MnSO4 as an electrolyte additive was further investigated using CV curves, as shown in Fig. 4 e, which were obtained at different scanning rates (0.1–0.5 mv·s−1) in the potential range of 0.8–1.8 V. The CV curves showed two distinct reduction peaks at 1.25 V and 1.39 V (peak 1, peak 2), which are related to the conversion reaction (Mn4+/Mn2+ two-electron reaction) and the insertion of Zn2+, respectively [28]. The oxidation peak (peak 3) is related to the extraction of Zn2+ [26]. With the increase of scanning rate, the CV curves can keep similar shapes, indicating that the anode materials have good reversibility. And with the increase of scanning frequency, the peak currents of the peaks gradually increase, and the potentials of peak 1 and peak 2 move to the low-voltage direction, while the potential of peak 3 moves to the high-voltage direction. The electrochemical kinetics of these cathodes at different scan rates can be determined by Eqs. (1) and (2) [29]:
where a and b are variable parameters obtained from the slope of lg(v)-lg(i), which indicates the dominance of diffusive processes when b is approximately equal to 0.5 and capacitive behavior when b is approximately 1 [25]. i denotes the peak current and v is the scanning rate. As can be seen from Fig. 4 f, the parameters b for peaks 1–3 are 0.63, 0.70, and 0.51, respectively, which are all closer to 0.5, confirming that both the insertion/extraction and transformation reactions of Zn2+ are controlled by diffusion.
As shown in Fig. 5 a, b, and c, the CV curves are given when 0.1 M MnSO4, 0.3 M MnSO4, and 0.6 M MnSO4 are used as electrolyte additives, respectively. After analyzing and calculating, the Tafel slopes of each peak of the CV curves are still close to 0.5 at this time, which again proves that the electrochemical behavior of the batteries is also dominated by diffusion. Next, the CV curves of electrolyte additives with different concentrations of MnSO4 were compared at the same scanning frequency, and Fig. 5 d gives the CV curves of electrolyte additives of 0.1 M MnSO4, 0.3 M MnSO4, and 0.5 M MnSO4 at a scanning frequency of 0.1 mv/s. The absolute areas of the closed CV curves were analyzed using Origin software, and we normalized the obtained data to show that the areas of the CV curves for electrolytes with 0.1 M MnSO4, 0.3 M MnSO4, and 0.5 M MnSO4 were 9.03, 10.45, and 13.78, respectively. It can be clearly seen that as the concentration of MnSO4 in the electrolyte increases from 0.1 to 0.5 M, the area wrapped by the CV curve and the ratio of peak current gradually increases, which is consistent with the previous performance of the discharge specific capacity of the batteries with different concentrations of MnSO4 additives. The batteries have the highest discharge specific capacity with the addition of 0.5 M MnSO4, and therefore the area wrapped by the CV curve and the peak current are also the maximum. Also in Fig. 5 d, it can be seen that as the concentration of MnSO4 in the electrolyte increases from 0.1 to 0.5 M, peak 1 of the CV curve is shifted to the left and peak 2 is shifted to the right, and the potentials of the reduction peaks of the CV curves (peak 1 and peak 2) corresponding to the addition of 0.1 M MnSO4 as an additive to the electrolyte are 1.26 V and 1.38 V, respectively. Compared to 1.25 V and 1.39 V for 0.5 M MnSO4, it indicates that the electrolyte at low concentration of MnSO4 addition is unfavorable for Zn2+ insertion reaction, but instead, it is favorable for the conversion reaction. Since the conversion reaction consumes MnOOH to produce by-products, this will in turn be detrimental to the H+ insertion reaction and limit the discharge performance of the batteries. One explanation is that low concentrations of MnSO4 are unfavorable for H+ insertion, which is mainly affected by the pH value [26], the pH of 2 M ZnSO4 + 0.1 M MnSO4 solution is about 4, while that of 2 M ZnSO4 + 0.5 M MnSO4 solution is about 3; acidic conditions are more favorable for the formation of a two-electron (Zn2+ and H+) insertion mechanism, which provides a higher reversible specific capacity. Further analyzing this figure, we can see that the peak current of the reduction peak 1 increases dramatically during the increase of the MnSO4 concentration in the electrolyte from 0.1 to 0.5 M, which well illustrates that the contribution of the conversion reaction to the discharge capacity of the batteries is significantly higher, and that the degree of conversion of MnO2 to Mn2+ is deeper. This can be verified from Fig. 4 b, where we calculated the proportion of the discharge capacity of the discharge stage corresponding to the conversion reaction (peak 1) (about 1.3 V or less) to the total capacity, and it can be obtained that the capacity contribution of the conversion reaction rises from 36 to 45% in the process of increasing the MnSO4 concentration in the electrolyte from 0.1 to 0.5 M, which is in agreement with the test of the CV curve results. Then the CV curves of Fig. 5 d were further refined, and it was found that a small oxidation peak appears to the left of the peak 3 oxidation peak at low concentrations of MnSO4 added to the electrolyte, which corresponds to the inverse process of the conversion reaction [26], suggesting that the Mn2+ in the electrolyte produces by-products to attach to the surface of the cathode material. When the MnSO4 concentration was increased from 0.5 M, this small oxidation peak was no longer obvious, which further proved that the conversion reaction basically proceeded in the positive direction and the two-electron conversion mechanism (Mn4+/Mn2+) deepened at the concentration of 0.5 M MnSO4, which provided the batteries with higher reversible specific capacity and cycle life.
