Abstract
Electrode-electrolyte interface side reactions cause the fast capacity fading of Ni-rich cathode, especially at high cutoff voltages and high temperatures. This is the main obstacle for their commercial application. Herein, a hybrid ZrO2-Li2ZrO3 (LZO) coating layer was fabricated through a wet process on LiNi0.7Co0.15Mn0.15O2 (NCM) cathode materials. The structure and chemical composition of the ZrO2-Li2ZrO3 coating layer were studied by the characterizations of XRD, SEM, TEM, and XPS. The ZrO2-Li2ZrO3-coated LiNi0.7Co0.15Mn0.15O2 cathode improved cycling stabilities at both 25 and 55 °C in the case of high cutoff voltage. In detail, ZrO2-Li2ZrO3-coated NCM (LZO-NCM) delivers a 187.40 mAh g−1 with 83.4% capacity retention after 100 cycles at 1C over 3.0–4.5 V, and it is far higher than that of uncoated sample (71.4%). At elevated temperature (55 °C), the ZrO2-Li2ZrO3-coated sample also maintains 79% capacity retention after 100 cycles (3.0–4.5 V, 1C), which is higher than the 61.5% capacity retention of the pristine electrode. The hybrid ZrO2-Li2ZrO3 coating could not only keep the bulk structure stability but also prevent the electrochemical resistance which varies after cycles. Thereby, the electrochemical properties were improved significantly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past decades, lithium-ion batteries (LIBs) have been rapidly developed and applied in most fields, especially in electric vehicles [1,2,3,4]. LIBs with high energy density are of significance in EVs to meet the requirement of longer endurance mileage [5, 6]. One of the most important factors affecting the LIB energy density is the cathode material, among which the Ni-rich cathodes have attracted much attention because of its relatively high capacity [7,8,9,10]. Generally, the increase of capacity can be achieved by increasing the nickel content in Ni-rich cathodes and/or enhancing the cutoff voltage [11,12,13,14,15].
However, Ni-rich cathodes such as LiNi0.7Co0.15Mn0.15O2 and LiNi0.8Co0.1Mn0.1O2 have so far been retarded by electrode-electrolyte interface side reactions, which leads to the low capacity retention with dramatic voltage plateau dropping, especially at high cutoff voltages and high temperatures [16,17,18,19]. In the higher voltage charging state, the highly oxidized and active Ni4+ on the surface of the material is prone to interface side reaction with electrolyte, leading to the surface structure instability of the cathode materials due to the irreversible phase transition on the surface [20,21,22]. In addition, the residual lithium on the particle surface of Ni-rich cathode materials can bring out more dissolution of the transition metals from the particle surface, leading to the capacity fading [23, 24]. Another problem with Ni-rich cathode materials is related to structural instability in high temperature. Transition metal ions dissolving into the electrolytes in high temperature aggravated the partial phase transformation to spinel phase, which decreases the safety performances [25, 26].
Surface coating has been proved to be one of the effective methods to solve some of the above problems [27,28,29,30,31]. By providing a shield at the material surface, the electrode surface is protected from reacting with the electrolyte, and the side reactions between the cathode and the electrolyte are prohibited so as to improve the electrochemical performances of the Ni-rich cathode materials [32,33,34,35]. The metal oxides and fast ion conductors have been developed as promising coatings. Metal oxides can be used as the HF scavenger in electrolyte, which protects the positive electrode material from HF corrosion and inhibits further decomposition of the electrolyte [32, 36]. The fast ionic conductors can improve the electrochemical performance by increasing the electrical conductivity [11, 37, 38]. In combination with the characteristics of the two cladding materials, in this paper, ZrO2-Li2ZrO3 (mark as LZO) multiphase layer was used to coat LiNi0.7Co0.15Mn0.15O2 via a wet process, and the effects of the LZO coating layer on LiNi0.7Co0.15Mn0.15O2 were systematically discussed. Furthermore, the electrochemical performance of prepared powder as cathodes was evaluated at both the room temperature and a higher temperature.
