Abstract
Electrochemical reduction of carbon dioxide is an attractive and challenging strategy to solve the problems of renewable energy storage and carbon neutral. However, activity, selectivity, and stability of present developing catalysts for production of C2H4 are not as high as required for practical applications. Herein, we report dezincified copper nanowires (pre-CuZn-CuNW) derived from an electrodeposited CuZn alloy by a wet chemical method for electrochemical reduction of carbon dioxide to C2H4. Compared with pre-Cu-CuNW (the copper nanowires derived from a bulk Cu), the pre-CuZn-CuNW significantly enhances the catalytic activity and the selectivity for C2H4. The partial normalized current density of the pre-CuZn-CuNW for production of C2H4 increases to 183 mA∙cm−2, which is about twice relative to pre-Cu-CuNW. The faradaic efficiency (FE) of the pre-CuZn-CuNW for C2H4 can be maintained over 46% for 24 h during carbon dioxide reduction reaction. The improved catalytic performance can be attributed to the formation of a special nanowire structure with larger electrochemical active surface area and higher intrinsic activity of the pre-CuZn-CuNW during the dealloying process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, under the theme of the era of carbon neutrality, more people have focused on energy and environmental problems caused by carbon dioxide [1]. Electrochemical reduction is also considered as the most effective CO2 conversion method [2,3,4]. It is worth mentioning that Berlingette and his research team [5] optimized the carbon dioxide reduction reaction (CO2RR) platform to improve the transmission of carbon dioxide in the H-type electrolytic cell, increasing the current density. Many metal catalysts have been used to study the reduction of CO2. Because of the wide application of ethylene (C2H4) and ethanol (C2H5OH) in industry, metal copper has good selectivity to C2+ products [6, 7], which makes the research on highly selective copper metal catalyst more meaningful [8].
Researchers focus on developing different nano-copper catalysts to improve the selectivity for C2H4 production [9,10,11]. Among them, oxide-derived copper (OD-Cu) has been extensively studied due to its excellent reduction of CO2 to C2 products [12]. Many factors are widely believed to cause the special properties of OD-Cu, such as higher grain boundary density [13], single crystal plane orientation [14], stronger local pH [8, 15], and product reabsorption [16]. When copper oxide derivatives prepared on copper foam was used as the substrate, the faradaic efficiency (FE) for C2+ production reached 70% [17]. The copper nanowire catalyst prepared on a copper mesh substrate was used for electrocatalytic reduction of CO2 at a lower overpotential [18]. Catalysts grown on these substrates lack hydrophobic permeability and cannot be used directly in flow cells. Although the use of Nafion plasma binder to prepare gas diffusion electrodes can greatly improve the current efficiency of the electroreduction reaction [19], the preparation process is complicated and some active centers of the catalyst are covered.
As one of the simple and controllable material preparation methods, electrodeposition can be used to prepare catalysts with various composition and micromorphology by adjusting electrolytes, applying potential, electrodeposition methods, etc. Pardal et al. performed a series of experiments to study the electrodeposition of copper for the electrochemical conversion of CO2 to C2 products [20]. By changing the concentration of H2SO4, the hydrogen evolution reaction was intensified, and the adhesion of hydrogen bubbles was promoted, resulting in the accumulation of copper particles around the bubbles to form a honeycomb surface. The current efficiency for C2H4 production increased significantly from around 7 to 15%. The electrolyte with 3,5-diamino-1,2,4-triazole (DAT) as an additive can be used to electrodeposit porous copper films, which retarded the deposition rate of copper and promoted the formation of copper catalysts with high surface area [21]. In this case, the FE for C2H4 production reached over 60% at − 0.5 V (vs RHE, same as below). Ryting et al. prepared Cu-Pd bimetallic catalysts on carbon paper (CP) by dynamic hydrogen bubble template electrodeposition. In an H-type electrolyzer, the FE for C2H4 production could reach 45.2% at − 1.2 V and the current density was 17.4 mA∙cm−2 [22].
