Abstract
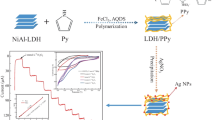
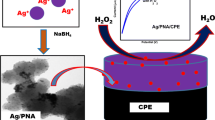
The NiAlO@PPy-Ag sensing material was designed and prepared via in-situ oxidative polymerization of pyrrole monomer on the NiAl-oxide (NiAlO), and then anchoring Ag nanoparticles (NPs) on the surface of the NiAlO@PPy carrier. It was determined that the NiAlO particles were encased by PPy chains and Ag NPs were homogeneously distributed on the NiAlO@PPy based on various structural characterization. Subsequently, the NiAlO@PPy-Ag was directly fabricated into a non-enzymatic sensor for the detection of H2O2, which sensor showed a high sensitivity and selectivity toward H2O2 with a low detection limit of 0.03 μmol∙L−1 and high sensitivity of 346.50 μA∙mmol−1∙cm−2, and excellent repeatability and reproducibility. The results demonstrated that the NiAlO@PPy-Ag was a promising electrocatalytic material for H2O2 detection in the biological, clinical and environmental fields.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrochemical biosensors, especially enzymatic sensor, have attracted much attention due to simplicity, high selectivity and sensitivity. However, the application of enzymatic sensors is limited because of high cost, complicated immobilization procedure and limited stability. Non-enzyme electrocatalytic materials are developed to avoid the issues [1,2,3]. All kinds of efforts have been used to improve the performances of non-enzyme sensor materials [4, 5]. Especially, Ni-based metal oxides have attracted enormous interest recently for the sensor research and practical application such as the detections of H2O2 [6, 7], glucose [4, 8] and uric acid [9], due to natural abundance, good biological compatibility and high electron transfer capability. For example, NiO anchored on carbon nanofibers displayed a good electrocatalytic activity towards H2O2 with a low detection limit (LOD) of 0.57 μmol∙L−1 and high sensitivity of 304.2 μA∙mol∙L−1∙cm−2 [10]. The non-enzyme sensor based on NiCoP displayed a good electrochemical sensing property with a LOD of 1.190 μmol∙L−1 and selectivity 225.7 μA∙mol∙L−1∙cm−2 toward H2O2 [11]. Nevertheless, it still faces the challenge of improving the stability and selectivity in applications of non-enzyme sensor.
Incorporating conducting polymers with the Ni-based metal oxides gives a promising way to strengthen the electrochemical sensing performances, in which conducting polymers can prevent the aggregation of metal oxides in favor of the electron transfer to increase the sensitivity and selectivity [12, 13]. Among the polymers, polypyrrole (PPy) as excellent electron donor is one of the most favorable conducting polymers due to flexible, ease of synthesis and high conductivity, which make it an interesting matrix for the organic-inorganic composites [14,15,16]. It is believed that the incorporation of the Ni-based metal oxides with PPy are expected to display new properties over their single component, making them potential for non-enzymatic sensor application. Moreover, Ag nanoparticles (NPs), as attractive non-enzymatic alternative, were decorated on the organic-inorganic composites can further enhance the electrocatalytic activity for the detection of H2O2 [17, 18].
In the present work, the NiAlO@PPy-Ag was synthesized by the incorporation of NiAl-oxide (NiAlO) and PPy together with the anchoring of Ag NPs, and used toward the detection of H2O2. To the best of our knowledge, little research is reported on the use of NiAlO@PPy-Ag as a sensor material for the detection of H2O2. The structure, morphology and electrochemical properties of the as-prepared samples were determined using various characterization techniques and electrochemical measurements. As a non-enzymatic H2O2 sensor material, the NiAlO@PPy-Ag exhibited superior sensing performances with a relatively wide potential range, high sensitivity and good stability.
Experimental
Materials
In the experiments, all reagents were of analytical grade without any further purification. 0.1 mol∙L–1 phosphate buffer solution (PBS) with pH 7.0 was prepared by dissolving KH2PO4 and K2HPO4 in distilled water, and distilled water was used to prepare all the solutions. A pH electrode (Mettler Toledo 5-2C) was used for pH measurements.