Experimental
Synthesis of LiNi0.7Mn0.15Co0.15O2
For the preparation of pristine LiNi0.7Co0.15Mn0.15O2 materials, commercial Ni0.7Co0.15Mn0.15(OH)2 (Hunan Brunep Recycling Corp., China) precursor was mixed thoroughly with LiOH·H2O (nLi/n(Ni + Co + Mn) = 1.04) and sintered at 820 °C in O2 flow for 10 h. Then, the pristine LiNi0.7Mn0.15Co0.15O2 materials (mark as NCM) were prepared.
Synthesis of ZrO2-Li2ZrO3-coated LiNi0.7Mn0.15Co0.15O2
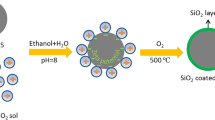
LZO-coated LiNi0.7Co0.15Mn0.15O2 (LZO-NCM) materials were synthesized in the following procedure showed in Fig. 1. A total of 200 g LiNi0.7Co0.15Mn0.15O2 powders was dispersed into 200 ml Zr(CH3COO)4 (~ 1.07 g) aqueous solution and stirred for 2 min to achieve good dispersion, and then NH3·H2O aqueous solution was slowly added into the above suspension to produce a slurry. Then, the slurry was dried at 60 °C in a vacuum oven. Lastly, the obtained solid was calcined at 600 °C in O2 flow for 6 h to obtain the final products. Based on the ICP-OES test, it can be considered that the mass ratio of ZrO2 (here, we assume that all Zr are in the state of ZrO2) to LiNi0.7Co0.15Mn0.15O2 is about 0.2 wt%.
Physical characterization
X-Ray diffraction (XRD, Panaco XˊPert PRO) with a Cu Kα radiation source was employed to identify the crystalline structures of the samples. The scanning rate was 8°/min. Particle morphology and elemental mapping of the prepared powders were observed by scanning electron microscopy (SEM, Hitachi S3400N, Japan), and the status of the coating layers of the samples was studied by transmission electron microscopy (TEM, Tecnai G12, 200 kV). X-Ray photoelectron spectroscopy (XPS, VG Multilab 2000) measurements were performed to get information on the elements’ composition on samples’ surface. Cycled coin half cells were disassembled in the argon glove box (MNIUIVESAR1220-100, MIKROUNA) and the powder scraped from the obtained electrodes washed by dimethyl carbonate (DMC) for XRD analysis.
Electrochemical testing
CR2016-type coin button cells were assembled to perform the electrochemical characterizations. Positive electrodes were prepared by mixing the cathode powders with 10 wt% acetylene black and 10 wt% polyvinylidene fluoride (PVDF) binder in the proper amount of N-methyl-2-pyrrolidone (NMP). The blended slurries were cast onto an aluminum current collector and dried under vacuum at 120 °C for 12 h. CCR2016-type coin button cells were fabricated in the Ar-filled glovebox (MNIUIVESAR1220-100, MIKROUNA, China). We used lithium foil as the negative electrode, Celgard 2400 as the separator, and 1 M LiPF6 dissolved in the liquid of EMC:EC:DMC = 1:1:1 vol ratio as the electrolyte.
The initial charge/discharge cycle at 0.1C (1C = 180 mA g−1) and the following cycle at 1C of the half cells were tested by Neware Test System (CT-4008-5V6A-S1, Shenzhen Neware Energy Tech Co., Ltd., China) at a voltage between 3.0 and 4.5 V at 25 °C and 55 °C. Cyclic voltammetry (CV, 3.0–4.5 V, 0.1 mV s−1) measurements were carried out on a CHI750E electrochemical workstation (CHI750E, Shanghai, China). After the first and 50th cycles, we performed electrochemical impedance spectra (EIS) of cells using CHI750E with an amplitude of 5 mV over a frequency range of 0.1 MHz to 10 mHz.
Results and discussion
In Fig. 2, the XRD patterns for NCM and LZO-NCM are well indexed to α-NaFeO2 structure in a hexagonal form with \( R\overline{3}m \) space group (JCPDS #09-0063) [38]. The slight shift to the lower angle for (003) peak of the LZO-NCM (Fig. 2b) indicates the possible phase variation on the surface structure as a result of a trace doping of Zr4+ ions into the NCM crystals [20]. The splitting peaks of (018)/(110) in Fig. 2c represent a well-ordered layered structure in both samples. The lattice parameters (c and a) of LZO-NCM are 14.2175 Å and 2.8714 Å, which are similar to those of the NCM sample (c = 14.2177 Å and a = 2.8715 Å). Such small changes reveal that the bulk structure of NCM is not affected by the LZO coating. As shown in Fig. 2d, weak ZrO2 signals (JCPDS #49–1642) appear in the pattern of LZO-NCM in the angle range of 26 ~ 29° (2θ) while none is observed for pristine NCM.