a CV curves with the addition of 0.1 M MnSO4; b CV curves with the addition of 0.3 M MnSO4; c CV curves with the addition of 0.6 M MnSO4; d CV curves with the addition of different concentrations of MnSO4 for a scanning frequency of 0.1 mv·s−1; e EIS Nyquist curves with different concentrations of MnSO4; f fitted Warburg slope plots
The EIS-fitted Nyquist curves of the batteries in the frequency range of 0.01 Hz ~ 10 kHz for different concentrations of MnSO4 solution as electrolyte additive are given in Fig. 5 d. Among them, the bulk-phase ion diffusion coefficient (Dion) is an important parameter affecting the dynamic performance of zinc-ion batteries with the following equation:
where R is the gas constant, T is the operating temperature of the batteries (298.15 K), n is the number of electrons transferred in each molecule, F is Faraday’s constant, A is the area of the electrode (1.54 cm2), and Cion is the concentration of ions in the active substance [30]. The bulk-phase ionic diffusion coefficient of Zn2+ in different electrolytes (DZn2+) can be Warburg factor (σ) determined, σ being the slope of the Z′-ω−1/2 curve, which can be obtained from the results of the fit in Fig. 5 f. After calculation, the DZn2+ in the electrolyte at the content of 0.1 M MnSO4 and 0.5 M MnSO4 are 1.45 × 10−12 cm2·s−1 and 2.74 × 10−12 cm2·s−1, respectively, which again illustrates that 2 M ZnSO4 + 0.5 M MnSO4 has a higher ion diffusion rate when used as an electrolyte, which is a great advantage for the improvement of discharge capacity. In addition, the excellent rate performance and cycling stability are inextricably linked to the chemical structure of the selected cathode materials [19]: (1) The layered structure of β-MnO2 is more favorable for the insertion of M+ and Zn2+ and the transfer of Mn2+ [31]; (2) the active sites of β-MnO2 particles attached to the β-MnO2/3D GPE-CNT hybrid after ball milling are more easily contacted with the electrolyte; (3) ball milling significantly reduces the particle size of β-MnO2 particles, which results in an increase in the specific surface area and an acceleration of the ion diffusion rate; (4) the generation of new chemical bonds enhanced the internal stability and interfacial adhesion of the hybrids; (5) the excellent electrical conductivity of graphene enhanced the conductivity of the cathode material, which in turn increased the charge transfer rate.