Preparation of substrate materials
The NiAl-oxide was prepared by urea method (urea/NO3− molar ratio of 4.0). In brief, Ni(NO3)2·6H2O and Al(NO3)3·9H2O (Ni2+ + Al3+ = 0.30 mol·L–1, Ni/Al molar ratio of 3.0) as well as urea were dissolved in distilled water under vigorous stirring at 105°C for 12 h, and then filtered, washed, and dried at 80°C for 24 h. The resulting product was calcined in air atmosphere at 400°C for 4 h, which was denoted as NiAlO.
The NiAlO (0.1 g·L–1) was immersed in the solution containing 0.1 g·L–1 sodium anthraquinone disulfonate as dopant under stirring in an ice bath for 15 min. 9.0 mL·L–1 pyrrole monomer was added and maintained for 30 min under stirring, and then the FeCl3 (initiator) solution (3.2 mL·L–1) was added dropwise into the reaction vessel to initiate the polymerization of pyrrole at 0~4°C for 6 h. The precipitate was filtered, washed and dried at 60°C for 24 h, which was denoted as NiAlO@PPy. For comparison, the pure polypyrrole (PPy) was also prepared through the above-mentioned process in the absence of the NiAlO.
In our preliminary tests, it was found that the optimized AgNO3 amount was 0.17 mass ratio of AgNO3 to NiAlO@PPy (seeing detailed information in Fig. S1). So, AgNO3 with 0.17 mass ratio of AgNO3 to NiAlO@PPy was dissolved in the NiAlO/PPy suspension under stirring, and then NaBH4 solution was mixed dropwise (0.5 molar ratio of AgNO3 to NaBH4) under stirring for 2 h. After reaction, the resulting product was centrifuged and washed thoroughly, and dried at 60°C overnight, which was denoted as NiAlO@PPy-Ag. The preparation process of the NiAlO, NiAlO@PPy and NiAlO@PPy-Ag are illustrated in Scheme 1.
Characterization
X-ray diffraction (XRD) patterns were collected on a Rigaku D/max-2550PC (λ=1.5406 Å) with Cu Kα radiation. The morphology was investigated by scanning electron microscopy (SEM, JEOL JSM-6700F) and transmission electron microscopy (TEM, JEM2100). The X-ray photoelectron spectroscopy (XPS) was carried out by Thermo Fisher Scientific K-Alpha. The composition was characterized by energy-dispersive spectrometry (EDS, Noran SystemSix).
Electrochemical measurements
The substrate material films were immobilized on bare glassy carbon electrodes (GCE). Before immobilization, the GCE with a diameter of 2.0 mm was pretreated according to the literature [19]. The active substance was dispersed in distilled water under ultrasonic treatment, and then the 10 μL dispersion solution containing 1.0 g·L–1 active substance was dropped onto the pretreated GCE. After immobilization, the electrode was washed in distilled water and then dried under infrared radiation for 5 min to obtain the modified electrodes. The electrodes modified by the NiAlO, PPy, NiAlO@PPy and NiAlO@PPy-Ag were designated as NiAlO/GCE, PPy/GCE, NiAlO@PPy/GCE and NiAlO@PPy-Ag/GCE, respectively.
All electrochemical experiments were performed on a CHI660D electrochemical workstation (Shanghai Chenhua Apparatus Co. Ltd., Shanghai, China) with working electrode, a platinum plate counter electrode and a saturated calomel electrode (SCE). The electrolyte was 0.1 mol∙L–1 KCl solution containing 5 mmol·L−1 K3[Fe(CN)6] and 5 mmol∙L−1 K4[Fe(CN)6]. CV measurements were performed between −0.2 and 0.6 V at the 12th cycle. EIS was between 0.01 and 100 KHz under open circuit voltage conditions. All experiments were carried out at room temperature. The H2O2 limit of detection (LOD, μmol·L−1) was calculated as follows [20],
where sB was the standard deviation of blank test, and m was slope of calibration curve.