Figure 3a and d show the SEM images of NCM and LZO-NCM materials, in which all samples display spherical-like particles with about ~ 1 μm primary particles. The surface of coated samples (Fig. 3d) became obscure with some tiny nanoparticles while the particles of pristine NCM are clean and smooth. In more detail, the TEM images were used to characterize the structural characteristics of the samples. Compared with Fig. 3b and e, an ultra-thin coating layer can be clearly found on the particle surface after LZO modification. From the HRTEM images of pristine NCM (Fig. 3c), a layered crystal structure with a clear lattice fringe of ~ 0.479 nm agrees well with the distance of (0 0 3) crystal facet of the LiNi0.7Mn0.15Co0.15O2 (JCPDS #09-0063) [28]. In addition, the corresponding FFT pattern (inset in Fig. 3c) shows an array of hexagonal symmetry dots, and revealed the single crystalline feature of the NCM. Furthermore, the surface shows an amorphous layer, which associates with the surface lithium residual (Li2CO3/LiOH). In addition, some different lattice fringes with interplanar spacing of ~ 0.16 nm and ~ 0.296 nm are also observed near the NCM boundaries (Fig. 3f), along with the corresponding FFT pattern (inset in Fig. 3f), which corresponds to the (2 0 2) planes of Li2ZrO3 (JCPDS #41-0324) and (1 1 1) planes of ZrO2 (JCPDS #49-1642), respectively. This demonstrates the existence of Li2ZrO3 and ZrO2 on the surface of coated samples.
The energy-dispersive X-ray spectroscopy (EDS) mappings are present to check the element distribution of the LZO-NCM. Figure 4 indicates that the Zr elements are completely overlapped with Ni, Co, and Mn, suggesting that LZO coating layers were fully distributed throughout the NCM surface. This LZO multiphase coating layer is designed to act not only as a protection layer but also as an ion conductive layer, with possible effects on alleviating side reactions and improving the electrochemical performance of the LiNi0.7Mn0.15Co0.15O2, which would be discussed later.
XPS analysis was performed to find out the surface chemical compositions of pristine NCM and LZO-NCM. The survey spectra of both samples show typical Ni, Mn, and Co peaks, but Zr peaks are only observed in the LZO-NCM sample (Fig. 5a). The spectra of Ni 2p of both samples are shown in Fig. 5b. The Ni 2p binding energies show no obvious variations, suggesting that LZO coating does not affect the bulk cathode. This phenomenon is consistent with the results discussed in the previous XRD analysis. From Fig. 5c, the binding energy (BE) of Zr 3d at 182.2 eV and 184.7 eV is consistent with the reported value in ZrO2 and Li2ZrO3 bulk material [39, 40]. Figure 5d exhibits the O 1s spectra of two samples. The BE located at 531.6 eV corresponds to the absorbed oxygen from surface lithium residual, while the small peak located at 529.8 eV is attributed to the oxygen in the metal framework [41]. As can be seen from Fig. 5d, compared with NCM, the O1s peak of LZO-NCM at 531.6 eV is slightly reduced than that of NCM, indicating that LZO coating may decrease amounts of the lithium residues, which may be favorable to improving the interfacial properties and leading to excellent electrochemical properties.
Figure 6 shows the initial charge/discharge curves at 0.1C and cycle performance at 1C of two samples between 3.0 and 4.5 V at 25 °C and 55 °C. As can be seen from Fig. 6a, the initial discharge capacities and corresponding coulombic efficiencies of two samples are 200.2 mAh g−1/89.14% (NCM) and 196.3 mA h g−1/89.57% (LZO-NCM), respectively. The phenomenon that discharge capacity of the LZO-NCM electrode is lower than that of NCM electrode is probably because the coating layer is electrochemically inactive in the voltage range. With respect to the cycling performance at 1C and 25 °C (Fig. 6b), the LZO-NCM sample exhibits a lower initial discharge capacity (187.4 mAh g−1) than that of the pristine (190.2 mAh g−1). But the LZO-NCM maintains a higher capacity retention (83.4%) than the pristine NCM (71.4%) after 100 cycles. In order to further assess the effectiveness of the LZO coating layer, the cycling performance of 1C rate was tested in a severe condition of 55 °C high temperature. Figure 6c shows the results of this test for the pristine NCM and LZO-NCM electrodes, from which their capacity retentions can be found as 61.5% and 79.0%, respectively. It can be observed that the LZO coating effectively improved the cycle performance of LiNi0.7Mn0.15Co0.15O2, which is consistent with the above analysis.
