Abstract
Electrocatalysts for hydrogen evolution made from earth’s rich elements are key to a sustainable and clean hydrogen economy. Now, using a straightforward way to make valid catalysts with preferable catalytic activity from inexpensive raw materials in large scale remains challenge. The preparation of nickel-sulfur hybrid nanoplate electrocatalyst has a deep consideration because of its ordinary preparation process, outstanding property, and fine stabilization. In this work, employing an improved chemical vapor deposition (CVD) synthesis method to fabricate a highly efficient and stable NiS2/NiS electrocatalyst prepared by changing the mass of nickel-sulfur ratios of 8:1 (NiS2/NiS-8), 4:1 (NiS2/NiS-4), 2:1 (NiS2/NiS-2), and 1:1 (NiS2/NiS-1), applied to hydrogen evolution reactions (HER). The acquired NiS2/NiS-4 showed excellent HER performance with an overpotential of 202 mV to drive 100 mA/cm2 in 1 M KOH solution with a Tafel slope of 69.0 mV/dec, also with 30 h stability testing. This vigorous catalyst, fabricated from commercial Ni foam, has a potential for industrialization of hydrogen economy and will stimulate the industrial development of nonprecious metal catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With environmental pollution and heavy consumption of fossil fuels, it becomes increasingly crucial to find a clean pollution-free energy source [1, 2]. Hydrogen, as a new type of energy, fabricated by electrochemical, is seen as a clean energy source comparable to fossil fuels [3] and has the merit of high calorific power and pollution-free combustion [4]. While, using cathode to produce molecular hydrogen during electrolysis is regarded as an effective way to manufacture large-scale hydrogen [5]. However, electrocatalyst is a slow process, and thus highly efficient cathode materials are desirable to ameliorate the energy conversion ratio. Nowadays, rare metals electrocatalysts display predominant activity, Pt-based materials are seen as the most impactful hydrogen evolution electrode, but their large-scale applications are rigorously hindered by the shortage and high cost [6]. Hence, it is significant to develop an efficient material to substitute the Pt-based group metals [7]; noble-metal-free earth-abundant materials have to be fabricated to depress the cost for the hydrogen production gas.
For acquiring preeminent hydrogen evolution electrocatalysts, a great deal of measure has been adopted to supersede noble metals by non-precious metal electrocatalysts, such as earth-abundant transition metal sulfides (Ni3S2, NiS2, MoS2, etc.) [8,9,10], carbides (Mo2C, WC, etc.) [11, 12], selenides (MoSe2, CoSe2, etc.) [13, 14], nitrides (Ni2N, Co4N, etc.) [15, 16], and phosphides (NiP2, NiP2/CoP2, etc.) [17, 18]. Well-designed nanostructures have been extensively explored, and they reveal highly efficient activity in terms of electrochemical water splitting. For example, bimetal (Ni-Mo) based sulfides [19,20,21,22,23,24] can be modulated by nitrogen incorporation to show a high efficiency and stable HER activity. There have been many reports on Ni3S2 [25], using Na2S·9H2O and commercial Ni foam with different temperatures to get 3D edge rich Ni3S2 thin film, for HER, because it is treated as one of the promising HER catalysts to replace Pt. NiS/Ni [26] also attracted vital research interest, because of its structural and electronic. Despite encouraging progress has been made, developing a kind of Pt-like electrocatalysts with good stability remains challenging. Comparatively, commercial nickel foam (NF) is promising for manufacturing efficient catalysts due to their structural integrity, rich large holes, and low price.
In this work, we synthesized highly active hydrogen evolution electrocatalyst nanoplates that were grown on Ni foam which is in situ conversion from Ni(OH)2/nickel foam. The as-prepared electrocatalyst consists of a mixture of NiS2/NiS nanoplate arrays that were synthesized in argon (Ar) atmosphere through a vapor phase sulfurization process, showing a good morphological property, which can assist the desorption of as-generated H2 bubbles in HER process with the many reactive active sites on the electrode surface. Furthermore, Ni foam, as the base of the electrocatalyst, has strong electron conductivity and is beneficial to the contact between the electrocatalyst and the electrolyte. A large number of literatures show that many heterogeneous interfaces are beneficial to the decomposition of water and improvement of the alkaline HER performance.