Results and discussion
Characterization of the as-prepared samples
Fig. 1 shows the XRD patterns of the NiAlO, pure PPy, NiAlO@PPy and NiAlO@PPy-Ag, where a broad reflection at 25.5° in the pure PPy was due to the aligned polypyrrole chains at the interplanar spacing of protonated PPy [15]. The NiAlO displayed the (111), (200), (220) and (311) planes attributing to NiO (JCPDS no. 65-5745) [5], where no Al2O3 phase was detected with the idea that Al3+ formed amorphous phases or dispersed into the NiO matrix [21]. The NiAlO@PPy clearly exhibited the coexistent presence of the characteristic reflections of NiO and PPy, suggesting that the coating of PPy had no impact on the crystallinity of the NiAlO. In the XRD pattern of the NiAlO@PPy-Ag, the new reflections at 38.0, 45.2, 64.4 and 77.3° corresponding to (111), (200), (220), and (311) planes of metallic Ago (JCPDS no. 04-0783) besides the crystal phases of NiO and PPy, showing that Ago NPs were anchored on the NiAlO@PPy [22].
The morphology of the NiAlO, pure PPy, NiAlO@PPy and NiAlO@PPy-Ag were observed by SEM. As seen in Fig. 2, the NiAlO demonstrated a flower-like layer structure consisting of individually layered platelets, while the pure PPy showed platy particles with average diameter about 0.6 μm. After coating PPY on the NiAlO NPs, the NiAlO@PPy particles still showed individually layered platelets, but the platelets tended to aggregate into cluster. The PPy chains acting as binders glued together with the NiAlO particles, which provided many active sites for the electrochemical reaction [23]. Clearly, Ago NPs (white arrow) were dispersed on the NiAlO@PPy-Ag, indicating that the metallic Ago NPs were anchored on the NiAlO@PPy-Ag surface. Furthermore. the TEM image ascertained that the presence of metallic Ago and PPy, in which the Ago NPs were anchored at the NiAlO nanoparticles (in dark), and PPy (in gray), as pointed by the white arrows (Fig. S2).
The elemental composition and surface state of the NiAlO@PPy-Ag were examined by XPS, and the results are shown in Fig. S3. The high-resolution Ni 2p spectrum appeared two main peaks at 873.3 eV (Ni 2p1/2) and 855.7 eV (Ni 2p3/2) with a spin-energy separation of 17.6 eV, suggesting a typical feature of the Ni2+ in the NiAlO [24]. the O 1s spectrum could be fitted into three peaks, which peaks at 531.4 eV was related to hydroxyl radical (−OH), and the other two peaks were assigned to lattice oxygen (Ol, Ni-O, Al-O, Ag-O) [25, 26]. The fitting N 1s spectrum presented three existence forms, corresponding to −N+H− (402.3 eV), –NH− (400.2 eV) and =NH− (398.4 eV) [15], respectively. The Ag 3d spectrum had two individual peaks corresponding to Ag 3d3/2(374.2 eV) and Ag 3d5/2 (368.1 eV), which were attributed to metallic Ago [15]. In the deconvoluted C1s spectrum, three peaks at 288.5, 286.2, and 284.8 eV belonged to the C=O, C-N and C-C/C=C groups [27], respectively. Finally, the Al 2p spectrum showed a peak at 72.4 eV, which was relating to the Al3+ species (Al-O) [28]. Furthermore, the elemental composition of the NiAlO@PPy-Ag was further verified by EDS, and the EDS results are shown in Table S1. As seen in Table S1 and Fig. S4, the NiAlO@PPy contained Ni, Al, C, O, N, Cl and S elements, while the Ag element (1.43% atomic content) was detected in the NiAlO@PPy-Ag except the elements in the NiAlO@PPy. The Cl atoms in the NiAlO@PPy and NiAlO@PPy-Ag were from initiator FeCl3, and S atoms should derive from the dopant sodium anthraquinone disulfonate during the polymerization of pyrrole monomer [29]. The XPS and EDS results supported the point from the XRD and SEM/TEM that Ag NPs was successfully anchored onto the NiAlO@PPy, in which PPy was coated on the surface of the NiAlO.