Herein, we report a gas diffusion electrode (GDE) composed of dezincified copper nanowires (pre-CuZn-CuNW) derived from an electrodeposited CuZn alloy. 5,5-dimethylhydantoin (DMH) is applied as a complexing agent in the electrolyte for directly electrodeposition of the CuZn alloy on the carbon paper. The formation of nanowires and the dezincification of alloy are proceeded in one-step that is dipping in an alkaline solution. The catalytic performances of dezincified Cu nanowires for electroreduction CO2 are studied in a flow cell. Electrochemical measurements demonstrate that the catalyst has excellent C2H4 selectivity with high activity and stability due to the increased electrochemical active surface area and intrinsic activity resulted by chemical dezincification.
Experimental
Materials and methods
Carbon paper and Fuma FAA-PK-130 anion exchange membrane were purchased from Toray Industries and Gaoss Union, respectively. Deionized water was produced using a Millipore system and used throughout. All chemicals were analytical-grade reagents and used without further purification.
The micromorphologies of catalysts were characterized via scanning electron micrographs (SEM) recorded by using a ZEISS system (SUPRA55010102) and transmission electron microscopy (TEM, JEM 2100, 200 kV). X-ray diffraction (XRD, D/max2550V with Cu Kα radiation of λ = 1.541841 Å) was used to determine the crystal structures. X-ray photoelectron spectroscopy (XPS) experiments were applied on an AXIS ULTRA DLD spectrometer to analyze elemental contents.
Cyclic voltammetry (CV), linear sweep voltammetry (LSV), and amperometric current–time (i-t) curve measurements were measured by an electrochemical workstation (CHI660E). A 0.1 M KClO4 was used in a H-type cell for the measurement of CV at various scan rates, 1 M KOH solution in N2 or CO2 saturated was used in a flow cell for the measurement of LSV at 20 mV∙s−1, and 1 M KOH solution in CO2 saturated was used in a flow cell for the measurement of i-t curve. The GDE prepared in this work, a platinum electrode, and Ag/AgCl were used as the working electrode, the counter electrode, and the reference electrode, respectively. The value of the potential versus Ag/AgCl was converted to the reversible hydrogen electrode (RHE) scale using the Eq. (1):
The CV curves were applied for measurement of electrochemical active surface area (ECSA). There is a proportional relationship between ECSA and electric double layer capacitance (Cdl), as shown in Eq. (2). Cs is usually located between 20–60 μF∙cm−2, and 60 μF∙cm−2 is used in this paper [23]. In the case of comparing ECSA of catalysts composed by same metal, the value of Cdl can be used to evaluate the order of the ECSA of the catalyst because Cs is a constant.
Since the CV curves were measured in the voltage range without oxidation–reduction reaction, the potential scanning range is relatively small, and the electric double-layer capacitance remains approximately unchanged. At this time, the relationship between the non-faradaic current (ic) and the scanning rate is shown in Eq. (3). Here, ic is electric double-layer current (the difference between the cathode current and the anode current from the CV curve). When the scanning rate is plotted with the ic, a straight line is obtained. The slope of the line is the electric double-layer capacitance (Cdl).
Preparation of copper nanowires
The preparation process of the GDE loaded with copper nanowires catalyst is shown in Fig. 1. CuZn alloy was electrodeposited on a piece of hydrophobic carbon paper from an electrolyte containing copper sulfate, zinc sulfate, DMH, and potassium citrate, as Supplementary S1.1 and Table S1 introduce. In the pre-experiment, three different complexing agent systems were compared. The copper nanowire catalyst derived from copper in the electrodeposition system with DMH as the complexing agent has better catalytic properties (Fig. S1), so the electrodeposition of CuZn alloy was carried out in the DMH system.