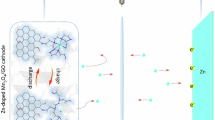
Combined with the electrochemical performance and capacity decay analysis, the β-MnO2/3D GPE-CNT aqueous zinc-ion batteries with MnSO4 additives in the electrolyte are consistent with the reaction mechanism for the coexistence of the H+, Zn2+ co-insertion/extraction, and the conversion reactions. The reaction principle are described as follows:
Cathode
Anode
where Eqs. (4) and (5) correspond to the insertion reactions of H+ and Zn2+, respectively. The products produced by the reactions of Eqs. (4) and (5) do not exist stably, which will react with SO42− and H2O in solution to produce by-products [24, 26]. Equation (6) can be understood as a conversion reaction equation that ignores the intermediate by-product production process, with the end result that anodic MnO2 is reduced to divalent Mn2+, providing higher discharge specific capacity. In fact, in order to obtain the direct conversion of MnO2/Mn2+, efforts have been made by many scholars, and most of them have proved that most of this direct conversion reacts only under high high-voltage state in acidic electrolyte [32,33,34], whereas this conversion is accompanied by the generation of by-products in the low-voltage state [26, 35, 36]. Therefore, if the direct conversion of MnO2/Mn2+ can be fully realized, the reversible specific capacity of the aqueous zinc-ion batteries will be increased to a much more appreciable magnitude. In addition, the MnSO4 additive in the electrolyte will help to maintain the reversibility of the conversion reaction, and effectively inhibit the structural collapse brought about by the dissolution of the anode MnO2 while ensuring the high discharge capacity brought about by the conversion reaction, so as to improve the stability of the cycle, which also explains why the β-MnO2/3D GPE-CNT batteries with 2 M ZnSO4 + 0.5 M MnSO4 as electrolyte have a 521.91 mAh·g−1 high discharge specific capacity and ultra 8000 turns long cycle life.
Conclusion
In summary, in this paper, β-MnO2/3D GPE-CNT were prepared by a simple and environmentally friendly ball milling method, and the nature of low crystallinity and particle size reduction greatly reduced the agglomeration phenomenon of the original β-MnO2 samples. This enhances the specific surface area of the cathode material, and the contact between the electrolyte and the cathode material is more adequate, which is correspondingly more favorable to the improvement of the Zn2+ diffusion rate. The excellent performance of this β-MnO2/3D GPE-CNT cathode material is one of the important reasons for the formation of the two-electron conversion mechanism. This two-electron conversion mechanism has a high theoretical specific capacity of 616 mAh·g−1 when the theoretical specific capacity is only 308 mAh·g−1 for the one-electron reaction pair (Mn4+/Mn3+). In addition, the relationship between the pre-addition of different concentrations of MnSO4 to the electrolyte and the discharge capacity as well as the cycling performance of the batteries was investigated, and the reaction mechanism of the cathode of the batteries was investigated by combining the analytical means of XRD, SEM, TEM, CV, EIS, etc. The results showed that Mn2+ in the electrolyte could not only effectively inhibit the dissolution of the anode manganese-based material, but also its concentration had a great influence on the maintenance of the Mn4+/Mn2+ conversion pathway. The batteries with the pre-addition of 0.5 M MnSO4 solution had a specific capacity of 521.91 mAh·g−1 at 100 mA·g−1 and 109.12 mAh·g−1 at 1A·g−1, and the capacity retention rate remained 83.8% after more than 8000 cycles. This provided an idea for the regulation of the electrolyte in Zn–Mn aqueous zinc-ion battery.
Data availability
No datasets were generated or analysed during the current study.
References
Pasta M, Wessells CD, Cui Y et al (2012) A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat Commun 3:1149. https://doi.org/10.1038/ncomms2139
Li M, Lu J, Chen Z et al (2018) 30 years of lithium-ion batteries. Adv Mater 33:1800561. https://doi.org/10.1002/adma.201800561