The discharge curves and corresponding dQ/dV profiles of NCM and LZO-NCM for different cycles at a rate of 1C and 25 °C between 3.0 V and 4.5 V are shown in Fig. 6d–g. Compared with Fig. 6d and f, we can see that LZO-NCM electrode exhibited higher stability for capacity and discharge voltage retention during cycling. Voltage fading and capacity loss resulted from the formation of a spinel-like and rock-like structural framework associated with the relocation of transition metals atomic in the Li layer. Differential capacity analyses were performed to understand the different voltage retention behaviors between NCM and LZO-NCM. From dQ/dV curves (Fig. 6e and g), the peak shift of LZO-NCM is smaller than that of the NCM sample, indicating the suppressed polarizations after LZO coating.
The cyclic voltammograms of the NCM and LZO-NCM after the first and the 50th cycles are shown in Fig. 7a and b. The sharp anodic/cathodic peaks around 3.7 V correspond to the oxidation/reductions of the Ni2+/Ni4+ and the small anodic/cathodic peaks around 4.3 V correspond to the structural transformations of hexagonal structures (H2–H3), respectively [42, 43]. The potential differences (ΔE) between the cathodic peak and the anodic peak of NCM after the 1st and 50th cycles are 0.074 and 0.206 V, respectively, which are higher than the compared result of LZO-NCM (0.055 and 0.165 V). As in previous reports [11, 44], a smaller ΔE which represents the electrochemical reversibility indicates a smaller reaction polarization. Obviously, the ΔE becomes smaller after LZO coating, indicating that the LZO coating was helpful in improving the electrochemical performance by reducing the electrochemical polarization.
To figure out the origin of positive effect of the LZO coating layer on cycling performance, the EIS for the NCM and LZO-NCM are analyzed and measured after the first and 50th cycles in full charge to 4.5 V state. In Fig. 8a and b, the Nyquist plots of two samples consist of a semicircle in high frequency and a semicircle in middle frequency. According to previous reports, Rs refers to the solution resistance, and Rf represents the surface interface resistance at high-frequency semicircle, while Rct is assigned to the charge transfer resistance at middle-frequency semicircle [45]. Based on the equivalent circuit (Fig. 8c), the calculated resistances are obtained and listed in Table 1. After cycles, the value of Rs in NCM sample was obviously increased while that in LZO-NCM sample basically remained stable. As presented in Table 1, for both samples, their Rct values are upregulated significantly after 50 cycles, but the Rct value of LZO-NCM is remarkably increased from 34.25 to 185.0 Ω after 50 cycles, while that of NCM increased from 20.23 to 824.6 Ω. This result further suggests that the LZO coating might effectively relieve the side reactions between electrolyte and cathode, thereby suppressing the enhancement of the impedance.
To further explore the reason for improved electrochemical performance of LZO-NCM, high-resolution XPS curves of C 1s, F 1s, and Li 1s of NCM and LZO-NCM electrodes were obtained after 100 cycles. In the C 1s spectra (Fig. 9a), C 1s was almost the same at the chemical bond properties expect for intensity. The peak around 683 eV in F 1s spectra is assigned to the LiF which may increase the interface impedance considerably [11]. The peak around 53.8 eV in Li 1s spectra is assigned to the Li2CO3/ROCOOLi which mainly originated from the side reactions between electrolyte and cathode [44]. As seen from Fig. 9b and c, the intensity of LiF and Li2CO3/ROCOOLi peaks in LZO-NCM are slightly less than those in NCM, indicating that more serious electrolyte decompositions occur on NCM surface. On the contrary, the side reactions can be significantly suppressed by the LZO layer on the NCM particle surface during cycling [46]. As can be seen in Fig. 9d, both electrodes can be indexed to the typical α-NaFeO2 layered structure by the diffraction after 100 cycles, but peak intensities of NCM became weaker than those in the LZO-NCM obviously, which confirm that the LZO coating may effectively strengthen structure of LiNi0.7Mn0.15Co0.15O2 [47].