Electrochemical performances of the substrate materials
To explore their potential application in electrochemical sensor devices, the electrochemical properties of the NiAlO, pure PPy, NiAlO@PPy and NiAlO@PPy-Ag were investigated by EIS (Fig. 3). The Nyquist curve of the bare GCE displayed a very small semicircle domain, implying a very low electron transfer resistance. The charge-transfer resistance (Rct) were calculated by fitting the EIS data to the suitable equivalent circuit (Fig. 3 inset, Chi square values ≤ 1.20×10−2). The sequence order of the Rct values was NiAlO/GCE (528.10 Ω) > PPy/GCE (173.50 Ω) > NiAlO@PPy/GCE (128.90 Ω) > NiAlO@PPy-Ag/GCE (90.53 Ω) > GCE (0.02 Ω). The hindered electron transfer for the NiAlO/GCE was caused by intrinsic poor electroconductivity. It was found that the Rct value markedly decreased after coating conductive polymer PPy on the NiAlO surface, and further fell after the anchoring of Ag NPs, revealing that the coating of PPy together with anchoring of Ag NPs could improve the electrical conductivity, namely the NiAlO@PPy-Ag/GCE had the fastest electron-transfer kinetics.
The electrochemical activity of the NiAlO, pure PPy and NiAlO@PPy-Ag was investigated, which displayed an apparent couple of reversible redox peaks in Fig. 4. As shown in Fig. 4 inset, the CV curves of the NiAlO/GCE and pure PPy/GCE at 10 mV·s−1 for 12 cycles exhibited a very weak current response, revealing a sluggish redox reaction kinetics and low sensitivity. When the NiAlO@PPy-Ag was immobilized on GCE, the current response in the system signifcantly increased, showing an enhanced sensitivity. Furthermore, the CV behavior of the NiAlO@PPy-Ag was evaluated at different scan rates (Fig. 4). The shape of the CV curves remained nearly constant at different scan rates, and the peak currents increased rose with rising the scan rate from 10 to 150 mV∙s−1, suggesting a good reversibility. The peak currents were linearly related to the square root of scan rate with high correlation coefficients (R2 ≥ 0.9589, Fig. 4 inset), indicating that the electron transfer reaction in the NiAlO@PPy-Ag was a diffusion-controlled process [30].
Electrocatalysis of the NiAlO@PPy-Ag towards H2O2
Electroactivity of the NiAlO@PPy/GCE and NiAlO@PPy-Ag/GCE towards the reduction of H2O2 were determined by CV and amperometric detection in 0.1 mol∙L–1 PBS solution (pH 7.0) at the 12th cycle, and the results are shown in Fig. 5. As seen in Fig. 5A inset, the NiAlO@PPy/GCE showed no reduction activity towards H2O2 in the reaction system containing 7.0 mmol∙L–1 H2O2. Interestingly, the cathodic peak current in the CV curves increased dramatically with increasing H2O2 concentration from 0 to 7.0 mmol∙L−1 at the. The result uncovered that Ag NPs played a key role in the H2O2 reduction, which endowed the NiAlO@PPy-Ag/GCE a high electrocatalytic performance toward the H2O2 reduction. On the other hand, the amperometric response of the NiAlO@PPy/GCE and NiAlO@PPy-Ag/GCE upon the successive addition of H2O2 in 0.1 mol∙L–1 PBS solution (pH=7.0) at −0.3 V was evaluated under stirring (Fig. 5B). The NiAlO@PPy/GCE was no response to H2O2, implying a low electrochemical response towards H2O2. On the contrast, the NiAlO@PPy-Ag showed a typical current-time (i-t) plot upon the successive addition of H2O2, and the amperometric response current increased with the adding of H2O2, indicating an excellent electrocatalytic activity to H2O2. So, the NiAlO@PPy-Ag/GCE was chosen as a sensor to detect H2O2 by Amperometric determination.