Borrowed the wet chemical oxidation method for growing copper hydroxide nanowires on copper foil [11], chemically dezincified Cu(OH)2 nanowires on carbon paper were obtained in one step by dipping the electrodeposited sample into the solution composing by NaOH and (NH4)2S2O8. By an annealing process with high-temperature sintering (Supplementary S1.2), Cu(OH)2 nanowires turn to CuO nanowires. The next step is to obtained Cu nanowires (pre-CuZn-CuNW) on the carbon paper under a constant electrochemical reductive potential for applying as the GDE of the flow cell (Supplementary S1.3).
In order to explore the role of zinc in the precursor CuZn alloy, Cu was electrodeposited on the carbon paper and copper nanowires (pre-Cu-CuNW) were prepared for comparison in the same steps as Fig. 1 shown, for comparison with CuZn alloy and its derived copper nanowires (pre-CuZn-CuNW).
CO2 reduction electrolysis and product analysis
A flow cell (purchased from Gaoss Union Photoelectric technology co. LTD) was used in the experiment, including a cathode chamber, an anode chamber, and a CO2 gas chamber. The anion exchange membrane was used to separate the cathode and anode to prevent cathodic liquid products from flowing into the anode chamber and to ensure the transmission of hydrixude ion transmission.
Both cathode and anode chambers had a precisely machined window (12.5 mm × 8 mm), so the geometric area of the working electrode was 1 cm2. The working electrode was a GDE that prepared as “Materials and methods” shown. For a GDE, one side of carbon nanofibers (Fig. S2a) was covered by the catalyst, while carbon black and polytetrafluoroethene (PTFE) were loaded on the other side in the factory (Fig. S2b). It means that the carbon black on the back side of the catalyst provided a thin gas diffusion layer to transmit carbon dioxide to the catalyst surface. The presence of PTFE prevented the catholyte from immersing the catalyst and kept the hydrophobicity of the GDE. Thus, a GDE provided three-phase interfaces for CO2 reduction reaction taking place. The concentration (C) of cathodic gas products, such as ethylene, carbon monoxide, and hydrogen, was detected by gas chromatography (GC) and the FE of a specific product was calculated by Eq. (4):
where n is the number of electrons transferred to produce one molecule of a product, F is the Faraday’s constant (96,500 C∙mol−1), C is the concentration of a product determined by GC (at%), v is the flow rate (0.5 ml·s−1) of CO2 bubbled into the electrolyte, P and T are the pressure (101,325 Pa) and temperature (298 K) of the gas sampled by the GC sample loop, respectively, j is the total current when sampling, and R is the gas constant (8.314 J·mol−1·K−1).
Results and discussion
Formation and characterization of copper nanowires derived from CuZn alloy
CuZn alloy was successfully electrodeposited on the carbon paper. From EDS elemental maps (Fig. S3), Zn is uniformly distributed on the carbon fibers. Figure 2a shows the XRD patterns of Cu, CuZn alloy, pre-Cu-CuNW, and pre-CuZn-CuNW on GDEs. The three groups of peaks at 43.3°, 50.4°, and 74.1° correspond to (111), (200), and (220) of Cu, and peaks appearing in 26.6° can be indexed to carbon. Compared with Cu, it is noticeable that the peak near 43° of CuZn pattern shifts leftwards. The peak shift is believed to be caused by the formation of CuZn alloy during electrodeposition. The intercalation of Zn into the Cu crystal lattice leads to an increase in the interplanar spacing of Cu and causes lattice distortion [24]. Therefore, it can be confirmed that CuZn alloy is obtained by the electrodeposition.