Conclusions
In summary, a hybrid ZrO2-Li2ZrO3-coated LiNi0.7Co0.15Mn0.15O2 cathode material was successfully achieved by a wet process and systematically investigated. The multifunctional ZrO2-Li2ZrO3 coating layer on NCM surface effectively reduced the side reactions between electrolyte and cathode, thereby significantly optimizing the interfacial structure of electrode and leading to lower impedance increment and enhanced electrochemical stabilities in high cutoff voltage and high temperature. The capacity retention of the LiNi0.7Co0.15Mn0.15O2 cathode was increased from 71.4 to 83.4% by coated ZrO2-Li2ZrO3 after 100 cycles between 3.0 and 4.5 V at 1C and 25 °C. Furthermore, this ZrO2-Li2ZrO3-coated cathode also showed higher capacity retention (79%) than the pristine (61.5%) after 100 cycles at 55 °C. Note that the multifunctional ZrO2-Li2ZrO3 layer can well enhance the electrochemical stabilities of Ni-rich layered oxides. We consider that it should shed some light for the surface design of Ni-rich cathodes with excellent electrochemical performance at high voltages and high temperatures.
References
Hu S, Pillai AS, Liang G, Pang WK, Wang H, Li Q, Guo Z (2019) Li-Rich layered oxides and their practical challenges: recent progress and perspectives. Electrochem Energy Rev 2:277–311. https://doi.org/10.1007/s41918-019-00032-8
Huang B, Liu D, Qian K, Zhang L, Zhou K, Liu Y, Kang F, Li B (2019) A simple method for the complete performance recovery of degraded Ni-rich LiNi0.70 Co0.15Mn0.15O2 cathode via surface reconstruction. ACS Appl Mater Interfaces 11:14076–14084. https://doi.org/10.1021/acsami.8b22529
Schipper F, Dixit M, Kovacheva D, Talianker M, Haik O, Grinblat J, Erickson EM, Ghanty C, Major DT, Markovsky B, Aurbach D (2016) Stabilizing nickel-rich layered cathode materials by a high-charge cation doping strategy: zirconium-doped LiNi0.6Co0.2Mn0.2O2. J Mater Chem A 4:16073–16084. https://doi.org/10.1039/c6ta06740a
Yang XQ, Tang ZF, Wang HY, Zou BK, Chen CH (2016) Improving the electrochemical performance of LiNi0.5Co0.2Mn0.3O2 by double-layer coating with Li2TiO3 for lithium-ion batteries. Ionics (Kiel) 22:2235–2238. https://doi.org/10.1007/s11581-016-1792-0
Zeng X, Zhu J, Yang L, Zhou L, Shao L, Hu S, Huang C, Yang C, Qian D, Xi X (2019) Electrochemical stabilities of surface aluminum-doped LiNi0.5Co0.2Mn0.3O2 single crystals under different cutoff voltages. J Electroanal Chem 838:94–100. https://doi.org/10.1016/j.jelechem.2019.02.051
Chen Y, Li Y, Li W, Cao G, Tang S, Su Q, Deng S, Guo J (2018) High-voltage electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material via the synergetic modification of the Zr/Ti elements. Electrochim Acta 281:48–59. https://doi.org/10.1016/j.electacta.2018.05.154
Dai S, Yuan M, Wang L, Luo L, Chen Q, Xie T, Li Y, Yang Y (2019) Ultrathin-Y2O3-coated LiNi0.8Co0.1Mn0.1O2 as cathode materials for Li-ion batteries: synthesis, performance and reversibility. Ceram Int 45:674–680. https://doi.org/10.1016/j.ceramint.2018.09.227
Li W, Liu X, Celio H, Smith P, Dolocan A, Chi M, Manthiram A (2018) Mn versus Al in layered oxide cathodes in lithium-ion batteries: a comprehensive evaluation on long-term cyclability. Adv Energy Mater 8:1703154. https://doi.org/10.1002/aenm.201703154