The experimental results show that the prepared hydrogen evolution catalyst possesses nice catalytic activity with a low starting overpotential and a corresponding Tafel slope of 69 mV/dec. And an overpotential of 202 mV was observed for the generation of HER current of 100 mA/cm2. Our catalyst also showed good stability after a test period up to 30 h. On the one hand, this work uses a simple method to prepare efficient and stable electric catalysts, and on the other hand, we provide a method for large-scale practice for converting transition metal nickel into sulfide.
Experimental
Preparation of NiS2/NiS electrocatalysts
NiS2/NiS electrocatalyst was synthesized by direct vulcanizing the hydrothermal Ni(OH)2/ Ni foam in a tube furnace in a direction perpendicular to the Ar gas flow. A piece of 1 cm × 3 cm × 2 mm Ni(OH)2/Ni foam was ultrasonically treated with ethanol and 3 M HCl, each for 30 min, to eliminate surface oxides and contamination, and then rinsed with distilled water. First, the treated Ni(OH)2/Ni foam was put into the oven with a temperature of 60 ℃, which sustains 6 h. Then, a ceramic boat loaded sulfur powder and a small piece of Ni(OH)2/Ni foam on the side near the sulfur powder were placed in the heat zone of tube furnace. The temperature was kept at 400 ℃ for 1 h for the growth. After that, the furnace was automatically turned off and naturally cooled down to room temperature under Ar atmosphere. For the best performing NiS2/NiS-4 sample, the nickel-sulfur mass ratio is 4:1. For comparison, other samples were also prepared with nickel-sulfur ratios which are 8:1 (NiS2/NiS-8), 2:1 (NiS2/NiS-2), and 1:1 (NiS2/NiS-1). All of the electrolytes are sulfured in tube furnace by tuning temperature to 400 ℃ for 1 h. The mass loading of catalyst NiS2/NiS-4 on NF was about 2.0 mg cm−2. The contrast electrode of Pt/C was fabricated by drop-coating Pt/C catalyst solution on Ni foam. Pt/C catalyst ink was prepared by dispersing 10 mg Pt/C (20 wt%) and 25 μL Nafion solution in 975 μL isopropanol. A 200 μL of powder ink was loaded onto as-cleaned NF (1 × 1 cm2), followed with the dry in air at room temperature. The average mass loading of Pt/C on NF is about 2.0 mg·cm−2.
Materials characterization
The crystal structures of the samples were characterized using X-ray diffraction (XRD, Smart Lab) and X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250XI). The surface morphology of as-prepared NiS/NiS2 were characterized by scanning electron microscopy (FESEM, JSM-7610F), energy-dispersive X-ray spectroscopy (EDX), and microstructure which were observed by transmission electron microscopy (TEM, FEI Tecnai F20).
Electrochemical characterization
The electrochemical property of hydrogen evolution reaction was investigated at room temperature and in a standard three-electrode system using a CHI660E electrochemical workstation (CH Instruments, Shanghai, China) and using 1 M KOH as the electrolyte under a nitrogen saturated atmosphere. NiS2/NiS, Hg/HgO, and graphite electrode were used as the working electrode, reference electrode, and counter electrode, respectively. All the measured potentials were converted to reversible hydrogen electrode (RHE). The conversion formula is E (vs RHE) = E (vs Hg/HgO) + 0.059*pH + 0.098 for Hg/HgO electrode in 1 M KOH electrolyte. Linear sweep voltammetry (LSV) was measured by changing the potential from − 0.9 to − 1.5 V vs Hg/HgO with a scan rate of 5 mV/s and managed with IR compensation unless being specifically indicated. The electrochemical double-layer capacitances (Cdl) data of NiS2/NiS electrodes were evaluated by the measurement of cyclic voltammetry (CV) at diverse scan rates (20, 40, 60, 80, 100, and 120 mV/s) in the range between 0.24 and 0.3 V (vs RHE). The electrochemical impedance spectroscopy (EIS) tests were taken in the frequency range of 100 kHz to 0.01 Hz. It should be emphasized that the KOH solution is treated by high purity N2 to remove oxygen before each electrochemical measurement. Long-term stability tests were conducted using galvanostatic technique with applied current density of 25 mA/cm2 in 1 M KOH.