The success of chemical dezincification requires quantitative analysis of the relative content of Zn before and after the reaction, so XPS measurement was used to verify the change of the relative content of Zn on the surface of the catalyst. The carbon element rich in the GDE can be used as a reference for comparing the content of Zn before and after the reaction. By measuring the relative contents of Zn and C in CuZn alloy and pre-CuZn-CuNW, the Zn atomic content is significantly reduced from 2.99 to 0.52% (Fig. 2b and c), which is consistent with the conclusion in literature [24] that chemical dezincification achieves dealloying. Figure 2d shows the XPS pattern of Zn 2p of pre-CuZn-CuNW. The characteristic peaks with binding energies of 1022.3 eV and 1045.3 eV correspond to the Zn 2p3/2 and Zn 2p1/2 regions. The characteristic peaks corresponding to Zn in the pre-CuZn-CuNW were not observed, which further indicated that the method of chemical dezincification successfully reduced the content of Zn in the catalyst. After the process of chemical dezincification, the left shift around 43° even occurs in the XRD spectrum, which can be explained by the fact that the vacancies left by the entry and exit of zinc atoms are still existing [24]. Zhang et al. found that the vacancies caused by zinc atoms would result in a special porous structure of catalysts with a large surface area [25].
The surface morphologies of pre-CuZn-CuNW were investigated by SEM and TEM. Compared with CuZn alloy (Fig. 3a), pre-CuZn-CuNW (Fig. 3b) has abundant nanowires and presents a radial structure. This special nanowire structure benefits from the use of NaOH and (NH4)2S2O8 in the second step, enabling the growth of nanowires at the same time as chemical dezincification. The diameter of pre-CuZn-NW is around 170 nm, which can be determined by TEM (Fig. 3c). The EDS elemental maps show the elemental distribution in the pre-CuZn-CuNW, including Cu, Zn, C, and O, as shown in Fig. 3d–g. The presence of Zn element is consistent with the XPS analysis of pre-CuZn-CuNW. The SEM images of bulk Cu and its derived pre-Cu-CuNW are shown in Fig. S4, while the distribution of the copper nanowires is not as regularly radial as that of pre-CuZn-CuNW.
Catalytic CO2RR performance of copper nanowires derived from CuZn alloy
Compared with Cu, CuZn alloy, and pre-Cu-CuNW, pre-CuZn-CuNW shows the best catalytic CO2RR performance. Their electrochemical testing curves are shown in Figs. 4, S5, and S6. LSV curves were carried out in 1 M KOH saturated CO2 or N2. As Figs. 4a and S5a show, the current density at a certain potential of pre-CuZn-CuNW for the reduction of CO2 is the highest in the potential range of 0 to − 0.6 V, which shows that the pre-CuZn-CuNW has the best catalytic activity for CO2RR. Pre-CuZn-CuNW exhibits the largest active specific surface area for electrocatalytic reduction of CO2. For calculating the Cdl and ECSA, CV curves were tested at various scan rates. According to Figs. 4b, S5b-d, S6a and b, and S7a, the values of Cdl and ECSA of four catalysts are listed in Table 1. For 1 cm2 samples in geometry, the ECSA of Cu, CuZn alloy, pre-Cu-CuNW, and pre-CuZn-CuNW are 0.20, 0.18, 0.33, and 0.41 cm2, respectively. Therefore, the pre-CuZn-CuNW has the largest ECSA among the above catalysts.
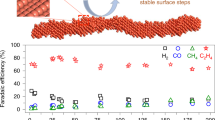
The pre-CuZn-CuNW also shows excellent selectivity for C2H4 and high current density. As seen from Fig. 5a, the FE for C2H4 production of the pre-CuZn-CuNW is 46% at − 0.8 V, while the FE for C2H4 production of other catalysts is less than 30% in potential range of − 0.4 to − 1.0 V as shown in Figs. S5e, S5g, and S6c. Furthermore, the pre-CuZn-CuNW exhibits good stability under − 0.8 V for 24 h. It can be seen from Fig. 5b that the total geometric current density of the catalyst pre-CuZn-CuNW is fluctuate and maintained stably at 170 ± 20 mA·cm−2 at − 0.8 V. The FEs for C2H4 production of the pre-CuZn-CuNW were taken every 6 h, which were stabilized above 50% and better than the initial. The pre-CuZn-CuNW exhibits a competitive selectivity and stability for C2H4 in this work compared with other similar research as Table 2 shown [26,27,28,29,30,31,32,33]. By calculating with ECSA, the partial normalized current densities for C2H4 production at − 0.8 V of Cu, CuZn, pre-Cu-CuNW, and pre-CuZn-CuNW are 49, 34, 97, and 183 mA·cm−2, respectively. It indicates that the pre-CuZn-CuNW has the best intrinsic activity for C2H4 production among these catalysts.