Qin C, Cao J, Chen J, Dai GL, Wu TF, Chen Y, Tang YF, Li AD, Chen Y (2016) Improvement of electrochemical performance of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode active material by ultrathin TiO2 coating. Dalt Trans 45:9669–9675. https://doi.org/10.1039/c6dt01764a
Zhang Y, Ren T, Zhang J, Duan J, Li X, Zhou Z, Dong P, Wang D (2019) The role of boracic polyanion substitution on structure and high voltage electrochemical performance of Ni-rich cathode materials for lithium ion batteries. J Alloys Compd 805:1288–1296. https://doi.org/10.1016/j.jallcom.2019.05.090
Chen Y, Li Y, Tang S, Lei T, Deng S, Xue L, Cao G, Zhu J (2018) Enhanced electrochemical properties of the Cd-modified LiNi0.6Co0.2Mn0.2O2 cathode materials at high cut-off voltage. J Power Sources 395:403–413. https://doi.org/10.1016/j.jpowsour.2018.05.088
Xu Y, Xiang W, Wu ZG et al (2018) Improving cycling performance and rate capability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials by Li4Ti5O12 coating. Electrochim Acta 268:358–365. https://doi.org/10.1016/j.electacta.2018.02.049
Du R, Bi Y, Yang W et al (2015) Improved cyclic stability of LiNi0.8Co0.1Mn0.1O2 via Ti substitution with a cut-off potential of 4.5 v. Ceram Int 41:7133–7139. https://doi.org/10.1016/j.ceramint.2015.02.026
Tornheim A, Trask SE, Zhang Z (2016) Evaluation of electrolyte oxidation stability on charged LiNi0.5Co0.2Mn0.3O2 cathode surface through potentiostatic holds. J Electrochem Soc 163:A1717–A1722. https://doi.org/10.1149/2.1051608jes
Dong P, Wang D, Yao Y, Li X, Zhang Y, Ru J, Ren T (2017) Stabilizing interface layer of LiNi0.5Co0.2Mn0.3O2 cathode materials under high voltage using p-toluenesulfonyl isocyanate as film forming additive. J Power Sources 344:111–118. https://doi.org/10.1016/j.jpowsour.2017.01.116
Feng Z, Rajagopalan R, Sun D et al (2020) In-situ formation of hybrid Li3PO4-AlPO4-Al(PO3)3 coating layer on LiNi0.8Co0.1Mn0.1O2 cathode with enhanced electrochemical properties for lithium-ion battery. Chem Eng J 382:122959. https://doi.org/10.1016/j.cej.2019.122959
Hou P, Li F, Sun Y, Li H, Xu X, Zhai T (2018) Multishell precursors facilitated synthesis of concentration-gradient nickel-rich cathodes for long-life and high-rate lithium-ion batteries. ACS Appl Mater Interfaces 10:24508–24515. https://doi.org/10.1021/acsami.8b06286
Wang M, Zhang R, Gong Y, Su Y, Xiang D, Chen L, Chen Y, Luo M, Chu M (2017) Improved electrochemical performance of the LiNi0.8Co0.1Mn0.1O2 material with lithium-ion conductor coating for lithium-ion batteries. Solid State Ionics 312:53–60. https://doi.org/10.1016/j.ssi.2017.10.017
Ren T, Zhang J, Wang D, Dong P, Duan J, Li X, Rao S, Huang D, Zhang Y (2018) Enhancing the high-voltage performances of Ni-rich cathode materials by homogeneous La2O3 coating via a freeze-drying assisted method. Ceram Int 44:14660–14666. https://doi.org/10.1016/j.ceramint.2018.05.092
Gao S, Zhan X, Cheng YT (2019) Structural, electrochemical and Li-ion transport properties of Zr-modified LiNi0.8Co0.1Mn0.1O2 positive electrode materials for Li-ion batteries. J Power Sources 410–411:45–52. https://doi.org/10.1016/j.jpowsour.2018.10.094
Li P, Zhao S, Zhuang Y, Adkins J, Zhou Q, Zheng J (2018) Improved electrochemical performance of LiNi0.8Co0.1Mn0.1O2 modified with 4-vinylbenzeneboronic acid. Appl Surf Sci 453:93–100. https://doi.org/10.1016/j.apsusc.2018.05.027