Optimization of amperometric determination
In order to ensure the performance of the NiAlO@PPy-Ag sensor, the effect of some parameters (active substance loading, applied potential and pH) were investigated by amperometric detection in the PBS solution containing 2.0 mmol∙L–1 of H2O2. Firstly, the effect of NiAlO@PPy-Ag loading on the amperometric response in initial pH 7.0 at −0.3 V (300 rpm) is shown in Fig. 6A, where a given volume of the suspension containing 1.0 g·L–1 NiAlO@PPy-Ag was dropped onto the GCE. The amperometric response increased obviously with the loading from 1 to 5 μL, and then decreased. Thus, 5 μL suspension was selected for the amperometric detection of H2O2. Second, the applied potential was studied at initial pH 7.0 in the range of −0.5 to 0.1 V. As seen in Fig. 6B, the amperometric response reached the highest above or equal to −0.3 V, and so the potential was determined to be−0.3 V as the applied potential. At a low potential, the background current decreased and the response toward the active substance was weakened, leading to the lessening in the reduction current [31]. Finally, initial pH in the detecting system was evaluated at −0.3 V (300 rpm) (Fig. 6C). The current response increased gradually with initial pH, and then achieved the maximum at pH 7.0. So, initial pH 7.0 in the PBS solution was selected for amperometric detection of H2O2.
Amperometric detection toward H2O2
The sensitivity of the NiAlO@PPy-Ag/GCE towards H2O2 was determined by amperometric detection at −0.3 V, where a typical steady-state i-t response plot with continuous addition of H2O2 every 30 s is shown in Fig. 7. As expected, well-defined stepwise increment in the amperometric response was observed upon the addition of H2O2. The sensor reached the steady-state current within 3 s, suggesting very fast response process in the NiAlO@PPy-Ag/GCE. The sensor had a wide linear range from 1.0×10−2 to 8.0 mmol·L−1 (R2 0.998, Fig. 7 inset), and the sensitivity was estimated to be 346.50 μA·mmol−1·cm−2 with the detection limit (LOD) of 0.03 μmol·L−1 (S/N=3). Furthermore, the analytical performance of the H2O2 sensor were compared with some other non-enzymatic H2O2 sensors reported in the literature [5–7, 10, 11, 39–44]. As listed in Table 1, analytical performance of the present NiAlO@PPy-Ag sensor was comparable to those of the H2O2 sensors.
The repeatability, reproducibility and stability of the as-prepared NiAlO@PPy-Ag/GCE were studied in 1.0 mmol·L−1 initial H2O2 concentration. Six successive amperometric detections were carried out to investigate the repeatability, where the response current (i) were 8.79, 8.92, 9.25, 9.15, 8.86 and 9.08 μA, respectively. The relative standard deviation (RSD) was found to be 1.99%, which indicated a satisfactory precision. Furthermore, six different modified sensors were prepared under the same condition, where the response current to the different electrodes were 8.86, 8.95, 9.06, 9.15, 9.10 and 8.93 μA, respectively. The RSD was 1.24%, confirming that the NiAlO@PPy-Ag/GCE could be reproducible. In order to investigate the stability, the sensor was stored in ambient condition and monitored over a period of 30 days. After 1 week, only 1.5% of the current signal was lost, 3.4% of the lost after 2 weeks, and maintained around 91.3% of the initial current signal for over 1 month. In a word, the results indicated that the NiAlO@PPy-Ag/GCE had a good repeatability, reproducibility and stability.
The influence of common interfering species on the analytical performance of the NiAlO@PPy-Ag/GCE was also evaluated. The amperometric response of the sensor toward addition of 1.0 mmol·L−1 H2O2 and succeeding NaCl, KCl, glucose, uric acid, ascorbic acid and dopamine (each 10 mmol·L−1) in a 0.1 mol·L−1 PBS solution (pH 7.0) was determined (Fig. 8). As seen in Fig. 8, the i-t responses of the mentioned interfering substance were quite negligible, demonstrating that the NiAlO@PPy-Ag sensor had a superior selectivity towards H2O2.