Results and discussion
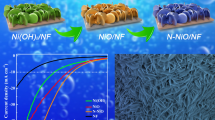
The NiS/NiS2 nanoplates were straightly fabricated in Fig. 1. The Ni(OH)2/Ni foam were converted to NiS2/NiS/NF through a chemical vapor deposition process. This preparation process is mild, simple, and very suitable for large-scale production.
Structural characterization
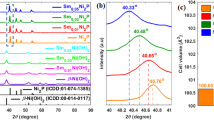
To further explore the crystal-line phases of the samples, the X-ray diffraction (XRD) patterns of NiS2/NiS-1, NiS2/NiS-2, NiS2/NiS-4, and NiS2/NiS-8 are presented in Fig. 2a. The results demonstrate that the NiS2/NiS/NF were successfully synthesized. The peaks in XRD pattern confirm the co-existence of NiS2, NiS, and Ni. The peaks at 2θ = 27.13°, 31.43°, 35.25°, 38.75°, 45.04°, 47.94°, and 53.38° are assigned to the (111), (200), (210), (211), (220), (221), and (311) planes of cubic structured NiS2 (PDF#88–1709), while the peaks at 2θ = 44.49°, 51.84°, and 76.38° are assigned to the (111), (200), and (220) planes of cubic structured Ni foam. The peaks marked with red lines are consistent with hexagonal structured NiS (PDF#75–0613), which the peaks at 2θ = 30.14°, 33.79°, 34.66°, 45.91°, 53.54°, and 61.09° are assigned to the (100), (002), (101), (102), (110), and (103)planes. And with the increasing of the mass of S powder, the intensity of XRD peaks is getting stronger. No other peaks were observed.
X-ray photoelectron spectroscopy (XPS) is used to explore the element composition and atomic valence states of the sample surface. As shown in Fig. 2b, the XPS spectrum shows the presence of Ni and S elements in NiS2/NiS. The Ni 2p spectrum (Fig. 2c) can be well fitted to 2p3/2 (located at 855.80 eV) and 2p1/2 (located at 873.08 eV), which indicates the existence of Ni3+ and one set of shakeup satellites, accompanying with the binding energy of 862.5 eV and 878.6 eV, respectively. In the S 2p region (Fig. 2d), the binding energies at 162.68 eV (S 2p3/2) and 163.08 eV (S 2p1/2) are ascribable to Ni-S bonds. The high binder energy at 167.6 eV is the satellite peak, which can be regarded as oxides formed on the surface of metal sulfide [27]. XPS analysis and XRD results demonstrate the presence of NiS2, NiS.
The surface morphology of the prepared catalysts was explored by SEM. The Ni(OH)2/Ni foam with a smooth and large scale of the surface was converted into NiS2/NiS nanoplates (Fig. 3a and d). Indicating that after the chemical vapor deposition, the NiS2/NiS presented a nanoplate arrays with a thick diameters about ~ 35 nm which supported a large scale of specific surface area and the surface of Ni foam getting tougher than others. In order to check the sulfuration degree of different mass of nickel-sulfur ratio, different samples were prepared and then analyzed. Compared to the nickel-sulfur mass ratio of 1:1, 2:1, and 8:1, nanoplates with a little difference were formed with the nickel-sulfur mass ratio of 4:1. The NiS2/NiS-4 formed much more nanoplates than others. Moreover, the single nanoplate is several dozens of nanometers in thickness and distributes uniformly. The special structure of the NiS2/NiS is referred to as “nanoplate”; this kind of heterojunction structure not only increases more accessible active sites which plays an important role in the catalytic performance [28, 29], enabling fast redox reaction, but also provides abundant paths for rapid mass transport and facilitates gas bubble diffusion, so the water splitting catalytic activities was better improved.