Liu X, Ren D, Su H et al (2018) Thermal runaway of lithium-ion batteries without internal short circuit. Joule 10:2047–2064. https://doi.org/10.1016/j.joule.2018.06.015
Wei X, Wei J, Song Y et al (2021) Potassium mediated Co-Fe-based Prussian blue analogue architectures for aqueous potassium-ion storage. Chem Commun 57:7019–7022. https://doi.org/10.1039/d1cc01852c
Wang M, Yagi S (2020) Layered birnessite MnO2 with enlarged interlayer spacing for fast Mg-ion storage. J Alloy Compd 820:153135. https://doi.org/10.1016/j.jallcom.2019.153135
Cang R, Zhao C, Ye K et al (2020) Aqueous calcium-ion battery based on a mesoporous organic anode and a manganite cathode with long cycling performance. Chemsuschem 13:3911–3918. https://doi.org/10.1002/cssc.202000812
Fang GZ, Zhou J, Pan A et al (2018) Recent advances in aqueous zinc-ion batteries. ACS Energy Lett 3:2480–2501. https://doi.org/10.1021/acsenergylett.8b01426
Zhang K, Han X, Hu Z, Zhang X, Tao Z, Chen J (2015) Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem Soc Rev 44:699–728. https://doi.org/10.1039/c4cs00218k
Wang J, Yang Y, Zhang Y, Li Y, Sun R, Wang Z, Wang H (2021) Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries. Energy Storage Mater 35:19–46. https://doi.org/10.1016/j.ensm.2020.10.027
Konarov N, Voronina JH, Jo Z, Bakenov YK, Sun ST (2018) Myung Present and future perspective on electrode materials for rechargeable zinc-ion batteries. ACS Energy Lett 3:2620–2640. https://doi.org/10.1021/acsenergylett.8b01552
Guo C, Liu H, Li J et al (2019) Ultrathin delta-MnO2 nanosheets as cathode for aqueous rechargeable zinc ion battery. Electrochim Acta 304:370–377. https://doi.org/10.1016/j.electacta.2019.03.008
Jiang B, Xu C, Wu C et al (2017) Manganese sesquioxide as cathode material for multivalent zinc ion battery with high capacity and long cycle life. Electrochim Acta 229:422–428. https://doi.org/10.1016/j.electacta.2017.01.163
H Chen, W Zhou, Zhu D et al (2020) Porous cube-like Mn3O4@C as an advanced cathode for low-cost neutral zinc-ion battery. J Alloys Compd 813. https://doi.org/10.1016/j.jallcom.2019.151812
Hu P, Yan M, Zhu T et al (2017) Zn/V2O5 aqueous hybrid-ion battery with high voltage platform and long cycle life. ACS Appl Mater Interfaces 9:42717–42722. https://doi.org/10.1021/acsami.7b13110
Zhang N, Jia M, Dong Y et al (2019) Hydrated layered vanadium oxide as a highly reversible cathode for rechargeable aqueous zinc batteries. Adv Func Mater 29:1807331. https://doi.org/10.1002/adfm.201807331
Jia Z, Wang B, Wang Y (2015) Copper hexacyanoferrate with a well-defined open framework as a positive electrode for aqueous zinc ion batteries. Mater Chem Phys 14:601–606. https://doi.org/10.1016/j.matchemphys.2014.11.014
Zhang L, Chen L, Zhou X et al (2015) Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: the zinc/zinc hexacyanoferrate system. Adv Energy Mater 5:1400930. https://doi.org/10.1002/aenm.201400930
Wang J, Wang JG, Liu H, Wei C, Kang F (2019) Zinc ion stabilized MnO2 nanospheres for high capacity and long lifespan aqueous zinc-ion batteries. J Mater Chem A 7:13727. https://doi.org/10.1039/c9ta03541a
Xin SH, Dong XP, Jin DL, Yang LY, Su DD (2023) β-MnO2/three-dimensional graphene-carbon nanotube hybrids as cathode for aqueous zinc-ion battery. J Alloy Compd 968:172115. https://doi.org/10.1016/j.jallcom.2023.172115
Cao J, Zhang DD, Zhang XY, Wang SM, Han JT, Zhao YS, Huang YH, Qin JQ (2020) Mechanochemical reactions of MnO2 and graphite nanosheets as a durable zinc ion battery cathode. Appl Surface Sci 534(1–12):147630. https://doi.org/10.1016/j.apsusc.2020.147630
Pan H, Shao Y, Yan P et al (2016) Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat Energy 1:16039. https://doi.org/10.1038/nenergy.2016.39
Soundharrajan V, Sambandam B, Kim S, Islam S, Jo J et al (2020) The dominant role of Mn2+ additive on the electrochemical reaction in ZnMn2O4 cathode for aqueous zinc-ion batteries. Energy Storage Mater 28:407–417. https://doi.org/10.1016/j.ensm.2019.12.021