Practicality of the sensor
To evaluate the possible applicability of the present sensor, the determination of H2O2 in the water samples from different sources was investigated. The standard addition method was used, for no response towards H2O2 was found in the water samples. The collected water samples were diluted using 0.1 mmol·L−1 PBS solution (pH 7.0) before the determinations, and all the determinations were carried out four times in parallel. As shown in Table 2, the calculated recovery and RSD indicated that the present NiAlO@PPy-Ag sensor had an appreciable practicality in the determination of H2O2.
Conclusions
The NiAlO@PPy-Ag material was successfully prepared via in-situ oxidative polymerization of pyrrole monomer on the NiAl-oxide (NiAlO), and then anchoring Ag nanoparticles (NPs) on the surface of the NiAlO@PPy carrier. The presence of Ag NPs and PPy was confirmed by XRD, EDS and SEM techniques. Such the architectures not only had respective merits of each component, but also showed a strong synergistic effect among the NiAlO, PPy and Ago, where the PPy shell provided more anchoring sites for Ago, and Ag NPs had excellent electrocatalytic reduction ability to H2O2 resulting in high response towards H2O2. The present @PPy-Ag sensor demonstrated an attractively electrocatalytic activity in the H2O2 reduction, which showed a wide linear detection range, low LOD, and high sensitivity. Furthermore, the sensor was also practically applied to determine H2O2 in the water samples from different sources. The present work provided a low cost, simple preparation, environmental friend and green synthetic method to prepare the NiAlO@PPy-Ag material, which had a great potential commercial application for H2O2 detection.
Data availability
The authors do not have the permission to share data.
References
Dong Q, Ryu H, Lei Y (2021) Metal oxide based non-enzymatic electrochemical sensors for glucose detection. Electrochim Acta 370:137744
Yang X, Qiu P, Yang J, Fan Y, Wang L, Jiang W, Cheng X, Deng Y, Luo W (2021) Mesoporous materials-based electrochemical biosensors from enzymatic to nonenzymatic. Small 17(9):1904022
Thatikayala D, Ponnamma D, Sadasivuni KK, Cabibihan J-J, Al-Ali AK, Malik RA, Min B (2020) Progress of advanced nanomaterials in the non-enzymatic electrochemical sensing of glucose and H2O2. Biosensors 10(11):151
Rafique N, Hannan Asif A, Hirani RAK, Wu H, Shi L, Zhang S, Sun H (2022) Binder free 3D core-shell NiFe layered double hydroxide (LDH) nanosheets (NSs) supported on cu foam as a highly efficient non-enzymatic glucose sensor. J Colloid Interface Sci 615:865–875
Liu M, An M, Xu J, Liu T, Wang L, Liu Y, Zhang J (2021) Three-dimensional carbon foam supported NiO nanosheets as non-enzymatic electrochemical H2O2 sensors. Appl Surf Sci 542:148699
Ma X, Tang K, Yang M, Shi W, Zhao W (2021) Metal-organic framework-derived yolk-shell hollow Ni/NiO@C microspheres for bifunctional non-enzymatic glucose and hydrogen peroxide biosensors. J Mater Sci 56(1):442–456
Islam MDF, Islam MT, Hasan MM, Rahman MM, Nagao Y, Hasnat MA (2022) Facile fabrication of GCE/Nafion/Ni composite, a robust platform to detect hydrogen peroxide in basic medium via oxidation reaction. Talanta 240:123202
Xiao L, Yang K, Duan J, Zheng S, Jiang J (2022) The nickel phosphate rods derived from Ni-MOF with enhanced electrochemical activity for non-enzymatic glucose sensing. Talanta 247:123587