The detailed morphology and microstructure of NiS2/NiS were characterized by the TEM (Fig. 3b). The surfaces of smooth nanoplates are converted to tough nanoplate, forming a hierarchical nanoplate structure, which would provide more active sites for electrochemical reactions occurring at the electrode/electrolyte interface. Figure 3e is an enlarged view of nanoplate with a thin diameter. The HRTEM images of NiS2/NiS nanoplate are presented in Fig. 3c and f with well-defined lattice fringes. The lattice spacings of 0.3 nm and 0.23 nm correspond to the (100) plane of NiS and the (211) plane of NiS2, respectively, confirming the chemical composition of NiS2/NiS. In addition, elemental mapping was performed to illustrate the element distribution and structural properties of the NiS2/NiS (Fig. 3g–i). It is observed that the Ni and S elements are all evenly distributed throughout the nanoplates. Differently from that, Ni shows high content in the edges of the nanoplates, and S shows high content in the center of the nanoplates.
Electrochemical performance
The electrocatalytic performance of NiS2/NiS was evaluated on a three-electrode system in 1 M KOH. Electrodes composed of bare NF, NiS2/NiS-1, NiS2/NiS-2, NiS2/NiS-4, NiS2/NiS-8, and a commercial Pt/C for comparison, and the results are shown in Fig. 4. Figure 4a displays the LSV curves of NF, NiS2/NiS-1, NiS2/NiS-2, NiS2/NiS-4, NiS2/NiS-8, and Pt/C. According to IR-corrected polarization curves, the bare NF exhibits negligible HER activity. The NiS2/NiS-4 electrode exhibits the best HER performance among all the samples. The current density of 100 mA/cm2 is about 202 mV for NiS2/NiS-4, which is smaller than NiS2/NiS-1 (372 mV), NiS2/NiS-2 (244 mV), and NiS2/NiS-8 (302 mV). Moreover, the cathodic current density of NiS2/NiS-4 could reach 250 mA/cm2 at about 245 mV. These values are comparable to those of most of the reported NiS2-based electrocatalysts toward HER in alkaline electrolyte, which demonstrate the potential of this heterostructure as an available candidate toward HER. It is worth nothing that the HER performance of NiS2/NiS has been improved significantly by adding the mass of S powder. This result demonstrates the existence of the synergistic effect between Ni and S.
Figure 4b compared the overpotential at the current density of 50 and 100 mA/cm2. At the 50 mA/cm2, the overpotentials of NF, NiS2/NiS-8, NiS2/NiS-4, NiS2/NiS-2, and NiS2/NiS-1 are 296, 148, 161, and 300 mV, respectively. The whole conductivity of NiS2/NiS-4 can be enhanced obviously. By comparing the overpotentials of different samples at different current densities, NiS2/NiS-4 shows the better electrocatalytic hydrogen analysis properties than other samples.
Electrochemical impedance spectra (EIS) was used to characterize the electrode kinetics of the interface reactions during the HER process, which was operated at the potential of − 200 mV vs RHE. Through Fig. 4c of the EIS, the size of the charge-transfer resistances (Rct) of each sample can be obtained. Obviously, the Rct of NiS2/NiS-1, NiS2/NiS-2, NiS2/NiS-4, and NiS2/NiS-8 are 4.7 Ω, 3.2 Ω, 1.8 Ω, and 4 Ω, respectively. NiS2/NiS-4 exhibits the lowest charge transfer resistance (Rct). This means an efficient charge transfer rate of NiS2/NiS-4, which is consistent with the fact that NiS2/NiS-4 exhibits an excellent electrocatalytic capability. The intimate nickel-sulfur ratio of 4:1 can take advantage of the high conductivity of Ni foam.