Zhang J, Ren T, Duan J, Li X, Dong P, Zhang Y, Wang D (2018) Enhanced high-voltage cycling stability of nickel-rich cathode materials by surface modification using LaFeO3 ionic conductor. JOM 71:1975–1980. https://doi.org/10.1007/s11837-019-03446-3
Dong S, Zhou Y, Hai C, Zeng J, Sun Y, Shen Y, Li X, Ren X, Qi G, Zhang X, Ma L (2019) Ultrathin CeO2 coating for improved cycling and rate performance of Ni-rich layered LiNi0.7Co0.2Mn0.1O2 cathode materials. Ceram Int 45:144–152. https://doi.org/10.1016/j.ceramint.2018.09.145
Chen T, Wang F, Li X, Yan X, Wang H, Deng B, Xie Z, Qu M (2019) Dual functional MgHPO4 surface modifier used to repair deteriorated Ni-rich LiNi0.8Co0.15Al0.05O2 cathode material. Appl Surf Sci 465:863–870. https://doi.org/10.1016/j.apsusc.2018.09.250
Hu GR, Deng XR, Peng ZD, Du K (2008) Comparison of AlPO4- and Co3(PO4)2-coated LiNi0.8Co0.2O2 cathode materials for Li-ion battery. Electrochim Acta 53:2567–2573. https://doi.org/10.1016/j.electacta.2007.10.040
Lee DJ, Scrosati B, Sun YK (2011) Ni3(PO4)2-coated Li[Ni0.8Co0.15Al0.05]O2 lithium battery electrode with improved cycling performance at 55°C. J Power Sources 196:7742–7746. https://doi.org/10.1016/j.jpowsour.2011.04.007
Li X, Zhang K, Wang M, Liu Y, Qu MZ, Zhao W, Zheng J (2018) Dual functions of zirconium modification on improving the electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2. Sustain Energy Fuels 2:413–421. https://doi.org/10.1039/c7se00513j
Huang J, Fang X, Wu Y, Zhou L, Wang Y, Jin Y, Dang W, Wu L, Rong Z, Chen X, Tang X (2018) Enhanced electrochemical performance of LiNi0.8Co0.1 Mn0.1O2 by surface modification with lithium-active MoO3. J Electroanal Chem 823:359–367. https://doi.org/10.1016/j.jelechem.2018.06.035
Xu CL, Xiang W, Wu ZG, Li YC, Xu YD, Hua WB, Guo XD, Zhang XB, Zhong BH (2018) A comparative study of crystalline and amorphous Li0.5La0.5TiO3 as surface coating layers to enhance the electrochemical performance of LiNi0.815Co0.15Al0.035O2 cathode. J Alloys Compd 740:428–435. https://doi.org/10.1016/j.jallcom.2017.12.193
Zhang Y, Xia G, Zhang J, Wang D, Dong P, Duan J (2020) Boosting high-voltage cyclic stability of nickel-rich layered cathodes in full-cell by metallurgy-inspired coating strategy. Appl Surf Sci 509:145380. https://doi.org/10.1016/j.apsusc.2020.145380
Zhang S, Ma J, Hu Z et al (2019) Identifying and addressing critical challenges of high-voltage layered ternary oxide cathode materials. Chem Mater 31:6033–6065. https://doi.org/10.1021/acs.chemmater.9b01557
Chen Y, Tang S, Deng S, Lei T, Li Y, Li W, Cao G, Zhu J, Zhang J (2019) Chemical coupling constructs amorphous silica modified LiNi0.6Co0.2Mn0.2O2 cathode materials and its electrochemical performances. J Power Sources 431:8–16. https://doi.org/10.1016/j.jpowsour.2019.05.042
Xu Y, Li X, Wang Z, Guo H, Huang B (2015) Structure and electrochemical performance of TiO2-coated LiNi0.80Co0.15Al0.05O2 cathode material. Mater Lett 143:151–154. https://doi.org/10.1016/j.matlet.2014.12.093
Wu F, Zhang X, Zhao T, Li L, Xie M, Chen R (2015) Multifunctional AlPO4 coating for improving electrochemical properties of low-cost Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 cathode materials for lithium-ion batteries. ACS Appl Mater Interfaces 7:3773–3781. https://doi.org/10.1021/am508579r
Chen Z, Qin Y, Amine K, Sun YK (2010) Role of surface coating on cathode materials for lithium-ion batteries. J Mater Chem 20:7606. https://doi.org/10.1039/c0jm00154f