a FEs for gas products and geometric current density for ethylene production of pre-CuZn-CuNW under different potential in CO2RR, b stability testing of pre-CuZn-CuNW at − 0.8 V (vs RHE) in 1 M KOH using a flow cell while the black curve corresponds to the CO2RR geometric current density and the orange histograms are the FEs for ethylene production at different sampling time
It is worth noting that the order of the catalytic performance for the catalysts from high to low is pre-CuZn-CuNW, pre-Cu-CuNW, Cu, and CuZn alloy. The nanowire catalyst derived from the CuZn alloy has the best activity and selectivity for C2H4 production, but the catalytic performance of the CuZn alloy is lower than that of the Cu. To illustrate the role of Zn during the preparation of the catalysts is interesting, compared with the Cu, the CuZn alloy exhibits a lower activity and selectivity for C2H4 in the CO2RR test while the partial geometric current density for C2H4 production was 6.2 mA·cm−2 and FE for C2H4 production was 17% at − 0.8 V (Fig. S5g). Because of the addition of zinc, the CuZn alloy has a smaller ECSA than that of the Cu catalyst. It indicates that the zinc element is useless for promoting the performance of the catalyst during the CO2RR. However, for the nanowires derived from the CuZn alloy, the chemical dezincification in the process of nanowires growth not only results in a special structure of pre-CuZn-CuNW with a larger surface area than that of pre-Cu-CuNW but also provides higher intrinsic activity for C2H4 because of more CO* dimerization [34].
Conclusions
Binder-free copper-based catalyst pre-CuZn-CuNW was loaded on carbon paper by electrodeposition, wet chemical oxidation, annealing, and electrochemical reduction. The pre-CuZn-CuNW has abundant nanowires in diameter of around 170 nm and presents a radial structure. The Zn atomic content in CuZn alloy is 2.99%, while in the pre-CuZn-CuNW, it is 0.52%. The vacancies were left in nanowires by the entry and exit of zinc atoms in Cu crystal lattice during CuZn codeposition and chemical dezincification process, which cause larger electrochemical active surface area and higher intrinsic activity of the pre-CuZn-CuNW. Compared with Cu, CuZn alloy, and pre-Cu-CuNW, pre-CuZn-CuNW exhibits the highest activity, selectivity, and stability for ethylene production. In a flow cell, at − 0.8 V (vs RHE), the FE for ethylene production of pre-CuZn-CuNW is over 46% for 24 h under a total geometric current density of 170 ± 20 mA∙cm−2, which is competitive among similar research and provides a possibility for subsequent industrial applications. In the future work, the proposed reaction mechanism could be verified by more experimental and theoretical methods. In addition, the selectivity and stability of the copper nanowires catalyst may be enhanced by adjusting the ratio of Cu to Zn in the electrodeposition system.