To evaluate the dynamic process involved in HER, the Tafel plots of all samples were measured, and the corresponding results are given in Fig. 4d. Evidently, NiS2/NiS-1, NiS2/NiS-2, NiS2/NiS-4, and NiS2/NiS-8 have the Tafel slope of 189.4 mV/dec, 110 mV/dec, 69 mV/dec, and 138 mV/dec, respectively. So, NiS2/NiS-4 displayed a smaller Tafel slope value than others. It is obvious that NiS2/NiS-4 exhibits a better dynamic process in HER.
To further explore the mechanism of enhanced conductivity of NiS2/NiS-4, Cdl was carried out to estimate the electrochemically active surface area (ECSA) of the samples [30]. Figure 5a, b, and c show the CV patterns of different nickel-sulfur mass ratios of 1:1, 2:1, 4:1, and 8:1. A series of scan rates were conducted to extract the linear relationship of current density difference (Δj/2) against scan rate. From Fig. 5d, it can be seen that NiS2/NiS-4 exhibits the Cdl of 31.6 mF/cm2 among other samples. As can be clearly seen, the NiS2/NiS-1, NiS2/NiS-2, and NiS2/NiS-8 show the Cdl of 15.2 mF/cm2, 25.4 mF/cm2, and 23.2 mF/cm2, respectively. This result indicates that NiS2/NiS-4 has a larger effective electrochemical area and exposes more available active sites on the surface, which is beneficial to the HER activity. Therefore, the catalytic properties of NiS2/NiS-4 could be ascribed to the large specific surface area.
Stability is another important parameter to measure the performance of the catalyst. Applying a current density of 25 mA/cm2 to the catalyst and the overpotential is stable for 30 h (Fig. 6a). No prominent change is found, so the nickel-sulfur electrocatalyst perform an outstanding electrocatalyst stability in alkaline solution. Accelerated cyclic voltammetry was carried out for 3000 cycles between − 0.5 V and 0 V vs RHE at the scan rate of 50 mV/s. Compared to the initial LSV curve, there is just a little change after 3000 cycles (Fig. 6b), which may show a nice potential to be a stable electrocatalyst and imply it as a durable hydrogen evolution electrode in large scale [31]. The consistent high resolution peak position of Ni 2P and S 2p for NiS2/NiS/Ni hybrid electrode before and after HER test indicates that the catalyst has good stability in Fig. 6c and d. The SEM images of NiS2/NiS/Ni also indicate that the catalyst remains a good stability (Fig. 6e and f).
Stability test of the catalyst. a Steady-state chronopotentiometry curve of NiS2/NiS-4 under a 25 mA/cm2 current density with a stable voltage for 30 h. b The polarization curves of NiS2/NiS-4 before and after 3000 CV cycles. c,d The XPS plot of nickel and sulfur element before and after 1000 CV circles. e,f The SEM image of NiS2/NiS-4 before and after 3000 cycles
Conclusion
In summary, a highly efficient electrocatalyst for hydrogen evolution is designed by chemical vapor deposition. Via controllably tailor the mass of S powder, the constructed NiS2/NiS nanoplates catalysts exhibit excellent catalytic activity for HER, with low Tafel slope of 69 mV/dec and a low overpotential of 202 mV to achieve 100 mA/cm2. Benefiting from the strongly coupled NiS2/NiS interface, the enriched electrons on in situ formed NiS2/NiS domains contribute to HER performance improvement. The promising HER catalytic activity of this electrode is the consequence of its numerous active sites from a large surface area, thereby promoting its catalytic activity. We believe that our study will significantly advance the development of efficient HER catalysts for eventually large-scale commercialization of hydrogen evolution.