Zhang N, Cheng F, Liu J et al (2017) Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat C 405. https://doi.org/10.1038/s41467-017-00467-x
Zhao S, Han B, Zhang DT et al (2018) Unravelling the reaction chemistry and degradation mechanism in aqueous Zn/MnO2 rechargeable batteries. J Mater Chem A 6:5733–5739. https://doi.org/10.1039/c8ta01031e
Jiang W, Xu X, Liu Y, Tan L et al (2020) Facile plasma treated β-MnO2@C hybrids for durable cycling cathodes in aqueous Zn-ion batteries. J Alloy Compd 827:154273. https://doi.org/10.1016/j.jallcom.2020.154273
Li GZ, Huang ZX, Chen J et al (2020) Rechargeable Zn-ion batteries with high power and energy densities: a two-electron reaction pathway in birnessite MnO2 cathode materials. J Mater Chem A 8:1966–1974. https://doi.org/10.1039/c9ta11985j
Chamoun M, Brant WR, Tai C et al (2018) Rechargeability of aqueous sulfate Zn/MnO2 batteries enhanced by accessible Mn2+ ions. Energy Storage Mater 15:351–360. https://doi.org/10.1016/j.ensm.2018.06.019
Cao J, Zhang D, Zhang X et al (2020) A universal and facile approach to suppress dendrite formation for Zn and Li metal anode. J Mater Chem A 8:9331–9344. https://doi.org/10.1039/d0ta02486d
Brezesinski T, Wang J, Tolbert SH et al (2010) Ordered mesoporous α-MoO3 with iso oriented nanocrystalline walls for thin-film pseudocapacitors. Nat Mater 9:146–151. https://doi.org/10.1038/nmat2612
Liu M, Zhao Q, Liu H, Yang J et al (2019) Tuning phase evolution of β-MnO2 during microwave hydrothermal synthesis for high-performance aqueous Zn ion battery. Nano Energy 64:103942. https://doi.org/10.1016/j.nanoen.2019.103942
Zhu CY, Li PZ, Xu GY, Cheng H, Gao G (2023) Recent progress and challenges of Zn anode modification materials in aqueous Zn-ion batteries. Coord Chem Rev 485:215142. https://doi.org/10.1016/j.ccr.2023.215142
Yang M, Chen R, Shen Y et al (2020) A high-energy aqueous manganese-metal hydride hybrid battery. Adv Mater 32:e2001106. https://doi.org/10.1002/adma.202001106
Zhang Y, Li M, Li H et al (2023) Catholyte modulation and Prussian blue/Berlin green redox mediator enabling efficient high-potential Mn2+/MnO2 reaction for aqueous hybrid batteries. Small Structures 5:2300257. https://doi.org/10.1002/sstr.202300257
Chao D, Zhou W, Ye C et al (2019) An electrolytic Zn-MnO2 battery for high-voltage and scalable energy storage. Angew Chem Int Ed 58:7823–7828. https://doi.org/10.1002/anie.201904174
Zeng XH, Liu JT, Mao JF et al (2020) Toward a reversible Mn4+/Mn2+ redox reaction and dendrite-free Zn anode in near-neutral aqueous Zn/MnO2batteries via salt anion chemistry. Adv Energy Mater 10:1904163. https://doi.org/10.1002/aenm.201904163
Zhong ZP, Li JY, Li LY et al (2022) Improving performance of zinc-manganese battery via efficient deposition/dissolution chemistry. Energy Storage Mater 46:165–174. https://doi.org/10.1016/j.ensm.2022.01.006
Funding
This study received financial supports from the Natural Science Foundation of Hebei Province, China, (E2021201032); 2023 Hebei Province and Hebei University School-level Student Innovation and Entrepreneurship Training project (S202310075044 and 2023232); Ministry of Education’s “Spring Fai Project” cooperative research project (202200397); and Hebei University 2022 Laboratory Opening Project (sy202266).
Author information
Authors and Affiliations
Contributions
Duolong Jin (First Author): Data Curation, Methodology, Investigation, Formal Analysis, Writing-Original Draft; Xiaoping Dong (Corresponding Author): Conceptualization, Funding Acquisition, Resources, Supervision, Writing-Review & Editing. Shenghai Xin: Visualization, Investigation; Liying Yang: Resources, Supervision; JianKai Liu: Validation; Data Curation Qianran Pang: Visualization; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, D., Dong, X., Xin, S. et al. Effect of MnSO4 concentration on the electrochemical performance of β-MnO2/3D graphene-carbon nanotube hybrids cathode for aqueous zinc-ion batteries. Ionics 30, 3329–3338 (2024). https://doi.org/10.1007/s11581-024-05537-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05537-x