Liang L, Hu G, Jiang F, Cao Y (2016) Electrochemical behaviours of SiO2-coated LiNi0.8Co0.1Mn0.1O2 cathode materials by a novel modification method. J Alloys Compd 657:570–581. https://doi.org/10.1016/j.jallcom.2015.10.177
Huang Y, Chen J, Ni J, Zhou H, Zhang X (2009) A modified ZrO2-coating process to improve electrochemical performance of Li(Ni1/3Co1/3Mn1/3)O2. J Power Sources 188:538–545. https://doi.org/10.1016/j.jpowsour.2008.12.037
Zhan X, Gao S, Cheng YT (2019) Influence of annealing atmosphere on Li2ZrO3 –coated LiNi 0.6Co0.2Mn0.2O2 and its high-voltage cycling performance. Electrochim Acta 300:36–44. https://doi.org/10.1016/j.electacta.2019.01.077
Wang D, Li X, Wang Z, Guo H, Huang Z, Kong L, Ru J (2015) Improved high voltage electrochemical performance of Li2ZrO3-coated LiNi0.5Co0.2Mn0.3O2 cathode material. J Alloys Compd 647:612–619. https://doi.org/10.1016/j.jallcom.2015.06.071
Kong JZ, Wang SS, Tai GA, Zhu L, Wang LG, Zhai HF, Wu D, Li AD, Li H (2016) Enhanced electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material by ultrathin ZrO2 coating. J Alloys Compd 657:593–600. https://doi.org/10.1016/j.jallcom.2015.10.187
Liu H, Yang Y, Zhang J (2006) Investigation and improvement on the storage property of LiNi0.8Co0.2O2 as a cathode material for lithium-ion batteries. J Power Sources 162:644–650. https://doi.org/10.1016/j.jpowsour.2006.07.028
Noh HJ, Youn S, Yoon CS, Sun YK (2013) Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J Power Sources 233:121–130. https://doi.org/10.1016/j.jpowsour.2013.01.063
Wu F, Tian J, Su Y, Wang J, Zhang C, Bao L, He T, Li J, Chen S (2015) Effect of Ni2+ content on lithium/nickel disorder for Ni-rich cathode materials. ACS Appl Mater Interfaces 7:7702–7708. https://doi.org/10.1021/acsami.5b00645
Meng K, Wang Z, Guo H, Li X, Wang D (2016) Improving the cycling performance of LiNi0.8Co0.1Mn0.1O2 by surface coating with Li2TiO3. Electrochim Acta 211:822–831. https://doi.org/10.1016/j.electacta.2016.06.110
Wang L, Zhao J, He X et al (2012) Electrochemical impedance spectroscopy (EIS) study of LiNi1/3Co1/3Mn1/3O2 for Li-ion batteries. Int J Electrochem Sci 7:345–353. https://doi.org/10.1149/2.jes120015
Hu SK, Cheng GH, Cheng MY, Hwang BJ, Santhanam R (2009) Cycle life improvement of ZrO2-coated spherical LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion batteries. J Power Sources 188:564–569. https://doi.org/10.1016/j.jpowsour.2008.11.113
Liang H, Wang Z, Guo H, Wang J, Leng J (2017) Improvement in the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material by Li2ZrO3 coating. Appl Surf Sci 423:1045–1053. https://doi.org/10.1016/j.apsusc.2017.06.283
Funding
The authors received financial support from the Government of Chongzuo, Guangxi Zhuang Autonomous Region (GC Joint Special Fund No. FA2019015), and Science and Technology Department of Guangxi Zhuang Autonomous Region (Gangxi Special Fund for Scientific Center and Talent Resources, No. AD18281073).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, W., Li, Y., Yang, L. et al. Enhanced the electrochemical performances of LiNi0.7Co0.15Mn0.15O2 cathodes by the hybrid ZrO2-Li2ZrO3 layer. Ionics 27, 479–489 (2021). https://doi.org/10.1007/s11581-020-03836-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03836-7