References
Wu Y, Liu X, Han D et al (2018) Electron density modulation of NiCo2S4 nanowires by nitrogen incorporation for highly efficient hydrogen evolution catalysis. Nat Commun 9(1):1425
Huang C, Cheng S, Yu L et al (2019) Electrolyzer with hierarchical transition metal sulfide and phosphide towards overall water splitting. Mater Today Phys 11:100162
Schenato M et al (2016) Effect of annealing and nanostructuring on pulsed laser deposited WS2 for HER catalysis. Appl Catal A-Gen 510:156–160
Xiong B, Chen L, Shi J (2018) Anion-containing Noble-metal-free bifunctional electrocatalysts for overall water splitting. Acs Catal 8(4):3688–3707
Wu Z-Z, Fang B-Z et al (2012) WS2 nanosheets as a highly efficient electrocatalyst for hydrogen evolution reaction. Appl Catal B-Environ 125:59–66
Benck JD et al (2016) Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. Acs Catal 4(11):3957–3971
Zhou H-Q, Yu F, Huang Y-F et al (2016) Efficient hydrogen evolution by ternary molybdenum sulfoselenide particles on self-standing porous nickel diselenide foam. Nat Commun 7(1):12765
Zhang D-W, Li J-W, Luo J-X et al (2018) Ni3S2 nanowires grown on nickel foam as an efficient bifunctional electrocatalyst for water splitting with greatly practical prospects. Nanotechnology 29(24):245402
Yin J, Jin J, Zhang H et al (2019) Atomic arrangement in metal-doped NiS2 boosts the hydrogen evolution reaction in alkaline media. Angew Chem Int Edit 131(51):18676–18682
Wang H, Xiao X, Liu Sh-Y et al (2019) Structural and electronic optimization of MoS2 edges for hydrogen evolution. J Am Chen Soc 141:18578–18584
Sun J-H, Liu J-N, Chen H et al (2020) Strongly coupled Mo2C and Ni nanoparticles with in-situ formed interfaces encapsulated by porous carbon nanofibers for efficient hydrogen evolution reaction under alkaline conditions. J Colloid Interf Sci 558:203720756
Han N, Yang K-R, Lu Z et al (2018) Nitrogen-doped tungsten carbide nanoarray as an efficient bifunctional electrocatalyst for water splitting in acid. Nat Commun 9(1):924
Oh NK, Kim C, Lee J et al (2019) In-situ local phase-transitioned MoSe2 in La0.5Sr0.5CoO3-δ heterostructure and stable overall water electrolysis over 1000 hours. Nat Commun 10:1723
Zhang G, Gao J, Wang G et al (2020) Ternary molybdenum sulfoselenide based hybrid nanotubes boosts potassium-ion diffusion kinetics for high energy/power hybrid capacitors. J Mater Chem A 8(28):13946–13954
Ma Z-Y, Li Z-C, Li S-H et al (2018) Nanostructured Ni2N thin films magnetron-sputtered on nickel foam as efficient electrocatalyst for hydrogen evolution reaction. Mater Lett 229:148–151
Yao N, Li P, Zhou Z et al (2019) Synergistically tuning water and hydrogen binding abilities over Co4N by Cr doping for exceptional alkaline hydrogen evolution electrocatalysis. Adv Energy Mater 9(41):1902449
Cao S, Chen Y, Wang C-J et al (2014) Highly efficient photocatalytic hydrogen evolution by nickel phosphide nanoparticles from aqueous solution. Chem Commun 50(72):10427–10429
Patel M, Ali M, Ahmad J, Dar M, Majid K, Lone S, Puthusseri D, Wahid M (2020) Aligned NiP2/CoP2 nanoneedle arrays obtained over carbon fiber paper by selective temperature control for efficient HER electrocatalysis. Mater Lett 278:128456