References
Zhu DD, Liu JL, Qiao SZ (2016) Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv Mater 28:3423–3452
Clark EL, Hahn C, Jaramillo TF, Bell AT (2017) Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J Am Chem Soc 139:15848–15857
Gao D, Zhang Y, Zhou Z, Cai F, Zhao X, Huang W, Li Y, Zhu J, Liu P, Yang F, Wang G, Bao X (2017) Enhancing CO2 electroreduction with the metal-oxide interface. J Am Chem Soc 139:5652–5655
Ju W, Bagger A, Hao GP, Varela AS, Sinev I, Bon V, Roldan Cuenya B, Kaskel S, Rossmeisl J, Strasser P (2017) Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat Commun 8:944
Weekes DM, Salvatore DA, Reyes A, Huang A, Berlinguette CP (2018) Electrolytic CO2 reduction in a flow cell. Acc Chem Res 51:910–918
Mistry H, Varela AS, Bonifacio CS, Zegkinoglou I, Sinev I, Choi YW, Kisslinger K, Stach EA, Yang JC, Strasser P, Cuenya BR (2016) Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat Commun 7:12123
Bertheussen E, Verdaguer-Casadevall A, Ravasio D, Montoya JH, Trimarco DB, Roy C, Meier S, Wendland J, Norskov JK, Stephens IE, Chorkendorff I (2016) Acetaldehyde as an intermediate in the electroreduction of carbon monoxide to ethanol on oxide-derived copper. Angew Chem Int Ed Engl 55:1450–1454
Roberts FS, Kuhl KP, Nilsson A (2016) Electroreduction of carbon monoxide over a copper nanocube catalyst: surface structure and ph dependence on selectivity. ChemCatChem 8:1119–1124
Dutta A, Rahaman M, Luedi NC, Mohos M, Broekmann P (2016) Morphology matters: tuning the product distribution of CO2 electroreduction on oxide-derived Cu foam catalysts. ACS Catal 6:3804–3814
Yang KD, Ko WR, Lee JH, Kim SJ, Lee H, Lee MH, Nam KT (2017) Morphology-directed selective production of ethylene or ethane from CO2 on a Cu mesopore electrode. Angew Chem Int Ed Engl 56:796–800
Ma M, Djanashvili K, Smith WA (2016) Controllable hydrocarbon formation from the electrochemical reduction of CO2 over Cu nanowire arrays. Angew Chem Int Ed Engl 55:6680–6684
Feng X, Jiang K, Fan S, Kanan MW (2016) A direct grain-boundary-activity correlation for CO electroreduction on Cu nanoparticles. ACS Cent Sci 2:169–174
Li CW, Ciston J, Kanan MW (2014) Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508:504–507
Gupta N, Gattrell M, MacDougall B (2005) Calculation for the cathode surface concentrations in the electrochemical reduction of CO2 in KHCO3 solutions. J Appl Electrochem 36:161–172
Varela AS, Kroschel M, Reier T, Strasser P (2016) Controlling the selectivity of CO2 electroreduction on copper: the effect of the electrolyte concentration and the importance of the local pH. Catal Today 260:8–13
Schouten KJP, Pérez Gallent E, Koper MTM (2014) The influence of pH on the reduction of CO and CO2 to hydrocarbons on copper electrodes. J Electroanal Chem 716:53–57
Lum Y, Yue B, Lobaccaro P, Bell AT, Ager JW (2017) Optimizing C-C coupling on oxide-derived copper catalysts for electrochemical CO2 reduction. J Phys Chem C 121:14191–14203
Raciti D, Livi KJ, Wang C (2015) Highly dense Cu nanowires for low-overpotential CO2 reduction. Nano Lett 15:6829–6835
Wu JJ, Sharma PP, Harris BH, Zhou XD (2014) Electrochemical reduction of carbon dioxide: IV dependence of the Faradaic efficiency and current density on the microstructure and thickness of tin electrode. J Power Sources 258:189–194