Azeredo NFB, Gonçalves JM, Rossini PO, Araki K, Wang J, Angnes L (2020) Uric acid electrochemical sensing in biofluids based on Ni/Zn hydroxide nanocatalyst. Microchim Acta 187(7):379
Cai J, Vasudevan SV, Wang M, Mao H, Bu Q (2022) Microwave-assisted synthesized renewable carbon nanofiber/nickel oxide for high-sensitivity detection of H2O2. Electroanal Chem 924:116876
Wang Z-Y, Chang H-W, Tsai Y-C (2023) Synthesis of bimetallic Ni-Co phosphide nanosheets for electrochemical non-enzymatic H2O2 sensing. Nanomaterials 13(1):66
John L, Benny AR, Cherian SY, Narahari A, Varghese A, Hegde G (2021) Electrochemical sensors using conducting polymer/noble metal nanoparticle nanocomposites for the detection of various analytes: a review. J Nanostruct Chem 11(1):1–31
Emir G, Dilgin Y, Ramanaviciene A, Ramanavicius A (2021) Amperometric nonenzymatic glucose biosensor based on graphite rod electrode modified by Ni-nanoparticle/polypyrrole composite. Microchem J 161:105751
Jain R, Jadon N, Pawaiya A (2017) Polypyrrole based next generation electrochemical sensors and biosensors: a review. TrAC Trends Anal Chem 97:363–373
Sun R, Zhang W, Zhang J, Zhao Y, Yuan H, Guan H, Huang C, Ma C, Ge J, Tian W, Hao L (2023) Flexible and conductive polypyrrole/Ag/cellulose paper bar with sensitive response to multiple stimulus of pH, mist, breath and finger press. Mater Lett 341:134260
Rizi KS, Hatamluyi B, Rezayi M, Meshkat Z, Sankian M, Ghazvini K, Farsiani H, Aryan E (2021) Response surface methodology optimized electrochemical DNA biosensor based on HAPNPTs/PPY/MWCNTs nanocomposite for detecting mycobacterium tuberculosis. Talanta 226:122099
Shafa M, Ahmad I, Hussain S, Asif M, Pan Y, Zairov R, Alothman AA, Ouladsmane M, Ullah Z, Ullah N, Lai C, Jabeen U (2023) Ag-Cu nanoalloys: an electrochemical sensor for H2O2 detection. Surf Interfaces 36:102616
Gholami M, Koivisto B (2019) A flexible and highly selective non-enzymatic H2O2 sensor based on silver nanoparticles embedded into Nafion. Appl Surf Sci 467-468:112–118
Han J, Zeng H-Y, Cao X, Chen C-R (2017) Cycling stability of iron-based layered double hydroxide thin-films for battery-type electrode materials. J Mater Sci Mater Electron 28(3):2754–2762
Yuan J, Xu S, Zeng H-Y, Cao X, Pan A-DG-F, Xiao D, P.-X. (2018) Hydrogen peroxide biosensor based on chitosan/2D layered double hydroxide composite for the determination of H2O2. Bioelectrochemistry 123:94–102
Zhao S, Yi H, Tang X, Kang D, Gao F, Wang J, Huang Y, Yang Z (2018) Removal of volatile odorous organic compounds over NiAl mixed oxides at low temperature. J Hazard Mater 344:797–810
Yin J, Qi X, Yang L, Hao G, Li J, Zhong J (2011) A hydrogen peroxide electrochemical sensor based on silver nanoparticles decorated silicon nanowire arrays. Electrochim Acta 56(11):3884–3889
Sharma RK, Rastogi AC, Desu SB (2008) Manganese oxide embedded polypyrrole nanocomposites for electrochemical supercapacitor. Electrochim Acta 53(26):7690–7695
Zhang K, Zeng H-Y, Wang M-X, Li H-B, Yan W, Wang H-B, Tang Z-H (2022) 3D hierarchical core-shell structural NiCoMoS@NiCoAl hydrotalcite for high-performance supercapacitors. J Mater Chem A 10(20):11213–11224
Huo Y, Wang Z, Zhang J, Liang C, Dai K (2018) Ag SPR-promoted 2D porous g-C3N4/Ag2MoO4 composites for enhanced photocatalytic performance towards methylene blue degradation. Appl Surf Sci 459:271–280