Huang C-Q, Yu L, Zhang W et al (2020) N-doped Ni-Mo based sulfides for high-efficiency and stable hydrogen evolution reaction. Appl Catal B-Environ 276:119137
Wang W-P, Wang W-J, Xu Y, Ren X-X, Liu X, Li Z-C (2021) Synthesis of Ni3S4/NiS2/FeS2 nanoparticles for hydrogen and oxygen evolution reaction. Appl Surf Sci 560:149985
Xu J-C, Rong J, Zheng Y-H, Zhu Y, Mao K-L, Jing Z-F, Zhang T, Yang D-Y, Qiu F-X (2021) Construction of sheet-on-sheet hierarchical MoS2/NiS2 heterostructures as efficient bifunctional electrocatalysts for overall water splitting. Electrochim Acta 385:138438
Li S-J, Yang G-L, Ge P, Lin H-W, Wang Q, Ren X-H, Luo S-Q, Philo D, Chang K, Ye J-H (2021) Engineering heterogeneous NiS2/NiS cocatalysts with progressive electron transfer from planar p-Si photocathodes for solar hydrogen evolution. Small Methods 5(4):2001018
Wang M, Jian K-L, Lv Z-P, Li D, Fan G-Q, Zhang R, Dang J (2021) MoS2/Co9S8/MoC heterostructure connected by carbon nanotubes as electrocatalyst for efficient hydrogen evolution reaction. J Mater Sci Technol 79:29–34
Li J-D, Arthur L, Liang J-X, Shi F, Li K, Jia J-P (2021) High proportion of 1 T phase MoS2 prepared by a simple solvothermal method for high-efficiency electrocatalytic hydrogen evolution. Chem Eng J 422:130100
Zhang H-N, Liu Y, Zhu C, Ma X-G (2020) Influence of annealing process on the electrochemical properties of Ni3S2 electrode for stable supercapacitors. J Energy Storage 32:101946. https://doi.org/10.1016/J.EST.2020.101946.
Yan C-Y, Huang J-W, Wu C-Y, Li Y-Y, Tan Y-C, Zhang L-Y, Sun Y-H, Huang X-N, Xiong J (2020) In-situ formed NiS/Ni coupled interface for efficient oxygen evolution and hydrogen evolution. J Mater Sci Technol 42(07):10–16
Kuang P-Y, He M, Zou H-Y, Yu J-G, Fan K (2018) 0D/3D MoS2 -NiS2 /N-doped graphene foam composite for efficient overall water splitting. Appl Catal B- Environ 254:15–25
Li D-J et al (2014) Molybdenum sulfide/N-doped CNT forest hybrid catalysts for high-performance hydrogen evolution reaction. Nano Lett 14(3):1228–33
Wu Y-Y, Liu Y-P, Li G-D et al (2017) Efficient electrocatalysis of overall water splitting by ultrasmall NixCo3−xS4 coupled Ni3S2 nanosheet arrays. Nano Energy 35:161–170
Mishra IK, Zhou H-Q et al (2018) Highly efficient hydrogen evolution by self-standing nickel phosphide-based hybrid nanosheet arrays electrocatalyst. Mater Today Phys 4:1–6
Liu F, He W-J, Li Y et al (2021) Activating sulfur sites of CoS2 electrocatalysts through tin doping for hydrogen evolution reaction. Appl Surf Sci 546:149101
Funding
This work was financially supported by the National Natural Science Foundation of China under Project No. 51602186, the Shaanxi Province Natural Science Foundation of China under Project No. 2020JM-599 and 2020JQ-870, and the Opening Fund of National and Local Joint Engineering Laboratory for Slag Comprehensive Utilization and Environmental Technology under Project No. SLGPT2019KF01-04.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, X., Li, W., Ai, T. et al. An efficient hydrogen evolution by self-supported nickel sulfur-based hybrid nanoplate electrocatalyst. Ionics 28, 353–360 (2022). https://doi.org/10.1007/s11581-021-04301-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-04301-9