Gonçalves MR, Gomes A, Condeço J, Fernandes TRC, Pardal T, Sequeira CAC, Branco JB (2013) Electrochemical conversion of CO2 to C2 hydrocarbons using different ex situ copper electrodeposits. Electrochim Acta 102:388–392
Chen X, Henckel DA, Nwabara UO, Li Y, Frenkel AI, Fister TT, Kenis PJA, Gewirth AA (2019) Controlling speciation during CO2 reduction on Cu-alloy electrodes. ACS Catal 10:672–682
Feng R, Zhu Q, Chu M, Jia S, Zhai J, Wu H, Wu P, Han B (2020) Electrodeposited Cu–Pd bimetallic catalysts for the selective electroreduction of CO2 to ethylene. Green Chem 22:7560–7565
Wang S, Zhang D, Li B, Zhang C, Du Z, Yin H, Bi X, Yang S (2018) Ultrastable in-plane 1T–2H MoS2 heterostructures for enhanced hydrogen evolution reaction. Adv Energy Mater 8:1801345–1801351
Peng Y, Wu T, Sun L, Nsanzimana JMV, Fisher AC, Wang X (2017) Selective electrochemical reduction of CO2 to ethylene on nanopores-modified copper electrodes in aqueous solution. ACS Appl Mater Interfaces 9:32782–32789
Shao X, Zhang Q, Zhang X, Yi J, Liu Y, Zhang J (2020) Nanoporous structured Sn-MWCNT/Cu electrodes fabricated by electrodeposition–chemical dezincification for catalytic CO2 reduction. Int J Energy Res 45:6273–6284
Gao D, Sinev I, Scholten F, Aran-Ais RM, Divins NJ, Kvashnina K, Timoshenko J, Roldan Cuenya B (2019) Selective CO2 electroreduction to ethylene and multicarbon alcohols via electrolyte-driven nanostructuring. Angew Chem Int Ed Engl 58:17047–17053
Wu M, Zhu C, Wang K, Li G, Dong X, Song Y, Xue J, Chen W, Wei W, Sun Y (2020) Promotion of CO2 electrochemical reduction via cu nanodendrites. ACS Appl Mater Interfaces 12:11562–11569
Loiudice A, Lobaccaro P, Kamali EA, Thao T, Huang BH, Ager JW, Buonsanti R (2016) Tailoring copper nanocrystals towards C2 products in electrochemical CO2 reduction. Angew Chem Int Ed Engl 55:5789–5792
Liang ZQ, Zhuang TT, Seifitokaldani A, Li J, Huang CW, Tan CS, Li Y, De Luna P, Dinh CT, Hu Y, Xiao Q, Hsieh PL, Wang Y, Li F, Quintero-Bermudez R, Zhou Y, Chen P, Pang Y, Lo SC, Chen LJ, Tan H, Xu Z, Zhao S, Sinton D, Sargent EH (2018) Copper-on-nitride enhances the stable electrosynthesis of multi-carbon products from CO2. Nat Commun 9:3828
Ma W, Xie S, Liu T, Fan Q, Ye J, Sun F, Jiang Z, Zhang Q, Cheng J, Wang Y (2020) Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C-C coupling over fluorine-modified copper. Nat Catal 3:478–487
Lv JJ, Jouny M, Luc W, Zhu W, Zhu JJ, Jiao F (2018) A highly porous copper electrocatalyst for carbon dioxide reduction. Adv Mater 30:e1803111
Lee SY, Chae SY, Jung H, Lee CW, Nguyen DLT, Oh H-S, Min BK, Hwang YJ (2020) Controlling the C2+ product selectivity of electrochemical CO2 reduction on an electrosprayed Cu catalyst. J Mater Chem A 8:6210–6218
Lee SY, Jung H, Kim NK, Oh HS, Min BK, Hwang YJ (2018) Mixed copper states in anodized Cu electrocatalyst for stable and selective ethylene production from CO2 reduction. J Am Chem Soc 140:8681–8689
Zheng Y, Vasileff A, Zhou X, Jiao Y, Jaroniec M, Qiao SZ (2019) Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J Am Chem Soc 141:7646–7659
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Zhang, R., Yao, Y. et al. Chemically dezincified copper nanowires catalysts with competitive selectivity for ethylene production by carbon dioxide reduction reaction. Ionics 28, 4817–4824 (2022). https://doi.org/10.1007/s11581-022-04723-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-022-04723-z