Cao S, Li Y, Tang Y, Sun Y, Li W, Guo X, Yang F, Zhang G, Zhou H, Liu Z, Li Q, Shakouri M, Pang H (2023) Space-confined metal ion strategy for carbon materials derived from cobalt benzimidazole frameworks with high desalination performance in simulated seawater. Adv Mater:2301011
Yi T-F, Qiu L-Y, Mei J, Qi S-Y, Cui P, Luo S, Zhu Y-R, Xie Y, He Y-B (2020) Porous spherical NiO@NiMoO4@PPy nanoarchitectures as advanced electrochemical pseudocapacitor materials. Sci Bull 65(7):546–556
Shishegari N, Sabahi A, Manteghi F, Ghaffarinejad A, Tehrani Z (2020) Non-enzymatic sensor based on nitrogen-doped graphene modified with Pd nano-particles and NiAl layered double hydroxide for glucose determination in blood. J Electroanal Chem 871:114285
Wang GX, Yang L, Chen Y, Wang JZ, Bewlay S, Liu HK (2005) An investigation of polypyrrole-LiFePO4 composite cathode materials for lithium-ion batteries. Electrochim Acta 50(24):4649–4654
Yan W, Zeng H-Y, Zhang K, Long Y-W, Wang M-X (2023) Ni-Co-Mn hydrotalcite-derived hierarchically porous sulfide for hybrid supercapacitors. J Colloid Interface Sci 635:379–390
Wang Q, Zheng J (2010) Electrodeposition of silver nanoparticles on a zinc oxide film: improvement of amperometric sensing sensitivity and stability for hydrogen peroxide determination. Microchim Acta 169(3):361–365
Aparicio-Martínez E, Ibarra A, Estrada-Moreno IA, Osuna V, Dominguez RB (2019) Flexible electrochemical sensor based on laser scribed graphene/Ag nanoparticles for non-enzymatic hydrogen peroxide detection. Sensors Actuators B Chem 301:127101
Li W, Liu J, Chen C, Zhu Y, Liu N, Zhou Y, Chen S (2022) High catalytic performance non-enzymatic H2O2 sensor based on Cu2O@Cu9S5 yolk-shell nanospheres. Appl Surf Sci 587:152766
Zhang Y, Wang Q, Liu D, Wang Q, Li T, Wang Z (2020) Cu2O-BiOI isotype (p-p) heterojunction: boosted visible-light-driven photoelectrochemical activity for non-enzymatic H2O2 sensing. Appl Surf Sci 521:146434
Li Y, Tang L, Deng D, Ye J, Wu Z, Wang J, Luo L (2019) A novel non-enzymatic H2O2 sensor using ZnMn2O4 microspheres modified glassy carbon electrode. Colloids Surf B Biointerfaces 179:293–298
Sheng ZM, Gan ZZ, Huang H, Niu RL, Han ZW, Jia RP (2020) M-Nx (M = Fe, Co, Ni, Cu) doped graphitic nanocages with high specific surface area for non-enzymatic electrochemical detection of H2O2. Sensors Actuators B Chem 305:127550
Carbone M, Aneggi E, Figueredo F, Susmel S (2022) NiO-nanoflowers decorating a plastic electrode for the non-enzymatic amperometric detection of H2O2 in milk: old issue, new challenge. Food Control 132:108549
Author information
Authors and Affiliations
Contributions
Wei Yan: Writing - review & editing, Software. Hong-Yan Zeng: Conceptualization, Methodology, Supervision. Kai Zhang: Data curation, Validation, Visualization. Kai-Min Zou: Writing - original draft preparation, Investigation.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known conflict of financial interests or personal relationships that could have appeared to influence the content reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 757 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, W., Zeng, HY., Zhang, K. et al. Fabrication of Ag nanoparticles decorated on the NiAl-oxide@PPy for non-enzymatic H2O2 sensing. Ionics 29, 3759–3767 (2023). https://doi.org/10.1007/s11581-023-05138-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05138-0